Abstract
Background
The purpose of this study is to describe measurements using a newly developed modified Goldmann convex tonometer (CT) 1 year after myopic laser refractive surgery. Intraocular pressure (IOP) measurements were compared with IOP values obtained by Goldmann applanation tonometer (GAT), and Ocular Response Analyzer (ORA).
Methods
Prospective double-masked study performed on thirty eyes of thirty patients that underwent laser in situ keratomileusis (LASIK; n = 19) or photorefractive keratectomy (PRK; n = 11). IOP was measured before and 3 and 12 months after surgery. Intraclass correlation coefficient (ICC) and Bland-Altman plot were calculated to assess the agreement between GAT, CT, IOPg (Goldmann-correlated IOP) and IOPcc (corneal-compensated IOP) from ORA.
Results
Twelve months after LASIK, IOP measured with CT showed the best correlation with IOP measured with GAT before surgery (GATpre) (ICC = 0.886, 95% CI: 0.703–0.956) (15.60 ± 3.27 vs 15.80 ± 3.22; p < 0.000). However, a moderate correlation was found for IOP measured with IOPcc and CT 12 months after LASIK (ICC = 0.568, 95% CI: − 0.185 – 0.843) (15.80 ± 3.22 vs 12.87 ± 2.77; p < 0.004). Twelve months after PRK, CT showed a weak correlation (ICC = − 0.266, 95% CI: − 3.896 – 0.663), compared to GATpre (17.30 ± 3.47 vs 16.01 ± 1.45; p < 0.642), as well as poor correlation (ICC = 0.256, 95% CI: − 0.332 – 0.719) with IOPcc (17.30 ± 3.47 vs 13.38 ± 1.65; p < 0.182).
Conclusions
Twelve months after LASIK, IOP measured with CT strongly correlated with GAT before surgery and could therefore provide an alternative method for measuring IOP after this surgery. More studies regarding this new convex prism are needed to assess its accuracy.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Background
Laser in situ keratomileusis (LASIK) is the most popular corneal refractive surgery performed in the last decade [1], with an estimated one million myopic patients undergoing LASIK every year [2]. In view of this circumstance, it is expected that clinical practice will involve an increasing number of patients that have undergone laser refractive surgery (LRS) in the past.
It is known that corneal biomechanics (CB) are altered after LRS [3, 4]. The long-term results of postoperative visual acuity and the safety of this frequent procedure have been widely reported [5, 6]. Regardless of the surgery performed, CB can vary with age, which may affect corneal topography, visual outcomes and variations in tonometry measurements [7,8,9,10]. Corneal stiffness may increase with time due to a growth in glycogen-induced cross-links, and lead to different wound healing responses for PRK and LASIK [11, 12]. Moreover, biases in IOP measurements may lead to glaucoma misdiagnoses, especially in myopic eyes that are also known as a risk factor for developing open-angle glaucoma [13, 14].
The Ocular Response Analyzer (ORA) is a non-contact tonometer that is less influenced by CB modifications after LRS [3] than the Goldmann applanation tonometer (GAT), which is still the gold standard for measuring intraocular pressure (IOP) in normal corneas [15,16,17]. However, IOP underestimations in GAT measurements after LRS have been widely described [18, 19], due to changes in corneal central thickness (CCT) and CB [4, 20]. To overcome this underestimation of IOP in myopic eyes post LRS, we have developed a new method for measuring IOP in this subgroup of patients: the modified Goldmann convex tonometer (CT) (Fig. 1A) [4]. Using finite element analysis, we demonstrated that the applicability of the Imbert-Fick law is compromised after myopic LRS. When GAT reaches the 3.06 mm area of applanation, the flattened centre of an operated cornea (OC) is consistent with the idea of Imbert-Fick behaviour, but not the corneal edges [4]. This indicates that there is lower resistance from the centre of an OC when we make physical contact with GAT, and as a result the IOP registered will be lower in these patients. Despite this, if a convex force is applied towards the centre of the ablated zone of an OC, a different phenomenon can be expected: initial contact pressure rises from the centre of the OC, resulting in a balance of forces comparable to that before surgery. By applying this force with the CT, we get to estimate the patient’s IOP from before their corneal procedure. In addition, this device functions exactly like GAT prisms and its measurements are reproducible by different observers [4], which implies that it is universal and simple to use, since it can be inserted in any slit-lamp or Perkins tonometer (Fig. 1B).
Recording pre-operative CCT and IOP measurements is very convenient for patient management after LRS [15] in addition to follow-up IOPm, taking into account that pre-operative measurement values do not remain stable throughout a patient’s life [4]. Given that IOP is the only risk factor in glaucoma progression that can be modified [21, 22], knowledge of the IOP baseline seems mandatory to create a prognosis profile [23, 24], even more so if LRS is not recent. Following our previous research in which we found a strong correlation between IOP using CT 3 months after myopic LASIK and GAT before surgery [4], we describe the clinical outcomes of this new Goldmann modified device in the long term, its correlations with GAT and with ORA by evaluating IOP measurements (IOPm) before and 3 and 12 months after myopic LRS.
Methods
Study design
A prospective, double-masked, single-centre, comparative study was carried out on a sample of thirty myopic subjects who were going in for femtosecond-assisted LASIK or photorefractive keratectomy (PRK) at the Barraquer Ophthalmology Centre in Barcelona. These subjects first underwent a general medical history review and a detailed eye examination. The study protocol adhered to the tenets of the Declaration of Helsinki and the centre’s Institutional Review Board approved this study. Written consent was obtained from all participants.
Exploration protocol
All right eyes were randomly selected, adding up to a total of 30 eyes. The inclusion criteria for the study were: myopic Caucasian patients over 18 years of age with a stable myopic refractive error of less than − 9 spherical dioptres (dpts), and myopic astigmatism of less than − 4 dpts. Subjects with previously diagnosed ocular pathology, previous ocular surgery or receiving treatment with medications that may affect IOP levels were excluded. All patients underwent a standardised examination at baseline, 24 h, 1 week, 1, 3, and 12 months after surgery. To simplify comparisons in the long term, only 3 IOPm controls were considered for the study: at baseline and 3 and 12 months after surgery. These included measurement of visual acuity (spherical equivalent refraction, SE), slit-lamp anterior biomicroscopy, posterior segment ophthalmoscopy and IOPm. Age, gender, and refractive error were recorded. Before recording IOPm, corneal topography was carried out by Pentacam, (Oculus, Wetzlar, Germany), to obtain anterior simulated keratometry (simK) and CCT. Moreover, maximum ablation depth (Max.Abl) and percentage of ablated tissue (PTA) were analysed. Corneal characteristics including Corneal Hysteresis (CH), Corneal Resistance Factor (CRF), Goldmann-correlated IOP (IOPg) and corneal-compensated IOP (IOPcc) were also obtained by ORA (Reichert Ophthalmic Instruments, New York) after 3 different measurements with a waveform score (WS) higher than 3.5 as recommended [25], and the best score was selected.
In each patient, IOPm were taken between 10:00 and 13:00 h (10 am and 1 pm) [18] and ORA was performed at least 10 min before GAT [26] and CT measurements. Each applanation tonometer measurement was carried out with a double-mask protocol on the same slit lamp Goldmann tonometer, which is regularly calibrated by a certified assistant. The main observer (AL) measured IOP with different devices – GAT and CT – randomly given by a second observer (MI), and with 2-min rests between measurements to avoid tonographic effects [27, 28].
Statistical analysis
Statistical analysis was performed using SPSS® (Statistical Package for the Social Sciences, version 22.0; SPSS Inc., Chicago, IL, USA), with the exception of the Bland-Altman analysis, for which jamovi 1.2 (jamovi project, 2020) was used. A nonparametric test was used due to the small sample size, and a significance level of 5% was considered in all analyses. We conducted descriptive analyses for all variables before surgery and 3 and 12 months after surgery. Descriptive values are presented as mean ± standard deviation (MD ± SD) unless stated otherwise. Comparisons between preoperative and postoperative values were performed using the Wilcoxon test, and comparisons between LASIK and PRK patients were performed using the Mann-Witney test.
GATpre was considered the current reference, and its correlation with IOPm using different tonometers was evaluated by: i) calculating the mean differences (md) between measurements with GATpre and all devices after surgery and testing for the absence of differences using the Wilcoxon test; ii) calculating the intraclass correlation coefficient (ICC) based on absolute agreement; values lower than 0.5, between 0.5 and 0.8, and greater than 0.8 were indicative of poor or weak, good, and excellent reliability, respectively [29]; and iii) constructing the Bland-Altman plot.
Results
Thirty eyes of thirty patients were enrolled in the study and met the inclusion criteria in pre- and post-controls. Nineteen (63.3%) patients underwent LASIK and eleven (36.6%) PRK. The mean age was 30.7 ± 6.5 years. Table 1 shows descriptive statistics of the normally distributed variables in the pre- and post-surgical evaluations.
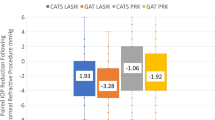
Before surgery, ORA and GAT IOPm where similar between groups. In contrast, CT overestimated IOP in relation to the other tonometers. After surgery, all tonometers significantly registered lower IOP values, except for IOPcc in the PRK subgroup, with IOPg recording the lowest compared to GATpre (Table 1). In the LASIK subgroup after 3 months (Fig. 2A), GAT and IOPcc underestimated IOP, whereas CT at 3 months provided similar values to those obtained with GATpre and ORA. After 12 months, CT values were like GAT and ORA before surgery, whereas the rest of the tonometers kept IOPm below its pre-surgery estimations (Fig. 2A).
Mean IOP changes over time obtained using different tonometer devices in pre- and post-refractive surgery evaluations after 3 and 12 months. CX: L, LASIK (A); CX:P, PRK (B); GAT, Goldmann applanation tonometer; CT, convex tonometer; IOPg, Goldmann-correlated intraocular pressure and IOPcc, corneal-compensated IOP. M, months
In the PRK subgroup (Fig. 2B), an IOP reduction was also found for all devices at 3 and 12 months after surgery. However, in this case, CT overestimated pre-surgery GAT, IOPg and IOPcc values; and IOPcc significantly registered the most stable IOPm compared to pre-surgery values.
The ICC, calculated for all patients and considering LASIK and PRK separately, presented poor or moderate correlation between GAT measurements before and after surgery (Table 2). The best correlations were observed in the LASIK subgroup between GATpre and CT at 3 months (15.60 ± 3.27 vs 14.05 ± 2.67; ICC = 0.808, 95% CI: 0.429–0.927; p < 0.000); and at 12 months (15.60 ± 3.27 vs 15.80 ± 3.22; ICC = 0.886, 95% CI: 0.703–0.956; p < 0.000). Moderate correlation was found for CT and IOPcc at 12 months (15.80 ± 3.22 vs 12.87 ± 2.77; ICC = 0.568, 95% CI: − 0.185 – 0.843; p < 0.004) (Table 2). The PRK subgroup – compared to GATpre – showed poor correlation for GAT and CT at 3 months (16.01 ± 1.45 vs 17.09 ± 2.55; ICC = − 0.196, 95% CI: − 1.817 – 0.619; p < 0.638) and also 12 months after surgery (16.01 ± 1.45 vs 17.30 ± 3.47; ICC = − 0.266, 95% CI: − 3.896 – 0.663; p < 0.642). A weak correlation was also found between CT and IOPcc at 3 and 12 months in the PRK subgroup (17.09 ± 2.55 vs 14.08 ± 2.83; ICC = 0.272, 95% CI: − 0.328 – 0.729; p < 0.116), (17.30 ± 3.47 vs 13.38 ± 1.65; ICC = 0.256, 95% CI: − 0.332 – 0.719; p < 0.182) respectively (Table 2).
Bland-Altman plots showed significant differences for GATpre and post measurements, and for CT and IOPcc after surgery. The mean differences for the entire cohort and between subgroups for IOPm before and after surgery can be found in Table 3. Considering LASIK separately, CT showed a small difference with GATpre at 3 months (md: − 1.11 mmHg, p = 0.440; LoA: − 3.32 – 5.53), the difference for CT at 12 months being the smallest (md: 0.158 mmHg, p = 0.438; LoA: − 5.58 – 5.26). On the other hand, CT showed small overestimations at 3 months in the PRK subgroup (md: 1.82 mmHg, p = 0.106; LoA: − 3.09 – 7.06) and at 12 months (md: 1.18 mmHg, p = 0.512; LoA: − 6.60 – 7.86). Additionally, CTpost significantly overestimated IOPccpost in LASIK and PRK respectively, at 3 months (md: 2.04 mmHg, p = 0.008; LoA: − 3.5 – 7.58) (md: 3.83 mmHg, p = 0.008; LoA: − 3.05 – 7.74), and 12 months (md: 2.91 mmHg, p < 0.000; LoA: − 2.53 – 8.35) (md: 3.89 mmHg, p = 0.03; LoA: − 2.95 – 8.04) respectively (graphics not shown).
Discussion
Tonometry nowadays has become a complex concern since a single tonometer cannot be referred universally for every CCT, nor can CB and prior surgical procedures such as LRS or transplants be dismissed when measuring IOP [30, 31].
A reduced CCT is not only an independent factor for developing glaucoma in the future [32], but also challenge for estimating IOP if such reduction is due to a laser-assisted procedure, regardless of the device used to obtain IOPm. It is known that after laser ablation, thicker corneas preserve more biomechanical properties than thinner corneas [33, 34], and high myopia has a greater decrease in CH and CRF properties after LASIK due to greater anterior flap stroma lamellae reduction [35]. Touboul et al. described that the lower the CH, the lower the IOP underestimation with GAT, defining CH as a risk factor for underestimating IOP [33]. Taking into account that CH is lower in glaucoma [21, 36, 37] and myopic LRS patients [20, 38], GAT should be avoided in this subgroup of patients since they will have low CH values regardless of glaucoma. Therefore, seems crucial to focus on using only specific tonometers with these patients.
This convex prism has already been tested 3 months after LRS in comparison with GAT, registering higher IOPm in PRK than in LASIK subjects (1.62 ± 2.65 mmHg vs − 0.19 ± 2.60 mmHg, respectively) [4], concurring with the third month outcomes of the present study (1.82 ± 2.55 mmHg vs − 1.11 ± 2.67 mmHg). Still, this new modified prism has never before been compared in the long term. In a global overview of our measurements, IOPm at 12 months were slightly higher than IOPm at 3 months. This could be related to a partial recovery of CB after this period. Nonetheless, this assumption must be interpreted with caution, since the main limitation of the present study is that the number of patients undergoing measurements is not appropriate for estimating significant IOP correlations with CB or biological factors such as age or degree of myopia. Nevertheless, our CTpost LASIK results concur with the outcomes referred to above, where a significant correlation with CRF and CH reduction was found between CTpost and GATpre in 73 patients 3 months after LASIK (0.15 ± 3.22 vs − 0.19 ± 2.60 mmHg, respectively) [4]. Likewise, despite our small sample in the PRK subgroup, our outcomes in the current study are comparable with those of our previous publication: we measured 29 PRK patients after 3 months and the IOP deviation was higher than in the LASIK subgroup [4], confirming that we have enough evidence to assure that the modified Goldmann CT applicability in PRK patients and in standard corneas is not reliable due to preservation of CB. Further studies in larger cohorts will focus on determining if this convex prism performs accurately in LASIK patients.
Biases in estimating IOP may come from diverse sources, like introducing different devices in our daily clinical routine or determining inaccurate IOPm for a same patient [15, 39]. To minimize the biases of GAT post LRS, we can use non-contact tonometers such as the ORA, the non-contact tonometer (NCT), and the Corvis ST; or contact tonometers like the Pascal Dynamic Tonometer (PDCT) [40]. Among these, bIOP (biomechanical corrected IOP) from Corvis ST has shown to be the most stable and accurate parameter after surface ablation or lamellar procedure [39, 41]. Nonetheless, ophthalmologists do not always have access to devices that evaluate post-operative IOP accurately. In applanation tonometry after myopic LRS, IOP estimations must be considered carefully since they do not seem to follow CCT reduction linearly due to CB modifications. We believe it could be related in part to that contact pressure profiles between applanation tonometry and the anterior corneal surface are very different in PRK compared to LASIK patients [4]. Since less corneal tissue is removed from the anterior stroma in low myopia, CB are expected to be less altered in PRK, and therefore these corneas may behave like non-OC. In this study, we have compared a non-contact tonometer with two contact tonometers. Considering all of them together, we have found a significant IOP decrease in the LASIK subgroup compared to PRK. These findings coincide with recent results for other devices. Chen et al. measured IOP after 3 months with GAT, ORA, PDCT and Corvis ST [41], and also found a significant IOP reduction in the GAT and ORA subgroups after femtosecond laser-assisted LASIK compared to TransPRK. In addition, Schallhorn et al., in a larger cohort of LASIK and PRK patients measured with NCT [34], found a higher reduction of IOP after 3 months of LASIK (− 4.57 ± 2.42 mmHg), compared to PRK (− 3.16 ± 2.53 mmHg). In our previous research, despite a strong correlation found between CT and LASIK compared to PRK, GAT IOPm were significantly lower in the first group (− 3.94 ± 2.17 mmHg vs − 2.62 ± 2.16 mmHg) [4].
This is the first time that this new convex prism is being compared to ORA IOPm. ORA has been proven to improve GAT measurements by introducing new parameters that can evaluate CB modifications and IOP after LRS [3, 42]. IOPcc is known for being less influenced by CCT variations, compared to the IOP underestimation of IOPg and GAT post LRS [3, 43, 44]. Mean differences between GAT and IOPcc readings in standard corneas are ±1.5 mmHg [45]. However, despite GAT and IOPcc show higher correlations than IOPg [26, 46], they should not be used interchangeably because there can be differences of more than 2 mmHg between them which could have clinical implications [45]. In our sample, although a significantly good correlation was found between IOPcc and CT in the LASIK subgroup after 1 year – compared to a very poor correlation in the PRK subgroup –, mean differences were significantly higher than > 2 mmHg in both groups. Moreover, even though IOPcc has shown better agreement with GAT than with IOPg before LRS, and is more stable after surgery [26, 45, 46], we believe that an agreement between the convex prism and IOPcc cannot be expected after LRS. To begin with, CTpost has already demonstrated bad agreement with GATpost [4]; besides, in our study, CTpost overestimated IOPcc and IOPg values at 3 and 12 months, having similar correlations with GAT and IOPcc after 1 year, but those correlations were weak compared to correlations with GAT before surgery. This could be associated to the fact that matching static with dynamic tonometry seems to be incongruous since it has been widely proven that comparing these devices is not accurate [26, 46, 47]. An exact comparison must not be extrapolated considering that the central areas from which the IOP is measured are different between them: in applanation tonometry after LRS, both GAT and CT contact pressure profiles are not consistent with 3.06 mm and could encompass a larger amount of tissue especially in LASIK [4] compared to the exact central corneal 3.0 mm diameter from which ORA estimates IOP, regardless of the surgical procedure performed [48, 49]. These results suggest that the three tonometers should not be used interchangeably after LRS due to clinically significant IOP variations between them.
Another important limitation affecting our work, is that modified Goldmann CT measurements do not apply for every OC and may not represent real IOP, in the same way that GAT is not suitable for every non-OC, except for standard corneas with CCT around 520 μm (microns) and accurate measurement technique [50]. Since true IOP can only be obtained from invasive intracameral readings [43, 51], and IOPm may have not been taken at the same time of day after several months entailing IOP fluctuations between measurements [52], IOP stability after LRS was taken as a reference for the quality of tonometer readings [43]. In our study, IOPcc in the PRK subgroup was the most stable IOPm. It seems obvious to affirm that GAT and CT measurements before and after surgery are not reliable compared to themselves. However, as previously discussed, CT appears to be ineffective in non-OC, but its use is to register IOP after surgery in correlation to GAT before surgery. Nevertheless, CTpost mean differences between IOPm at 3 and 12 months were not significantly different, and they registered high correlations with GATpre values in the LASIK subgroup, concluding that convex prism IOPm in the sample presented may be considered acceptably stable after this procedure. A future study in larger LASIK populations will be carried out comparing the Goldmann CT and other tonometers to shed some light on its accuracy.
Conclusions
In summary, though GAT is still the current reference technique in standard corneas, a certain IOPm variability is expected with applanation tonometry due to influencing corneal factors, especially after LRS [53, 54]. The new modified Goldmann CT is the first applanation tonometer to offer precise IOP estimations in LASIK patients after 3 [4] and 12 months in relation to GAT before surgery. It could be an affordable and effortless method for monitoring IOP by providing an additional reference to IOP assessment and therefore diminish the risk of glaucoma progression in this subtype of patients.
Availability of data and materials
The research data generated during the current study cannot be shared due to exclusive intellectual property rights of the new Goldmann “CT” convex tonometer secured by a Spanish patent filed with number P201631280 (granted patent), and a European patent filed with number 3520682 (granted patent). However, the data are available from the corresponding author upon reasonable request.
Abbreviations
- CB:
-
Corneal biomechanics
- CCT:
-
Corneal central thickness
- CH:
-
Corneal hysteresis
- CI:
-
Confidence interval
- CPP:
-
Contact pressure profile
- CRF:
-
Corneal resistance factor
- CT:
-
Convexly-shaped tonometer
- dpts:
-
Dioptres
- GAT:
-
Goldmann applanation tonometer
- ICC:
-
Intra-class correlation coefficient
- IOP:
-
Intraocular pressure
- IOPcc:
-
Corneal-compensated IOP
- IOPg:
-
Goldmann-correlated IOP
- IOPm:
-
IOP measurements
- LASIK:
-
Laser-assisted in situ keratomileusis
- LoA:
-
Limits of agreement
- LRS:
-
Laser refractive surgery
- M:
-
Months
- Max.Abl:
-
Maximum ablation depth
- md:
-
Mean differences
- MD ± SD :
-
Mean ± standard deviation
- NCT:
-
Non-contact tonometer
- OC:
-
Operated cornea
- ORA:
-
Ocular Response Analyser
- p :
-
Probability value
- PDCT:
-
Pascal Dynamic Tonometer
- PRK:
-
Photorefractive keratectomy
- PTA:
-
Percentage of ablated tissue
- SER:
-
Spherical equivalent refraction
- simK:
-
Simulated keratometry
- Z:
-
Wilcoxon test
References
Lin MY, Chang DCK, Shen YD, Lin YK, Lin CP, Wang IJ. Factors influencing intraocular pressure changes after laser in situ keratomileusis with flaps created by femtosecond laser or mechanical microkeratome. PLoS One. 2016;11(1):1–11.
Park JH, Jun RM, Choi KR. Significance of corneal biomechanical properties in patients with progressive normal-tension glaucoma. Br J Ophthalmol. 2015;99(6):746–51.
Shin J, Kim TW, Park SJ, Yoon M, Lee JW. Changes in biomechanical properties of the cornea and intraocular pressure after myopic laser in situ keratomileusis using a femtosecond laser for flap creation determined using ocular response analyzer and Goldmann applanation tonometry. J Glaucoma. 2015;24(3):195–201.
Iglesias M, Yebra F, Kudsieh B, Laiseca A, Santos C, Nadal J, et al. New applanation tonometer for myopic patients after laser refractive surgery. Sci Rep. 2020;10(1):7053. https://doi.org/10.1038/s41598-020-64013-4.
Dai ML, Wang QM, Lin ZS, Yu Y, Huang JH, Savini G, et al. Posterior corneal surface differences between non-laser in situ keratomileusis (LASIK) and 10-year post-LASIK myopic eyes. Acta Ophthalmol. 2018;96(2):e127–33.
D’Arcy FM, Kirwan C, O’Keefe M. Ten year follow up of laser in situ keratomileusis for all levels of myopia. Acta Ophthalmol. 2012;90(4):335–6.
Kida T, Liu JHK, Weinreb RN. Effect of 24-hour corneal biomechanical changes on intraocular pressure measurement. Investig Ophthalmol Vis Sci. 2006;47(10):4422–6.
Celebi ARC, Kilavuzoglu AE, Altiparmak UE, Cosar Yurteri CB. Age-related change in corneal biomechanical parameters in a healthy Caucasian population. Ophthalmic Epidemiol. 2018;25(1):55–62. Disponible en. https://doi.org/10.1080/09286586.2017.1351997.
Duch S, Serra A, Castanera J, Abos R, Quintana M. Tonometry after laser in situ keratomileusis treatment. J Glaucoma. 2001;10(4):261–5 Disponible en: http://www.ncbi.nlm.nih.gov/pubmed/11558808.
Narayanaswamy A, Chung RS, Wu RY, Park J, Wong WL, Saw SM, et al. Determinants of corneal biomechanical properties in an adult Chinese population. Ophthalmology. 2011;118(7):1253–9.
Pedersen IB, Bak-Nielsen S, Vestergaard AH, Ivarsen A, Hjortdal J. Corneal biomechanical properties after LASIK, ReLEx flex, and ReLEx smile by Scheimpflug-based dynamic tonometry. Graefes Arch Clin Exp Ophthalmol. 2014;252(8):1329–35.
Santhiago MR, Wilson SE, Hallahan KM, Lin M, Jr RA, Singh V, et al. Changes in custom biomechanical variables after femtosecond laser in situ keratomileusis and photorefractive keratectomy for myopia. J Cataract Refract Surg. 2014;40(6):918-28. Disponible en:https://doi.org/10.1016/j.jcrs.2013.11.030.
Matlach J, Bender S, König J, Binder H, Pfeiffer N, Hoffmann EM. Investigation of intraocular pressure fluctuation as a risk factor of glaucoma progression. Clin Ophthalmol. 2019;13:9–16.
Leske MC, Wu SY, Hennis A, Honkanen R, Nemesure B. Risk factors for incident open-angle Glaucoma. The Barbados Eye Studies. Ophthalmology. 2008;115(1):85–93.
Bagnasco L, Bagnis A, Bonzano C. EGS_guidelines_5_English.Pdf. European Glaucoma society terminology and Guidelines for Glaucoma. 5th ed; 2020. p. 49.
Nakao Y, Kiuchi Y, Okumichi H. Evaluation of biomechanically corrected intraocular pressure using Corvis ST and comparison of the Corvis ST, noncontact tonometer, and Goldmann applanation tonometer in patients with glaucoma. PLoS One. 2020;15(9 September):1–9. Disponible en. https://doi.org/10.1371/journal.pone.0238395.
Fu D, Li M, Knorz MC, Wei S, Shang J, Zhou X. Intraocular pressure changes and corneal biomechanics after hyperopic small-incision lenticule extraction. BMC Ophthalmol. 2020;20(1):2–7.
Fernández J, Rodríguez-Vallejo M, Martínez J, Tauste A, Piñero DP. Corneal biomechanics after laser refractive surgery: Unmasking differences between techniques. J Cataract Refract Surg . 2018;44(3):390–8. https://doi.org/10.1016/j.jcrs.2017.10.054.
Li H, Wang Y, Dou R, Wei P, Zhang J, Zhao W, et al. Intraocular pressure changes and relationship with corneal biomechanics after SMILE and FS-LASIK. Investig Ophthalmol Vis Sci. 2016;57(10):4180–6.
Luce DA. Determining in vivo biomechanical properties of the cornea with an ocular response analyzer. J Cataract Refract Surg. 2005;31(1):156–62.
Schweitzer JA, Ervin M, Berdahl JP. Assessment of corneal hysteresis measured by the ocular response analyzer as a screening tool in patients with glaucoma. Clin Ophthalmol. 2018;12:1809–13.
Matsuura M, Hirasawa K, Murata H, Yanagisawa M, Nakao Y, Nakakura S, et al. The relationship between corvis ST tonometry and ocular response analyzer measurements in eyes with glaucoma. PLoS One. 2016;11(8):1–13. Disponible en. https://doi.org/10.1371/journal.pone.0161742.
Chihara E, Takahashi H, Okazaki K, Park M, Tanito M. The preoperative intraocular pressure level predicts the amount of underestimated intraocular pressure after LASIK for myopia. Br J Ophthalmol. 2005;89(2):160–4.
Ernest PJ, Schouten JS, Beckers HJ, Hendrikse F, Prins MH, Webers CA. An evidence-based review of prognostic factors for glaucomatous visual field progression. Ophthalmology. 2013;120(3):512–9. Disponible en. https://doi.org/10.1016/j.ophtha.2012.09.005.
McMonnies CW. Assessing corneal hysteresis using the ocular response analyzer. Optom Vis Sci. 2012;89(3):343–9.
Mccann P, Hogg RE, Wright DM, Mcguinness B, Young IS, Kee F, et al. Comparison of Goldmann applanation and Ocular Response Analyser tonometry : intraocular pressure agreement and patient preference. Eye. 2019; Disponible en. https://doi.org/10.1038/s41433-019-0556-2.
Tonnu PA, Ho T, Sharma K, White E, Bunce C, Garway-Heath DF. A comparison of four methods of tonometry: method agreement and interobserver variability. Br J Ophthalmol. 2005;89(7):847–50.
Zimmermann M, Pitz S, Schmidtmann I, Pfeiffer N, Wasielica-Poslednik J. Tonographic effect of ocular response analyzer in comparison to Goldmann applanation tonometry. PLoS One. 2017;12(1):1–15. Disponible en. https://doi.org/10.1371/journal.pone.0169438.
Landis JR, Koch GG. The Measurement of Observer Agreement for Categorical Data. 2012;33(1):159–74.
Roberts CJ. Concepts and misconceptions in corneal biomechanics. J Cataract Refract Surg. 2014;40(6):862–9. Disponible en. https://doi.org/10.1016/j.jcrs.2014.04.019.
Vincent SJ, Vincent RA, Shields D, Lee GA. Comparison of intraocular pressure measurement between rebound, non-contact and Goldmann applanation tonometry in treated glaucoma patients. Clin Exp Ophthalmol. 2012;40(4):e163–70. https://doi.org/10.1111/j.1442-9071.2011.02670.x.
Kozobolis V, Konstantinidis A, Sideroudi H, Labiris G. The Effect of Corneal Refractive Surgery on Glaucoma. J Ophthalmol. 2017;2017:8914623. https://doi.org/10.1155/2017/8914623.
Touboul D, Roberts C, Kérautret J, Garra C, Maurice-Tison S, Saubusse E, et al. Correlations between corneal hysteresis, intraocular pressure, and corneal central pachymetry. J Cataract Refract Surg. 2008;34(4):616–22.
Schallhorn JM, Schallhorn SC, Ou Y. Factors that influence intraocular pressure changes after myopic and hyperopic lasik and photorefractive keratectomy: A large population study. Ophthalmology. 2015;122(3):471–9. Disponible en. https://doi.org/10.1016/j.ophtha.2014.09.033.
Guo H, Hosseini-Moghaddam SM, Hodge W. Corneal biomechanical properties after SMILE versus FLEX, LASIK, LASEK, or PRK: a systematic review and meta-analysis. BMC Ophthalmol. 2019;19(1):167.
Khawaja AP, Chan MPY, Broadway DC, Garway-Heath DF, Luben R, Yip JLY, et al. Corneal biomechanical properties and glaucoma-related quantitative traits in the EPIC-Norfolk eye study. Investig Ophthalmol Vis Sci. 2013;55(1):117–24.
Mangouritsas G, Mourtzoukos S, Mantzounis A, Alexopoulos L. Comparison of Goldmann and Pascal tonometry in relation to corneal hysteresis and central corneal thickness in nonglaucomatous eyes. Clin Ophthalmol. 2011;5(1):1071–7.
Hwang ES, Stagg BC, Swan R, Fenzl CR, McFadden M, Muthappan V, et al. Corneal biomechanical properties after laser-assisted in situ keratomileusis and photorefractive keratectomy. Clin Ophthalmol. 2017;11:1785–9.
Lee H, Roberts CJ, Im KT, Ambrósio R, Elsheikh A, Yong Kang DS. Changes in biomechanically corrected intraocular pressure and dynamic corneal response parameters before and after transepithelial photorefractive keratectomy and femtosecond laser–assisted laser in situ keratomileusis. J Cataract Refract Surg. 2017;43(12):1495–503.
Stamper RL. A history of intraocular pressure and its measurement. Optom Vis Sci. 2011;88(1):16–28.
Chen SH, Lopes BT, Huang W, Zheng XB, Wang JJ, Zhu R, et al. Effectiveness of 4 tonometers in measuring IOP after femtosecond laser-assisted LASIK, SMILE, and transepithelial photorefractive keratectomy. J Cataract Refract Surg. 2020;46(7):967–74.
Fan F, Li C, Li Y, Duan X, Pan D. Intraocular pressure instrument reading comparisons after LASIK. Optom Vis Sci. 2011;88(7):850–4.
Bao F, Huang W, Zhu R, Lu N, Wang Y, Li H, et al. Effectiveness of the Goldmann Applanation tonometer, the dynamic contour tonometer, the ocular response analyzer and the Corvis ST in measuring intraocular pressure following FS-LASIK. Curr Eye Res. 2020;45(2):144–52. Disponible en. https://doi.org/10.1080/02713683.2019.1660794.
Susanna BN, Ogata NG, Daga FB, Susanna CN, Diniz-Filho A, Medeiros FA. Association between rates of visual field progression and intraocular pressure measurements obtained by different Tonometers. Ophthalmology. 2019;126(1):49–54.
Cook JA, Botello AP, Elders A, Fathi Ali A, Azuara-Blanco A, Fraser C, et al. Systematic review of the agreement of tonometers with Goldmann applanation tonometry. Ophthalmology. 2012;119(8):1552–7. Disponible en. https://doi.org/10.1016/j.ophtha.2012.02.030.
Bayoumi NHL, Bessa AS, El Massry AAK. Ocular response analyzer and Goldmann applanation tonometry: A comparative study of findings. J Glaucoma. 2010;19(9):627–31.
Lanza M, Rinaldi M, Carnevale UAG, Di Staso S, Sconocchia MB, Costagliola C. Analysis of differences in intraocular pressure evaluation performed with contact and non-contact devices. BMC Ophthalmol. 2018;18(1):1–6.
Chen S, Chen D, Wang J, Lu F, Wang Q, Qu J. Changes in ocular response analyzer parameters after LASIK. J Refract Surg. 2010;26(4):279–88.
Kling S, Hafezi F. Corneal biomechanics – a review. Ophthalmic Physiol Opt. 2017;37(3):240–52.
Damji KF, Muni RH, Munger RM. Influence of corneal variables on accuracy of intraocular pressure measurement. J Glaucoma. 2003;12(1):69–80.
Feltgen N, Leifert D, Funk J. Correlation between central corneal thickness, applanation tonometry, and direct intracameral iop readings. Br J Ophthalmol. 2001;85(1):85–7.
Kim SH, Lee EJ, Han JC, Sohn SW, Rhee T, Kee C. The effect of diurnal fluctuation in intraocular pressure on the evaluation of risk factors of progression in normal tension glaucoma. PLoS One. 2016;11(10):1–14.
Ramm L, Herber R, Spoerl E, Raiskup F, Pillunat LE, Terai N. Intraocular Pressure Measurement Using Ocular Response Analyzer, Dynamic Contour Tonometer, and Scheimpflug Analyzer Corvis ST. J Ophthalmol. 2019;2019:3879651. https://doi.org/10.1155/2019/3879651.
Shousha SMA, Steit MAHA, Hosny MHM, Ewais WA, Shalaby AMM. Comparison of different intraocular pressure measurement techniques in normal eyes, post surface and post lamellar refractive surgery. Clin Ophthalmol. 2013;7(1):71–9.
Acknowledgements
The authors would like to thank Emilio Iglesias Touriño MD, PhD, an ophthalmologist whose extraordinary knowledge has been indispensable for the development of the new Goldmann CT device from the beginning of our project.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Author information
Authors and Affiliations
Contributions
MI contributed to ensure the double-masking. She also collected, analysed, and interpreted the sample data and was a major contributor in writing the original manuscript. BK analysed and interpreted the clinical results. AL collected IOP measurements before, and 3 and 12 months after surgery. CS interpreted the statistical analysis. RPCM, JN and RB reviewed the manuscript. All authors read and approved the final manuscript.
Authors’ information
Not applicable.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
An informed consent was obtained from all participants and/or their legal guardian/s before their participation in the study, and the Institutional Review Board of the Barraquer Ophthalmology Centre approved this study.
Consent for publication
The written consent of all participants approved by the Institutional Review Board of the centre included a statement for consent to publish any personal data regarding their refractive surgery, as well as any additional related information.
Competing interests
Each of the co-authors has seen and agrees with the financial and non-financial competing interests statement presented by MI (as corresponding author) on behalf of all the authors of the paper:
MI has personal conflict of interest being the inventor of the new Goldmann “CT” applanation tonometer. She had full access to all the data in this study and takes complete responsibility for the integrity of the data and the accuracy of the data analysis. MI has exclusive personal rights to the intellectual property of this invention secured by the patents referred to above, and is the owner of every figure presented in this study.
The rest of the co-authors declare no financial competing interests.
MI and the rest of the co-authors declare they do not have non-financial competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Iglesias, M., Kudsieh, B., Laiseca, A. et al. Intraocular pressure after myopic laser refractive surgery measured with a new Goldmann convex prism: correlations with GAT and ORA. BMC Ophthalmol 22, 79 (2022). https://doi.org/10.1186/s12886-022-02309-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12886-022-02309-x