Abstract
Background
Breast cancer is the most common cancer-affecting women globally, with disproportionally high mortality rates in lower-income countries, including Ethiopia. The stage at diagnosis is a well-defined predictive system that determines the likelihood of breast cancer mortality. Early-stage breast cancer at diagnosis is associated with better treatment outcomes as compared with late stage. Although there are numerous primary studies on women with breast cancer with different proportions of early-stage breast cancer, there is currently no summary data on what proportion of breast cancer was diagnosed at early-stage in Ethiopia. This study focused on a pooled proportion of early-stage breast cancer at diagnosis in Ethiopia.
Methods
By using key terms, Medline through Pub-Med, Google Scholar, Science Direct, HINARI and Medley were searched about breast cancer in Ethiopia, and a total of 288 articles were retrieved. After screening the articles and quality of each article was assessed using Newcastle–Ottawa Scale. Finally, 41 articles were used for the final pooled proportion. A random effects model was used to estimate the pooled prevalence and heterogeneity of included studies that were then assessed by using prediction interval.
Results
Pooled proportion of early-stage breast cancer at diagnosis in Ethiopian hospitals was found to be 36% with a 95% confidence interval ranging from 31 to 41% and a 95% prediction interval ranging from 28 to 45%.
Conclusion
Most breast cancer patients (64%) in Ethiopia are diagnosed at a late-stage. This contributes to the high mortality rates of breast cancer among women in Ethiopia. Therefore, in line with recommendations by the World Health Organization, we recommend that there should be an emphasis in Ethiopia to focus on early detection of breast cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is one of the most common cancers with leading causes of cancer mortality among women worldwide [1]. It is also the most common cancer affecting women in the Sub-Saharan region including Ethiopia. In Ethiopia, breast cancer commonly affects the younger women as compared to older women in low-income countries. Moreover, this cancer is also the leading cancer mortality among women in developing countries as well as in Ethiopia [2, 3].
There are multiple factors contributing to a higher breast cancer mortality rate (e.g., staging at diagnosis, type of breast cancer, therapeutic advance and compliance to breast cancer treatment) [4, 5]. Stage of breast cancer at diagnosis is the main predictor of breast cancer mortality [2]. There is stark gap in breast cancer survival rates between low- and high-income countries. Women in low-income countries suffer from disproportionately higher rates of breast cancer mortality in comparison to high-income countries. Breast cancer survival rates are increasing in developed countries because of early-stage diagnosis with the help of community awareness, wide spread screening, and advances in treatment options [4, 5]. According to the WHO, at least 60% of breast cancer should be diagnosed at early-stage to achieve a reduction of 2.5% in breast cancer mortality every year by 2040 [6].
In Ethiopia, 71% of breast cancer patients are diagnosed late, stages III and IV, leading to an increase in the likelihood of mortality [3, 7, 8]. Adequate management of all stages breast cancer requires a hospital facility that includes a multidisciplinary team (surgery, medical oncology, radiation oncology) to reduce mortality; which is lacking in most Ethiopian hospitals [3]. Therefore, in low-income countries like Ethiopia, early detection of breast cancer is vital to reduce breast cancer mortality. There is no country level summary data in Ethiopia on what proportion of breast cancer is detected in the early-stages in Ethiopia [9,10,11,12]. Therefore, the aim of this study is to conduct a systematic review and meta-analysis on the proportion of early-stage breast cancer at diagnosis in Ethiopia.
Methods
Search strategies
This systematic review and meta-analysis was conducted to estimate the pooled proportion of early breast cancer at diagnosis in Ethiopia. The protocol for this review was registered in the International Prospective Register of Systematic Reviews (PROSPERO) the University of York Centre for Reviews and Dissemination (registration number 339368),) on June 3rd, 2022. To adhere to the scientific standard, the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines were used [13, 14].
International databases: MEDLINE through Pub-Med, Google Scholar, Science Direct, HINARI and medley were systematically searched. Also, references of identified articles were searched to increase the chance of detecting missed articles in grey literature. To search relevant articles for this study, we used the following Mesh terms: “Breast” OR “Lactation” OR “Mammoplasty” OR “Mastectomy” OR “Mammography” OR “Mammary Glands, Animal” AND “Neoplasms” OR “Cancer” OR “Malignancy” OR “Tumors” OR “Tumor” OR “Malignant Neoplasms” OR “Malignant Neoplasm” OR “Neoplasm” OR “Neoplasia” AND “Ethiopia”. The key terms were used separately and/or in combination using Boolean operators like: “OR” or “AND”. The literature search from those databases was done from May 22nd to June 21st, 2022. All papers published until June 21st, 2022 were included in this systematic review and meta-analysis. Following every search, all identified citations were collated and uploaded into EndNote version 4/2020 and duplicates removed. Following a pilot test, titles and abstracts were then screened by two independent authors (KB and GB) for assessment against the inclusion and exclusion criteria for the review (Table 1).
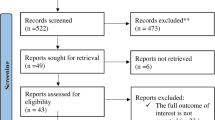
Potentially relevant studies were retrieved in full document. The full texts of selected citations were assessed in detail against the inclusion criteria by KB and GB. Reasons for excluding of papers at full text that did not meet the inclusion criteria were recorded and reported in the systematic review. Any disagreements that arose between the authors at stage of search, title and abstract screening, as well as full text screening of the selection process was resolved through discussion. If no resolution was found, we used an additional third author (FN). The results of the search and the study inclusion process were reported in the final systematic review and presented in our PRISMA flow diagram (Fig. 1).
Eligibility criteria
Participants
We included all studies, which were conducted in Ethiopia and describe the stage of breast cancer on initial diagnosis
Study designs
For this review, we included all observational studies reporting stages of breast cancer conducted in Ethiopia.
Language
We included all articles reported in English language only.
Setting
The setting was restricted to Ethiopia.
Exclusion criteria
Articles that used any other staging system different from TNM staging or did not mention the staging of breast cancer were excluded. Additionally, studies that did not include stage IV breast cancer were excluded from this review.
Outcome of interest
Primarily, this study aimed to estimate the pooled data on what proportion of breast cancer was diagnosed at the early-stage in Ethiopia. The proportion of early breast cancer patients was calculated by dividing the number of early breast cancer diagnoses (stage 1 and stage 2) to the total amount of breast cancer patients for whom TNM staging system was done [15].
Operational definition
Breast cancer staging was usually done by TNM staging system. We used the term “early-stage” of breast cancer for stage I and stage II tumors and “advanced stage” of breast cancer for stage III and stage IV tumors based on the TNM staging system [6, 16].
Data extraction
KB and GB independently extracted all the data required using a standardized data extraction format on excel. The data extraction format included the primary author, year of publication, study period, site of studies, regional state, study design, sample size, response rate, proportion of early breast cancer and total breast cancer for whom staging was done.
Quality assessment
Newcastle–Ottawa Scale (NOS) quality assessment tool for observational studies was adapted to assess the quality of each independent study. The tool has three main sections. The section of the tool graded out of 5 stars and mainly focuses on the methodological quality of each original study. The second component of the tool graded out of 2 stars and mainly focused on the comparability of each study. The last section of the tool graded out of 3 stars and was used to assess the outcomes and statistical analysis of each original study used for data analysis [17]. KB and GB independently assessed the quality of included research articles using the stated tool. Differences in the scoring of articles between the two reviewers were addressed by discussion. For the differences that could not be resolved by agreement between the two authors, we used a third author (FN) as a result. After reviewing various literatures, we declared that articles scoring ≥ 7 points out of 10 stars were considered to be good quality and therefore included in this study.
Data management and analysis
Microsoft Excel spreadsheets were used for data extraction while STATA Version 16 and compressive meta-analysis (CMA) software was used for data analysis. The descriptive data was presented using a table to describe the characteristics of all included studies. For analysis we used the random effects model. The magnitude of each original study as well as the overall magnitude of pooled proportion is described using a forest plot graph. The horizontal line of the forest plot shows the 95% CI, and the blue box represents the weight of each study. Presence of heterogeneity was confirmed by Cochrane Q-statistics and the degree of heterogeneity was explored by using predictive interval (PI) using 95%. In identifying the degree of heterogeneity, a predictive interval was used to evaluate the dispersion or variation of effect size across included studies. I2 statistics is a proportion not an absolute index for variability but can be used to describe what proportion of the observed variance reflects variation in true effects rather than due to sampling error or random error. It cannot be used to make a conclusion on the heterogeneity of the disease burden but, can provide estimates (Borenstein M, Higgins J, Rothstein HR, Hedges LV: I 2 is not an absolute measure of heterogeneity in a meta-analysis, Unpublished). To identify the source of heterogeneity we did a subgroup analysis based on study design, site of study, years of publication and sample size. Additionally, meta-regression was conducted using sample size and year of publication as study level covariate.
Publication bias was explored by using a funnel plot to assess symmetry by observation and statistically, we used Egger’s test to objectively assess the presence or absence of publication bias or small study effect.
Results
Characteristics of the included studies
A systematic search of the electronic databases yielded a total of 288 articles. After the removal of duplicates (n = 92), articles were screened by title and abstract. A total of 138 articles were excluded based on non-compliance with their titles and abstracts. As described in the Fig. 1, a total of 57 full articles were accessed and screened based on compliance with the inclusion and exclusion criteria. Finally, 43 research articles fulfilled the inclusion criteria from which 2 articles were excluded because of repetition of same sample for a different study objective and 41 of them were checked and fulfilled the optimal quality standard evaluated by the NOS tool. In this systematic review and meta-analysis, 41 studies published between 2006 and 2022 were used to estimate the pooled proportion of early breast cancer at diagnosis conducted in Ethiopia. A total of 10,123 breast cancer patients were included in the study. All original studies estimated the proportion of early-stage of breast cancer using an observational study design. The sample size of each study ranged from 13 to 787 (Table 2).
Meta -analysis
This meta-analysis found that the pooled proportion of early-stage breast cancer among all breast cancer patients for whom TNM staging was carried out at diagnosis in Ethiopian hospital was found to be 36% (95% CI: 31%—41%) and 95% PI was ranging from 28 to 45% (Fig. 2).
Degree of heterogeneity (dispersion of effect size) is best measured by predictive interval. By using estimate of between-study variance Tau^2 = 0.02 and upper confidence level 0 0.41 (upper confidence level of pooled proportion), we calculated 95%, PI by using CMA software and it was found to be in range of 0.28 to 0.45 (28%—45%). This shows that if studies were carried out on proportion of early-stage breast cancer detection in Ethiopian hospital in 95% of the case proportion of those studies falls between 28% and 45. This 95% PI range (28%—45%) is low for early breast cancer proportion at diagnosis. Hence, there is only a mild degree of heterogeneity (Fig. 3).
Subgroup analysis and meta regression
In this systematic review and meta-analysis, both subgroup analysis and meta regression were carried out. Accordingly, subgroup analysis described in Table 3 and meta regression described in Table 4.
Publication bias
Publication bias was checked graphically and statistically. On visual inspection of standard funnel plot, it looks there was publication bias (Fig. 4). As the result publication bias was statically evaluated by using the Egger test and it showed that there was no publication bias (p = 0.1486).
Discussion
This systematic review and meta-analysis was conducted to estimate the proportion of early-stage breast cancer at diagnosis among Ethiopian hospitals. Early-stage at diagnosis is one of strongest determinants of survival after breast cancer treatment. When breast cancer is diagnosed at early-stages, there is a higher chance of good outcomes and a higher survival rates [5]. This study showed the pooled prevalence of early-stage breast cancer at diagnosis in Ethiopia was 36.0% which is lower than the target set by GBCI of 60% of breast cancers diagnosed at stages I and II [6]. This pooled proportion of early-stage breast cancer at diagnosis in our study is slightly higher than what was reported in a systematic review and meta-analysis in 17 Sub-Saharan African countries in 2014 (33%). The difference might be because of time and population difference [5]. This low proportion of early-stage breast cancer at diagnosis in Ethiopia contribute to high mortality rates from breast cancer and hence makes it difficult to attain the breast cancer annual mortality reduction target of 2.5% by 2040 envisioned by WHO [6].
Subgroup analysis by site of study showed that there is a slightly higher proportion of early-stage breast cancer detection (39.9%) in Addis Ababa, the capital city, as compared to outside Addis Ababa (29%). This variation between study sites could relate to a difference in the community breast cancer awareness and the availability of diagnostic facilities in Addis Ababa as compared areas outside of the capital city.
There are well-established strategies to improve the early detection of breast cancer. Among these, community health education and awareness about early signs and symptoms of breast cancer, health professionals education at primary healthcare centers on these early signs and breast cancer screening are important [6].
Since breast cancer advanced care is limited to few hospitals in Ethiopia, the Government should adopt and work practically to implement some of said strategies at the primary healthcare level to facilitate the early detection of breast cancer cases. The Ministry of Health must focus on addressing the delays in diagnosis of symptomatic breast cancers to reduce the current high mortality rates breast cancer in Ethiopia. Additionally, there is also strong need for expansion of mammography for the screening of breast cancer to detect it at the early-stage in order to lower the number of preventable deaths of women with a breast cancer diagnosis. We recommend that the Ministry of Health give special focus on methods of early diagnoses of breast cancer to reduce the current high mortality rates of women with breast cancer in Ethiopia. Additionally, in settings with diagnostic capacity and access to effective cancer treatment, screening mammography could be considered.
Conclusion
Early breast cancer detection rates in Ethiopia are very low. To reduce breast cancer mortality rates to meet GBCI’s targets, the Ethiopian government should work strongly on the early detection of breast cancer. The Ethiopian Ministry of Health, different non-governmental organizations, Ethiopian Women’s Affair and Health Workers, and health care professionals should work on incorporating evidence-based methods of early breast cancer detection in Ethiopia.
Availability of the data and materials
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
Data availability
The Data can be provided on a reasonable request.
Abbreviations
- CI:
-
Confidence interval
- NOS:
-
Newcastle-Ottowa Scale
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- TNM:
-
Tumor Node Metastasis
- WHO:
-
World Health Organization
References
McGuire A, Brown JA, Malone C, McLaughlin R, Kerin MJ. Effects of age on the detection and management of breast cancer. Cancers. 2015;7(2):908–29.
Wang Y-J, Wang F, Yu L-X, Xiang Y-J, Zhou F, Huang S-Y, et al. Worldwide review with meta-analysis of women’s awareness about breast cancer. Patient Educ Couns. 2022;105(7):1818–27.
Tiruneh M, Tesfaw A, Tesfa D. Survival and Predictors of Mortality among Breast Cancer Patients in Northwest Ethiopia: A Retrospective Cohort Study. Cancer Management and Research. 2021;13:9225.
Cianfrocca M, Goldstein LJ. Prognostic and predictive factors in early-stage breast cancer. Oncologist. 2004;9(6):606–16.
Jedy-Agba E, McCormack V, Adebamowo C, Dos-Santos-Silva I. Stage at diagnosis of breast cancer in sub-Saharan Africa: a systematic review and meta-analysis. Lancet Glob Health. 2016;4(12):e923–35.
Eber-Schulz P, Tariku W, Reibold C, Addissie A, Wickenhauser C, Fathke C, Hauptmann S, Jemal A, Thomssen C, Kantelhardt EJ. Survival of breast cancer patients in rural Ethiopia. Breast Cancer Res Treat. 2018;170:111–8.
Tesfaw A, Tiruneh M, Tamire T, Yosef T. Factors associated with advanced-stage diagnosis of breast cancer in north-west Ethiopia: a cross-sectional study. Ecancermedicalscience. 2021;15:1214.
Yoseph M, Gebresadik A, Alemayehu A. Late diagnosis of breast cancer and associated factors among women attending Hawassa University comprehensive and specialized hospital Southern Ethiopia. Res Sq. 2021:1–18. https://doi.org/10.21203/rs.3.rs-832493/v1.
Deressa BT, Cihoric N, Badra EV, Tsikkinis A, Rauch D. Breast cancer care in northern Ethiopia - cross-sectional analysis. BMC Cancer. 2019;19(1):393.
Ersumo T. Breast cancer in an Ethiopian population, Addis Ababa. East and Central African Journal of Surgery. 2006;11(1):81–6.
Gadisa DA, Assefa M, Tefera GM, Yimer G. Patterns of anthracycline‐based chemotherapy‐induced adverse drug reactions and their impact on relative dose intensity among women with breast cancer in Ethiopia: a prospective observational study. J Oncol. 2020;2020(1):2636514.
Gebregzabher E, Seifu D, Tigneh W, Bokretsion Y, Bekele A, Abebe M, et al. Detection of High- and Low-Risk HPV DNA in Archived Breast Carcinoma Tissues from Ethiopian Women. Int J Breast Cancer. 2021;2021:2140151.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372.
Bacha RH, Jabir YN, Asebot AG, Liga AD. Risk Factors Affecting Survival Time of Breast Cancer Patients: The case of Southwest Ethiopia. J Res Health Sci. 2021;21(4):e00532.
Rosen RD, Sapra A. TNM classification. StatPearls [Internet]: Tampa: StatPearls Publishing; 2021.
Ginsburg O, Yip C-H, Brooks A, Cabanes A, Caleffi M, Dunstan Yataco JA, et al. Breast cancer early detection: A phased approach to implementation. Cancer. 2020;126(S10):2379–93.
Yesufe AA, Assefa M, Bekele A, Ergete W, Aynalem A, Wondemagegnehu T, et al. Adequacy of pathologic reports of invasive breast cancer from mastectomy specimens at Tikur Anbessa Specialized Hospital Oncology Center in Ethiopia. J Global Oncol. 2018;4:1–12.
Abebe E, Mekonen W, Seifu D, Bekurtsion Y, Bekele A. Assessment of Proliferation Index and Pathological Features as Prognostic Potential of Breast Cancer in Ethiopia. J Cancer Sci Ther. 2019;11:106–14.
Bogale AS, Yalew AW, Getachew S, editors. Determinants of survival among patients with breast cancer in hawassa, southern ethiopia: a retrospective study, 2019. Addis Ababa: 31st EPHA Annual Conference; 2020.
Aberaraw R, Boka A, Teshome R, Yeshambel A. Social networks and quality of life among female breast cancer patients at Tikur Anbessa specialized hospital, Addis Ababa, Ethiopia 2019. BMC Womens Health. 2020;20(1):50.
Gebrehiwot AG, Melka DS, Kassaye YM, Gemechu T, Lako W, Hinou H, et al. Exploring serum and immunoglobulin G N-glycome as diagnostic biomarkers for early detection of breast cancer in Ethiopian women. BMC Cancer. 2019;19(1):1–18.
Gebretsadik A, Bogale N, Negera DG. Epidemiological Trends of Breast Cancer in Southern Ethiopia: A Seven-Year Retrospective Review. Cancer Control. 2021;28:10732748211055262.
Gebremariam A, Assefa M, Addissie A, Worku A, Dereje N, Abreha A, et al. Delayed initiation of adjuvant chemotherapy among women with breast cancer in Addis Ababa. Ethiopia Breast Cancer Res Treat. 2021;187(3):877–82.
Degu A, Kebede K. Drug-related problems and its associated factors among breast cancer patients at the University of Gondar Comprehensive Specialized Hospital, Ethiopia: A hospital-based retrospective cross-sectional study. J Oncol Pharm Pract. 2021;27(1):88–98.
Hassen AM, Hussien FM, Asfaw ZA, Assen HE. Factors Associated with Delay in Breast Cancer Presentation at the Only Oncology Center in North East Ethiopia: A Cross-Sectional Study. J Multidiscip Healthc. 2021;14:681.
Hassen AM, Taye G, Gizaw M, Hussien FM. Quality of life and associated factors among patients with breast cancer under chemotherapy at Tikur Anbessa specialized hospital, Addis Ababa, Ethiopia. PLoS ONE. 2019;14(9).
Teshome B, Trabitzsch J, Afework T, Addissie A, Kaba M, Kantelhardt EJ, et al. Perceived barriers to timely treatment initiation and social support status among women with breast cancer in Ethiopia. PLoS ONE. 2021;16(9).
Gizaw M. Information needs of breast cancer patients attending care at Tikur Anbessa Specialized Hospital. Addis Ababa Ethiopia: Addis Ababa university; 2017.
Shenkutie B, Mekonnen Y, Seifu D, Abebe E, Ergete W, Damie A, et al. Biological and clinicopathological characteristics of breast cancer at Tikur Anbessa specialized hospital, Addis Ababa, Ethiopia. J Cancer Sci Ther. 2017;2017(9):12.
Tesfay B, Getinet T, Derso EA. Joint modeling of longitudinal change in tumor cell level and time to death of breast cancer patients: In case of Ayder comprehensive specialized Hospital Tigray, Ethiopia. Cogent Medicine. 2021;8(1):1874090.
Dagne S, Abate SM, Tigeneh W, Engidawork E. Assessment of breast cancer treatment outcome at tikur anbessa specialized hospital adult oncology unit, Addis Ababa, Ethiopia. European Journal of Oncology Pharmacy. 2019;2(2):e13.
Koboto DD, Deribe B, Gebretsadik A, Ababi G, Bogale N, Geleta D, et al. Quality of life among breast cancer patients attending Hawassa University comprehensive specialized hospital cancer treatment center. Breast Cancer: Targets and Therapy. 2020;12:87. https://doi.org/10.21203/rs.3.rs-832493/v1
Hadgu E, Seifu D, Tigneh W, Bokretsion Y, Bekele A, Abebe M, et al. Breast cancer in Ethiopia: evidence for geographic difference in the distribution of molecular subtypes in Africa. BMC Womens Health. 2018;18(1):1–8.
Abebe E, Demilie K, Lemmu B, Abebe K. Female Breast Cancer Patients, Mastectomy-Related Quality of Life: Experience from Ethiopia. Int J Breast Cancer. 2020;2020:8460374.
Tadesse E, Seifu D, Menon M, Tigeneh W, Obsa T, Neges B, et al. Assessment of Estradiol, Progesterone and Lipid Profile among Breast Cancer Patients in Ethiopia: Hospital-based Comparative Cross-Sectional Study. 2021.
Kantelhardt EJ, Mathewos A, Aynalem A, Wondemagegnehu T, Jemal A, Vetter M, et al. The prevalence of estrogen receptor-negative breast cancer in Ethiopia. BMC Cancer. 2014;14:895.
Letta G. Magnitude of Breast and Cervical Cancer and Associated Risk Factors of Breast Cancer in Addis Ababa. Ethiopia: Addis Ababa University; 2013.
Gebremeskel K. Assessment of Malnutrition Using Biochemical Markers among Female Breast Cancer Patients Attending Tikur Anbessa Specialized Hospital. Ethiopia: Addis Ababa University; 2017.
Tesfaw LM, Teshale TA, Muluneh EK. Assessing the incidence, epidemiological description and associated risk factors of breast cancer in western Amhara, Ethiopia. Breast Cancer Management. 2020;9(3):BMT47.
Yohannes M. Arginase Activity in Blood and Breast Tissue of Breast Cancer Patients at Selected Hospitals in Addis Ababa. Ethiopia: Addis Ababa University; 2015.
Getu MA, Wang P, Kantelhardt EJ, Seife E, Chen C, Addissie A. Translation and validation of the EORTC QLQ-BR45 among Ethiopian breast cancer patients. Sci Rep. 2022;12(1):605.
Mehdi M, Menon MK, Seyoum N, Bekele M, Tigeneh W, Seifu D. Blood and tissue enzymatic activities of GDH and LDH, index of glutathione, and oxidative stress among breast cancer patients attending referral hospitals of Addis Ababa, Ethiopia: hospital‐based comparative cross‐sectional study. Oxid Med Cell Longev. 2018;2018(1):6039453.
Yoseph R. Retrospective Analysis of Breast Cancer Cases Operated in Jush within Four Years Time Period, Jimma. Ethiopia Int J Sci. 2021;10(10):4–21.
Tekle G, Dutamo Z. Survival Analysis of Determinants of Breast Cancer Patients at Hossana Queen Elleni Mohammad Memorial Referral Hospital, South Ethiopia: Bayesian Application of Hypertabastic Proportional Hazards Model. Int J Public Health. 2019;5(2):108–18.
Tesfaw A, Getachew S, Taylor L, Kantelhardt EJ, Addissie A. Abstract C105: Late-stage diagnosis of breast cancer and associated factors at rural hospitals in Ethiopia: A mixed-method study. A mixed-method study AACR. 2020;29(6):C105.
Shiferaw WS, Aynalem YA, Akalu TY, Demelew TM. Incidence and Predictors of Recurrence among Breast Cancer Patients in Black Lion Specialized Hospital Adult Oncology Unit, Addis Ababa, Ethiopia: Retrospective Follow-up Study with Survival Analysis. Journal of cancer prevention. 2020;25(2):111.
Wako Z, Mengistu D, Dinegde NG, Asefa T, Wassie M. Adherence to adjuvant hormonal therapy and associated factors among women with breast cancer attending the Tikur Anbessa Specialized Hospital, Addis Ababa Ethiopia, 2019: a cross-sectional study. Breast Cancer: Targets and Therapy. 2021;13:383.
Acknowledgements
We would like to thank all authors of included study as well as the participants of each primary study. Additionally, we thank Madda walabu University for providing as with all necessary need including adequate training to conduct this research.
Funding
No funding was obtained for this systematic review and meta-analysis.
Author information
Authors and Affiliations
Contributions
KB, FN and GB participated in protocol development and design, data extraction, study selection, analysis, and manuscript writing. TH, AF, DD, BG, LG, BL, and HG were involved in quality assessment, manuscript writing, and revising. finally, all authors review the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing of interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Bekele, K., Nugusu, F., Beressa, G. et al. Proportion of early-stage breast cancer at diagnosis in Ethiopia: a systematic review and meta-analysis. BMC Cancer 24, 1017 (2024). https://doi.org/10.1186/s12885-024-12768-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-024-12768-8