Abstract
Background
This study aims to investigate the clinical characterization and causative genetic defect of a four-generation Chinese family with autosomal dominant aniridia.
Methods
The recruited family members underwent comprehensive routine and ophthalmic examinations, and Sanger sequencing was performed to screen the mutation in PAX6.
Results
A novel heterozygous PAX6 deletion c.435_445delTAGCGAAAAGC (p.Ser146ThrfsX9) in exon 7 was identified in all affected individuals, but this was absent in any of the unaffected family members and in the 200 unrelated controls.
Conclusion
A novel deletion in the PAX6 gene was identified in a Chinese family associated with aniridia, which expands the spectrum of the PAX6 mutation and its associated phenotype.
Similar content being viewed by others
Background
Aniridia is a congenital ocular abnormality, in which the most characteristic feature is iris hypoplasia associated with the gene mutation of paired box-6 (PAX6) in most cases. In order to investigate the clinical characterization and causative genetic defect in this four-generation Chinese family with autosomal dominant aniridia, the recruited family members underwent comprehensive routine and ophthalmic examinations, and Sanger sequencing was performed to screen the mutation in PAX6. A novel heterozygous PAX6 deletion c.435_445delTAGCGAAAAGC (p.Ser146ThrfsX9) in exon 7 was identified in all affected individuals, but this was absent in any of the unaffected family members and in the 200 unrelated controls. Hence, it was concluded that a novel deletion in the PAX6 gene was identified in a Chinese family associated with aniridia, which expands the spectrum of the PAX6 mutation and its associated phenotype.
Aniridia is a serious congenital abnormality in the iris, which is usually bilateral. The main clinical features include a partially or completely missing iris, and decreased vision and nystagmus in some patients. Aniridia can be accompanied by other ocular diseases, such as cataracts, clouding of the cornea, glaucoma, macular abnormality, optic nerve hypoplasia and so on. Approximately 13% of aniridia cases occur in WAGR (Wilms tumor, aniridia, genitourinary disorders, and mental Retardation) syndrome [1]. Furthermore, the prevalence rate of congenital aniridia is approximately 1:64,000–1:96,000 worldwide [2], and approximately 1:100,000 in China [3]. Moreover, two-thirds of aniridia cases have an obvious hereditary history, and are almost in the pattern of autosomal dominant inheritance, while the remaining cases refer to sporadic cases [4,5,6].
Approximately 80% congenital aniridia cases are caused by the gene mutation of human paired box-6 (PAX6) [7,8,9]. The Human PAX6 Allelic Variant Database (http://lsdb.hgu.mrc.ac.uk/home.php?select_db=PAX6) has recorded over 400 PAX6 mutations, to date [10]. Most of these mutations are frameshift mutations, splicing site mutations, or nonsense mutations, which have been considered to produce truncated or nonsense transcripts, leading to haploinsufficiency associated with ocular abnormal signs, while other mutations were missense [11,12,13]. The PAX6 protein is a functionally conserved transcription factor that can recognize and bind to a specific DNA sequence to regulate the expression of target genes, and is a transcription activation function that can activate downstream genes regulated by the PAX6 protein [14, 15].
In the present study, all exons and flanking regions of PAX6 were screened in a Chinese family with aniridia, and a novel heterozygous deletion was detected.
Methods
Participants and clinical data
The Institutional Review Board (IRB) of Tongji Eye Institute of Tongji University, School of Medicine (Shanghai, China), and the Second Affiliated Hospital of Nanchang University (Nanchang, Jiangxi Province, China) approved the present study. All participating family members provided an informed written consent, and were endorsed by their respective IRB. The whole procedure of the present study adhered to the tenets of the Declaration of Helsinki.
Family 12,002, a four-generation family with aniridia, was recruited for the present study. This family included four affected and eight unaffected members. Routine and ophthalmological examinations (including vision, cornea, iris, lens, fundus, intraocular pressure, etc.) were performed on the affected individuals, as well as on the unaffected family members. In addition, 200 ethnically matched healthy individuals with no direct or collateral ties and no ocular and systemic underlying diseases were recruited.
Mutation analysis
The peripheral venous blood genomic DNA of the family and healthy members of the family was extracted by phenol-chloroform extraction. The experimental procedures were conducted according to the protocol of the RelaxGene Blood DNA extraction kits (TianGen, Beijing, China). All 14 exons of PAX6 plus the flanking regions were amplified by polymerase chain reaction (PCR). The primers for the amplification were designed by the web-based version of the Primer 3 program. The PCR amplification was performed using a BIO-RAD T100 ThermalCycler PCR amplifier. The primer sequences and amplification conditions are presented in Table 1. Sanger sequencing was performed for all members of Family 12,002 with available DNA samples using an ABI3730 Automated Sequencer (PE Biosystems, Foster City, CA, USA). The sequencing results were sequenced using the DNASar software to screen for PAX6 gene mutation sites and mutation types, and compare these with PAX6 gene sequences from another cohort of 200 unrelated ethnically-matched controls.
Results
Clinical findings
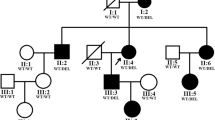
Family 12,002 is a four-generation family, and aniridia continuously occurred in the II, III and IV generations. This was consistent with the features of an autosomal dominant inheritance pattern (Fig. 1). All affected members had bilateral eye disease. The common eye abnormalities included low vision in both eyes, complete absence of the iris, and horizontal tremor in the eye. The different patient-specific eye manifestations were as follows: the proband (II:4) was a 46-year-old male with corneal haze, nystagmus, ptosis, strabismus, glaucoma, photophobia and cataract, and was lens ectopic (Fig. 2a and b); the niece of the proband (III:2) was 25 years old, has binocular cataract, and the left corneal matrix was turbid (Fig. 2c and d); her son (IV:1) had a developing cataract at five years old (Fig. 2e and f). There was no other disease or abnormalities in all the affects.
Mutation analysis
The PAX6 gene sequence of the proband was compared with the PAX6 gene sequence of healthy individuals. The coding and splicing regions of PAX6 were analyzed by bi-directional sequencing. The PAX6 gene exon 7 was found to have a heterozygous deletion mutation in proban c.435_445 delTAGCGAAAAGC (p.Ser146ThrfsX9). This mutation encodes the linker region between PD and HD, forms a premature termination codon (PTC), and finally results in PAX6 underdosage (II:4, Fig. 3). The mutation was detected in all other affected members in the family, but not in any of the unaffected members and in the 200 unrelated controls from the same ethnic background.
In addition, the identified mutation has not been recorded in the databases of probably benign variations, including the database of single nucleotide polymorphisms (dbSNP), Exome Variant Server database, or the 1000 genomes project dataset. Furthermore, the mutation was absent in either the Human PAX6 Allelic Variant Database (http://lsdb.hgu.mrc.ac.uk/home.php?select_db=PAX6), or reported literatures. These results suggest that this mutation of PAX6 is a novel causative mutation for autosomal dominant congenital aniridia.
Discussion
Congenital aniridia is a clinically rare hereditary eye disease. The most prominent clinical feature is the degeneration of the iris tissue, which is characterized by partial or complete loss of the iris, with or without foveal dysplasia and nystagmus. It often occurs with developmental glaucoma, congenital cataract and corneal abnormalities. The PAX6 (paired box gene 6) gene is the most important gene that leads to congenital aniridia. At least 90% of iris-free cases are caused by heterozygous mutations in the PAX6 gene. Furthermore, few studies have found that FOXC1 and PITX2 gene mutations can also lead to aniridia. [11]. PAX6 belongs to the Pax gene family, and is located on human chromosome 11. Its encoded transcription factor is highly conserved during biological evolution, and participates in the embryonic development of various tissues and organs, especially in the development of the eye [10].
The clinical manifestations of aniridia are diverse. According to literature statistics, in the LOVDPAX6 gene database, 90% of disease-causing mutations lead to the aniridia phenotype, while the remaining 10% causes follicular dysplasia, Peters Syndrome and small eyeballs. [4]. Studies have shown that the variant aniridia phenotype is mostly caused by missense mutations, which cause the production of the underlying genes. These underlying genes can exert some of the functions of the PAX6 gene and affect the development of the eyeball. The present study found that these mutations was mostly concentrated in exon 5-exon 7 of a highly conserved PD area. In this family, three of four affected members in the family had cataract. In addition to aniridia, one family member had ptosis and glaucoma, while another family member had corneal opacity. Furthermore, the heterozygous deletion mutation c.435_445delTAGCGAAAAGC was detected in all four affected members, while this was absent in all unaffected members. This shows that the mutation co-segregates with aniridia in Family 12,002. In aniridia cases, approximately 50–85% had progressive cataract, while 6–75% had glaucoma [16]. The reason why cataract occurs with aniridia remains unknown. The investigators assumed that the dysplasia of the iris may have allowed more light to pass through the crystalline lens that protects the retina, causing the clouding of the lens capsule to occur. However, this remains to be proven.
The PAX6 gene spans for approximately 23 Kb on chromosome 11p13, contains 14 exons, and encodes a protein of 422 amino acids, which belongs to a highly conserved family of transcription factors. The PAX6 protein has two DNA-binding domains, the paired domain (PD) and homeobox domain (HD), and a linker region connects these two kinds of domains [14]. In its C-terminal end, the proline-serine-threonine-rich (PST) activation domain regulates the expression of PAX6 downstream genes via phosphorylation [17]. The heterozygous deletion in the PAX6 gene (c.435_445delTAGCGAAAAGC) identified in the present study was located in exon 7, and was predicted in the protein level to cause the replacement of the serine codon at position 150aa by a threonine codon and a putative stop codon 9 amino acid downstream (p.Ser146ThrfsX9). Furthermore, the mutation located in exon 7 encodes the linker region between PD and HD, and forms a premature termination codon (PTC). One possibility that PTC may bring about is that the mutant PAX6 may produce a truncated protein with the absence of the HD and PST domain, which is considered functionally inactive. Another possibility is that it may cause nonsense-mediated mRNA decay (NMD), and both of which may finally result in PAX6 underdosage [18,19,20,21,22].
Conclusion
In conclusion, the phenotype of aniridia patients in a four-generation family was analyzed, and a novel deletion in PAX6 (c.435_445delTAGCGAAAAGC) was identified as the cause of aniridia in this family. This mutation induced the production of truncated proteins and the generation of null alleles, which resulted in a single-dose deficiency, leading to the absence of the iris. This expands the mutation spectrum of the PAX6 gene and its phenotype. The present study may be helpful in the genetic diagnosis of aniridia.
Abbreviations
- dbSNP:
-
Database of single nucleotide polymorphisms
- HD:
-
Homeobox domain
- IRB:
-
Institutional review board
- NMD:
-
Nonsense-mediated mRNA decay.
- PAX6:
-
Paired box-6
- PCR:
-
Polymerase chain reaction
- PD:
-
Paired domain
- PST:
-
Proline-serine-threonine-rich
- PTC:
-
Premature termination codon
- WAGR:
-
Wilms tumor, aniridia, genitourinary disorders, and mental Retardation
References
Neuhaus C, Betz C, Bergmann C, Bolz HJ. Genetics of congenital aniridia. Der Ophthalmologe : Zeitschrift der Deutschen Ophthalmologischen Gesellschaft. 2014;111(12):1157–63. https://doi.org/10.1007/s00347-014-3059-3 German. PMID: 25475187.
Nelson LB, Spaeth GL, Nowinski TS, Margo CE, Jackson L. Aniridia. A review. Surv Ophthalmol. 1984;28(6):621–42 PMID: 6330922.
Hu DN. Prevalence and mode of inheritance of major genetic eye diseases in China. J Med Genet. 1987;24(10):584–8 PMID: 3500313.
Jordan T, Hanson I, Zaletayev D, Hodgson S, Prosser J, Seawright A, Hastie N, van Heyningen V. The human PAX6 gene is mutated in two patients with aniridia. Nat Genet. 1992;1(5):328–32. https://doi.org/10.1038/ng0892-328 PMID: 1302030.
Brauner SC, Walton DS, Chen TC. Aniridia. Int Ophthalmol Clin. 2008;48(2):79–85. https://doi.org/10.1097/IIO.0b013e318169314b PMID: 18427263.
Yahalom C, Sharon D, Dalia E, Simhon SB, Shemesh E, Blumenfeld A. Combined occurrence of autosomal dominant Aniridia and autosomal recessive albinism in several members of a family. Ophthalmic Genet. 2015;36(2):175–9. https://doi.org/10.3109/13816810.2015.1005318 PMID: 25687215.
Ton CC, Hirvonen H, Miwa H, Weil MM, Monaghan P, Jordan T, van Heyningen V, Hastie ND, Meijers-Heijboer H, Drechsler M, et al. Positional cloning and characterization of a paired box- and homeobox-containing gene from the aniridia region. Cell. 1991;67(6):1059–74 PMID: 1684738.
Gronskov K, Olsen JH, Sand A, Pedersen W, Carlsen N, Bak Jylling AM, Lyngbye T, Brondum-Nielsen K, Rosenberg T. Population-based risk estimates of Wilms tumor in sporadic aniridia. A comprehensive mutation screening procedure of PAX6 identifies 80% of mutations in aniridia. Hum Genet. 2001;109(1):11–8 PMID: 11479730.
Robinson DO, Howarth RJ, Williamson KA, van Heyningen V, Beal SJ, Crolla JA. Genetic analysis of chromosome 11p13 and the PAX6 gene in a series of 125 cases referred with aniridia. Am J Med Genet A. 2008;146A(5):558–69. https://doi.org/10.1002/ajmg.a.32209 PMID: 18241071.
Brown A, McKie M, van Heyningen V, Prosser J. The human PAX6 mutation database. Nucleic Acids Res. 1998;26(1):259–64 9399848.
Fantes J, Redeker B, Breen M, Boyle S, Brown J, Fletcher J, Jones S, Bickmore W, Fukushima Y, Mannens M, et al. Aniridia-associated cytogenetic rearrangements suggest that a position effect may cause the mutant phenotype. Hum Mol Genet. 1995;4(3):415–22 PMID: 7795596.
Chien YH, Huang HP, Hwu WL, Chien YH, Chang TC, Lee NC. Eye anomalies and neurological manifestations in patients with PAX6 mutations. Mol Vis. 2009;15:2139–45 19898691.
Kokotas H, Petersen MB. Clinical and molecular aspects of aniridia. Clin Genet. 2010;77(5):409–20. https://doi.org/10.1111/j.1399-0004.2010.01372.x Epub 2010 Jan 6. Review. PMID: 20132240.
Mishra R, Gorlov IP, Chao LY, Singh S, Saunders GF. PAX6, paired domain influences sequence recognition by the homeodomain. J Biol Chem. 2002;277(51):49488–94 PMID: 12388550.
Li H, Leung CK, Wong L, Cheung CY, Pang CP, Weinreb RN, Lam DS. Comparative study of central corneal thickness measurement with slit-lamp optical coherence tomography and visante optical coherence tomography. Ophthalmology. 2008;115(5):796–801 e792 PMID: 17916376.
Ivanov I, Shuper A, Shohat M, Snir M, Weitz R. Aniridia: recent achievements in paediatric practice. Eur J Pediatr. 1995;154(10):795–800 PMID: 8529675.
Singh S, Chao LY, Mishra R, Davies J, Saunders GF. Missense mutation at the C-terminus of PAX6 negatively modulates homeodomain function. Hum Mol Genet. 2001;10(9):911–8 PMID: 11309364.
Nelson SM, Park L, Stenkamp DL. Retinal homeobox 1 is required for retinal neurogenesis and photoreceptor differentiation in embryonic zebrafish. Dev Biol. 2009;328(1):24–39. https://doi.org/10.1016/j.ydbio.2008.12.040 PMID: 19210961.
Fisher M, Nakajima K, Odeleye AO, Zimmerman KB, Fish MB, Yaoita Y, Chojnowski JL, Lauderdale JD, Netland PA, Robert M. Xenopus pax6 mutants affect eye development and other organ systems, and have phenotypic similarities to human aniridia patients Takuya Nakayama. Dev Biol. 2015;408(2):328–44. https://doi.org/10.1016/j.ydbio.2015.02.012 Grainger Dev Biol. Author manuscript; available in PMC 2016 Dec 15.Published in final edited form as: Published online 2015 Feb 25. PMCID: PMC4549229.
Bobilev AM, McDougal ME, Taylor WL, Geisert EE, Peter A, Netland JD. Assessment of PAX6 alleles in 66 families with aniridia. Clin Genet. 2016;89(6):669–77. https://doi.org/10.1111/cge.12708 Published online 2016 Jan 25. Lauderdale Clin Genet. Author manuscript; available in PMC 2017 Jun 1.Published in final edited form as: PMCID: PMC4873406.
Souzeau E, Rudkin AK, Dubowsky A, Casson RJ, Muecke JS, Mancel E, Whiting M, Mills RAD, Burdon KP, Craig JE. PAX6 molecular analysis and genotype–phenotype correlations in families with aniridia from Australasia and Southeast Asia. Mol Vis. 2018;24:261–73 Published online 2018 Mar 28. PMCID: PMC5873721.
Zhang R, Linpeng S, Wei X, Li H, Huang Y, Guo J, Wu Q, Liang D, Wu L. Novel variants in PAX6 gene caused congenital aniridia in two Chinese families. Eye (Lond). 2017;31(6):956–61. https://doi.org/10.1038/eye.2016.326 Published online 2017 Feb 3 PMCID: PMC5518831.
Acknowledgments
Not applicable.
Funding
Not applicable.
Availability of data and materials
All data generated and analyzed during this study were included in this manuscript.
Author information
Authors and Affiliations
Contributions
QJJ and GZX conceptualized and designed the study, drafted the initial manuscript, and reviewed and revised the manuscript. LM, ZZL,CJJ and LF designed the data collection instruments, collected data, carried out the initial analyses, and reviewed and revised the manuscript. ZQ coordinated and supervised data collection, and critically reviewed the manuscript for important intellectual content. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study followed the tenets of the Declaration of Helsinki.This study was conducted with approval from the Ethics Committee of the Second Affiliated Hospital of Nanchang University. A written informed consent was obtained from each participant.
Consent for publication
A written informed consent was obtained from each patient for the publication of this case report and any accompanying images.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Qiu, JJ., Zhang, Q., Geng, Zx. et al. Identification of a novel PAX6 mutation in a Chinese family with aniridia. BMC Ophthalmol 19, 10 (2019). https://doi.org/10.1186/s12886-018-1009-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12886-018-1009-6