Abstract
Background
To develop a clinical model for predicting high axillary nodal burden in patients with early breast cancer by integrating ultrasound (US) and clinicopathological features.
Methods and materials
Patients with breast cancer who underwent preoperative US examination and breast surgery at the Affiliated Hospital of Nantong University (centre 1, n = 250) and at the Affiliated Hospital of Jiangsu University (centre 2, n = 97) between January 2012 and December 2016 and between January 2020 and March 2022, respectively, were deemed eligible for this study (n = 347). According to the number of lymph node (LN) metastasis based on pathology, patients were divided into two groups: limited nodal burden (0–2 metastatic LNs) and heavy nodal burden (≥ 3 metastatic LNs). In addition, US features combined with clinicopathological variables were compared between these two groups. Univariate and multivariate logistic regression analysis were conducted to identify the most valuable variables for predicting ≥ 3 LNs in breast cancer. A nomogram was then developed based on these independent factors.
Results
Univariate logistic regression analysis revealed that the cortical thickness (p < 0.001), longitudinal to transverse ratio (p = 0.001), absence of hilum (p < 0.001), T stage (p = 0.002) and Ki-67 (p = 0.039) were significantly associated with heavy nodal burden. In the multivariate logistic regression analysis, cortical thickness (p = 0.001), absence of hilum (p = 0.042) and T stage (p = 0.012) were considered independent predictors of high-burden node. The area under curve (AUC) of the nomogram was 0.749.
Conclusion
Our model based on US variables and clinicopathological characteristics demonstrates that can help select patients with ≥ 3 LNs, which can in turn be helpful to predict high axillary nodal burden in early breast cancer patients and prevent unnecessary axillary lymph node dissection.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Background
Axillary lymph node (ALN) status is a critical prognostic factor in the therapeutic plan of breast cancer patients because it can determine the extent of surgery and assess the necessity of chemotherapy or radiotherapy [1,2,3,4]. Axillary lymph node dissection (ALND) is used to assess ALN status, provide accurate staging of axillary lymph nodes and eliminate potential metastatic lymph nodes, and it is the standard surgical method for patients associated with greater lymph node burden [3,4,5,6]. However, ALND can cause severe complications, such as shoulder dyskinesia and arm lymphedema, which can have a negative impact on quality of life [7,8,9,10,11,12]. Hence, avoiding excessive ALND becomes a pressing issue. The results of the ACOSOG Z0011 trial showed no statistically significant differences between the ALND and no-ALND groups in terms of local recurrence rate and 10-year overall survival rate in patients with < 3 axillary lymph node metastases [13]. Patients with ≥ 3 axillary lymph node metastases (high axillary nodal burden) are more likely to have local recurrence, and are thus considered suitable for neoadjuvant chemotherapy or ALND [14,15,16,17]. Therefore, preoperative prediction of lymph node metastasis and identification of patients with high axillary nodal burden are crucial to determine the appropriate therapeutic management.
Preoperative imaging, including ultrasound (US), mammography, computed Tomography, magnetic resonance imaging, positron emission tomography etc., has become increasingly important and more widely used in assessing ALN metastasis in patients with breast cancer [18,19,20,21]. Compared with other imaging modalities, ultrasound is more cost-effective, non-invasive and reproducible [14, 15, 22]. However, Hieken et al. showed a false positive rate of 79.8% for suspicious axillary ultrasound results according to pathological examination [23]. Therefore, it is insufficient to assess ALN burden by axillary ultrasound alone. Fortunately, the US characteristics of primary breast lesion are reported to be obviously associated with high ALN metastasis [24, 25]. In addition, certain clinicopathological features of patients with breast cancer are related to ALN metastasis [26,27,28]. The purpose of this study was to integrate the ultrasonic features of lymph nodes and primary lesions with clinicopathological characteristics to identify independent predictors and develop a model to predict high-burden lymph node (≥ 3) in patients with breast cancer and prevent unnecessary ALND.
Materials and methods
This study design followed the international regulations according to the Declaration of Helsinki. Our research was approved by the Ethical Committee of the Affiliated Hospital of Nantong University (2022-K108-01) and Affiliated Hospital of Jiangsu University (KY2021K1213), and written informed consent was obtained from participants.
Patient enrolment
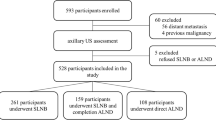
Patients who underwent breast surgery and US examination at the Affiliated Hospital of Nantong University (centre 1, n = 1232) and at the Affiliated Hospital of Jiangsu University (centre 2, n = 566) between January 2012 and December 2016 and between January 2020 and March 2022, respectively, were deemed eligible for this study (n = 1798). Patients were included if they: [1] had clinical N1 or N0; [2] had a preoperative breast US and axillary US performed within two weeks of surgery, which recorded US characteristics of the primary breast tumour and axillary lymph nodes; [3] had breast surgery and axillary lymph node dissection; [4] had pathology which documented the number of axillary lymph node metastasis; [5] and had a pathology tumour size that was T1 or T2. Exclusion criteria were as follows: [1] patients with a history of other malignant tumours and ipsilateral axillary surgery history; [2] patients who underwent preoperative chemotherapy, radiotherapy or immunotherapy and [3] absence of clinicopathological or US information. Finally, 347 patients were included in this study (Fig. 1).
US analysis
All patients included in this study underwent a preoperative breast and axillary US examination within two weeks of surgery using the GE LOGIQ E9 with a linear array transducer (12–15 MHz). Patients were positioned flat with bilateral arms raised to fully expose the bilateral breasts and axilla. US parameters, such as gain, depth, focal length, etc., were adjusted to enable a clear display of the primary lesion. The primary lesion or lymph node used for the assessment was situated in the central part of the ultrasound screening. Subsequently, we scanned the lesion from multiple angles and acquired images of the primary lesion and lymph nodes. The images were then stored for further analysis.
US features of the primary tumour and ALNs were observed independently by two radiologists who were blinded to any information that could interfere or bias with their task. Potential disagreements or differences were arbitrated by a third experienced radiologist to reach a consensus. The US features of the primary tumour were analysed, including quadrants (upper outer, upper inner, lower outer and lower inner quadrants), margins (circumscribed or non-circumscribed), orientation (parallel or non-parallel), shape (regular or irregular), attenuation (weak or not) and calcification (with or without). Furthermore, axillary US measured the longitudinal to transverse ratio (< 2 or ≥ 2) and cortical thickness (< 3 mm or ≥ 3 mm), and determined the absence of hilum (yes or not).
Clinicopathological analysis
Surgical-histopathologic data included the number of metastatic lymph nodes (< 3 or ≥ 3), T stage (≤ 2 cm or 2–5 cm), histological grade (I, II or III) and the expression of estrogen receptor (ER), progesterone receptor (PR), CerbB-2, P53 and Ki-67 from histopathology reports. ER and PR positivity were defined as the expression of greater than 1% [29], while Ki67 positivity was defined as the expression of greater than 14% [30]. CerbB-2 receptor of 3 + in HE or 2 + in gene amplification was defined as human epidermal growth factor receptor 2 (HER-2) positive [29, 31].
Statistical analysis
Statistical analysis was conducted using the SPSS software (ver. 24.0; SPSS Inc., Chicago, IL, USA), the MedCalc software (ver.19.07) the R software (ver 4.0.1). The enrolled patients were randomly divided into the training group and the verification group according to the ratio of 7:3. X2 tests or Fisher’s exact test were performed between the training and the validation groups. Univariate logistic regression analysis was used to identify factors that could significantly affect the training group. Multivariate logistic regression analysis was applied to determine independent predictors of the number of axillary lymph node metastasis and incorporate them into the model. The area under the area under curve (AUC), accuracy (ACC), sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) was used to assess model discrimination. Delong test was conducted to compare different diagnostic models across the training and validation cohort for nomogram, cortical thickness, lymphatic gate, and T stage by MedCale. Finally, a calibration diagram was drawn to evaluate the ability of calibration and the fit of the model was assessed by the Hosmer–Lemeshow goodness-of-fit test.
Results
Baseline characteristics
Table 1 shows the characteristics of the research population. Patients were divided into the training group (n = 243) and the verification group (n = 104) with a ratio of 7:3. Fifty-six (23.0%) and 30 (28.8%) patients had ≥ 3 lymph node metastases of primary breast cancer in the training and validation cohorts, respectively. There were no significant differences in the US characteristics and clinicopathological parameters between the two groups. The mean ages of the training and validation groups were 55.84 ± 10.74 and 55.54 ± 11.66, respectively.
Univariate and multivariate analyses
In the univariate analysis, variables that were significantly associated with ≥ 3 lymph node metastases included cortical thickness (p < 0.001), longitudinal to transverse ratio (p = 0.001), absence of hilum (p < 0.001), T stage (p = 0.002) and Ki-67 (p = 0.039) (Table 2 and 3). The remaining factors were not found to be significant for the identification of high-burden lymph nodes (all p > 0.05). In the multivariate logistic regression analysis, cortical thickness (p = 0.001), absence of hilum (p = 0.042) and T stage (p = 0.012) are shown in Table 4. Ki-67 and longitudinal to transverse ratio were not independent predictors. Cortical thickness, absence of hilum and T stage were considered as independent predictors of HBN, and these parameters were then incorporated into the predictive model to create a nomogram (p < 0.05).
Development of the nomogram
Based on the results of the multivariate logistic regression analysis, cortical thickness, absence of hilumand T stage were incorporated to create a nomogram (Fig. 2). The nomogram had an AUC of 0.749 (95% CI: 0.676–0.823), sensitivity of 71.4%, specificity of 75.9%, PPV of 67.7% and NPV of 81.1% (Table 5). The AUCs of cortical thickness, lymphatic gate and T stage were 0.690 (95% CI: 0.609–0.770), 0.639 (95% CI: 0.551–0.728) and 0.621 (95% CI: 0.539–0.703), respectively (Fig. 3). The AUC of model was greater than the AUCs of cortical thickness (p = 0.003), lymphatic gate (p < 0.001) and T stage (p < 0.001) (Table 5). The C-index of this model was 0.749 (95% CI: 0.677–0.820). The Hosmer–Lemeshow-Goodness-of-Fit test had a p-value of 0.995 and the calibration plot is shown in Fig. 3. Through bootstrap validation, the C-index of the nomogram was considered 0.68. The decision curve analysis (DCA) showed good net benefits in the training set in Fig. 4.
Validation of the nomogram
The validation group model consisted of 104 patients. The AUC of the prediction model for the validation group was 0.783 (95% CI: 0.685–0.881) (Fig. 5 and Table 6). The C-index of the validation group was 0.783(95% CI:0.685–0.881). The AUC of model was greater than the AUCs of cortical thickness (p = 0.009), lymphatic gate (p = 0.001) and T stage (p = 0.007) (Table 6). The P-value of the Hosmer–Lemeshow-Goodness-of-Fit test was 0.783. The DCA had good net benefits in the validation group (Fig. 6).
Discussion
Although US examination is currently one of the most widely used and important imaging technologies, it is not completely accurate in predicting high-burden lymph nodes [32, 33]. Predicting high lymph node burden can guide individualised treatment strategies with respect to the application of neoadjuvant chemotherapy and selection of the type of axillary surgery (SLNB vs. ALND) [34]. Therefore, prediction of lymph node metastasis and identification of patients with high axillary lymph node loads are both essential and challenging processes. The main strength of this study is that we successfully integrated US and clinicopathological features of lymph nodes and primary lesion ultrasound and establish a nomogram that could predict a high axillary lymph node burden.
In our study, the cortical thickness and lymphatic hilum of lymph nodes and the T stage of the primary lesion were found to be independent predictors of high-burden lymph nodes. Based on these three parameters, we established a nomogram to predict high-burden node (HBN), and our results showed that its AUC was 0.749, i.e. a satisfactory predictive value. In the nomogram, cortical thickness was more important. The point of cortical thickness, which was ≥ 3 mm, was 100 points, and thus greater than the lymphatic hilum of lymph nodes and T stage. The US characteristics of the lesion proved impossible to identify high-burden lymph nodes. However, according to the studies performed by Torstenson and Ansari, the distance between the tumour and nipple and the distance between the tumour and skin were significantly correlated with positive lymph node metastasis [35, 36]. In Yi’s study, the distance from the nipple was interconnected with high-burden lymph nodes [24]. Since this was a retrospective study, the US report failed to count the distance from the primary lesion to the nipple, which may have caused a degree of impact on the diagnostic efficiency. To our knowledge, only a limited number of studies have combined ultrasound of axillary lymph nodes, ultrasound of primary lesions and clinicopathological characteristics. Our study could comprehensively evaluate the relationship between these three parameters and high burden lymph nodes metastasis.
There are still some limitations in our research. First, this study was a retrospective study. In the axillary ultrasound report, we mainly focused on lymph nodes with large size or potential malignancies, while some lymph nodes with small size but abnormal morphology were ignored. Consequently, there may be sample selection deviation. Second, due to the small sample size, the low-burden group included a significantly greater number of cases compared to the high-burden group, which may influenced our results. Third, we did not statistically analyse the blood flow in the lymph nodes and the primary lesion. In addition, the clinical and pathological factors used in developing the nomogram were obtained postoperatively, making it challenging to directly predict the burden of lymph nodes before surgery. However, the study added information about ultrasound results, and future research may require additional studies incorporating MRI, molybdenum target and clinically assessable pathological factors before surgery and establish a preoperative prediction model. Therefore, future studies with a larger sample size and more comprehensive characteristics are expected to confirm the clinical application of the present model.
Conclusion
In conclusion, we established a nomogram integrating the US and clinicopathological features of axillary lymph nodes and primary breast lesions to predict high-burden lymph nodes metastasis. Lymph node cortical thickness, lymphatic hilum and T stage were found to be important indicators for predicting high-burden lymph nodes metastasis, and these parameters are expected to be helpful in clinical practice in terms of reducing unnecessary ALND and identifying the patients requiring neoadjuvant chemotherapy.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Abbreviations
- US:
-
Ultrasound
- ALN:
-
Axillary lymph node
- ALND:
-
Axillary lymph node dissection
- ER:
-
Estrogen receptor
- PR:
-
Progesterone receptor
- Her-2:
-
Human epidermal growth factor receptor 2
- AUC:
-
Area under curve
- ACC:
-
Accuracy
- SN:
-
Sensitivity
- SP:
-
Specificity
- PPV:
-
Positive predictive value
- NPV:
-
Negative predictive value
- CI:
-
Confidence interval
- DCA:
-
Decision curve analysis
References
Qiu SQ, Zeng HC, Zhang F, Chen C, Huang WH, Pleijhuis RG, et al. A Nomogram to predict the probability of axillary lymph node metastasis in early breast cancer patients with positive axillary ultrasound. Sci Rep. 2016;6:21196. https://doi.org/10.1038/srep21196.
Kim H, Shin MJ, Kim SJ, Kim IJ, Park I. The relation of visualization of internal mammary lymph nodes on lymphoscintigraphy to axillary lymph node metastases in breast cancer. Lymphat Res Biol. 2014;12(4):295–300. https://doi.org/10.1089/lrb.2013.0039.
Tandon M, Ball W, Kirby R, Soumian S, Narayanan S. A comparative analysis of axillary nodal burden in ultrasound/biopsy positive axilla vs ultrasound negative sentinel lymph node biopsy positive axilla. Breast Dis. 2019;38(3–4):93–6. https://doi.org/10.3233/bd-160230.
Abreu EB, Martinez P, Betancourt L, Romero G, Godoy A, Bergamo L. Treatment plan for breast cancer with sentinel node metastasis. E cancer medical science. 2014;8:383. https://doi.org/10.3332/ecancer.2014.383.
Isozaki H, Yamamoto Y, Murakami S, Matsumoto S, Takama T. Impact of the surgical modality for axillary lymph node dissection on postoperative drainage and seroma formation after total mastectomy. Patient Saf Surg. 2019;13:20. https://doi.org/10.1186/s13037-019-0199-z.
Collins M, O’Donoghue C, Sun W, Zhou JM, Ma Z, Laronga C, et al. Use of axillary lymph node dissection (Alnd) in patients with Micrometastatic breast cancer. J Surg Res. 2017;215:55–9. https://doi.org/10.1016/j.jss.2017.03.039.
Akezaki Y, Tominaga R, Kikuuchi M, Kurokawa H, Hamada M, Aogi K, et al. Risk factors for lymphedema in breast cancer survivors following axillary lymph node dissection. Progress in rehabilitation medicine. 2019;4:20190021. https://doi.org/10.2490/prm.20190021.
Galimberti V, Botteri E, Chifu C, Gentilini O, Luini A, Intra M, et al. Can we avoid axillary dissection in the micrometastatic sentinel node in breast cancer? Breast Cancer Res Treat. 2012;131(3):819–25. https://doi.org/10.1007/s10549-011-1486-2.
Hayes S, Janda M, Cornish B, Battistutta D, Newman B. Lymphedema secondary to breast cancer: how choice of measure influences diagnosis, prevalence, and identifiable risk factors. Lymphology. 2008;41(1):18–28.
Voogd AC, Ververs JM, Vingerhoets AJ, Roumen RM, Coebergh JW, Crommelin MA. Lymphoedema and reduced shoulder function as indicators of quality of life after axillary lymph node dissection for invasive breast cancer. Br J Surg. 2003;90(1):76–81. https://doi.org/10.1002/bjs.4010.
Goyal A, Newcombe RG, Chhabra A, Mansel RE. Morbidity in breast cancer patients with sentinel node metastases undergoing delayed axillary lymph node dissection (Alnd) compared with immediate Alnd. Ann Surg Oncol. 2008;15(1):262–7. https://doi.org/10.1245/s10434-007-9593-3.
Basta MN, Fox JP, Kanchwala SK, Wu LC, Serletti JM, Kovach SJ, et al. Complicated breast cancer-related lymphedema: evaluating health care resource utilization and associated costs of management. Am J Surg. 2016;211(1):133–41. https://doi.org/10.1016/j.amjsurg.2015.06.015.
Olson JA Jr, McCall LM, Beitsch P, Whitworth PW, Reintgen DS, Blumencranz PW, et al. Impact of immediate versus delayed axillary node dissection on surgical outcomes in breast cancer patients with positive sentinel nodes: results from American college of surgeons oncology group trials Z0010 and Z0011. J Clinic Oncol : Official J American Soc Clinical Oncol. 2008;26(21):3530–5. https://doi.org/10.1200/jco.2007.15.5630.
Ha SM, Chang JM, Kim SY, Lee SH, Kim ES, Kim YS, et al. Prediction of axillary nodal burden in patients with invasive lobular carcinoma using Mri. Breast Cancer Res Treat. 2021;186(2):463–73. https://doi.org/10.1007/s10549-020-06056-9.
Montemurro F, Maggiorotto F, Valabrega G, Kubatzki F, Rossi V, Magistris A, et al. Omission of axillary dissection after a positive sentinel node dissection may influence adjuvant chemotherapy indications in operable breast cancer patients. Ann Surg Oncol. 2012;19(12):3755–61. https://doi.org/10.1245/s10434-012-2505-1.
Caudle AS, Cupp JA, Kuerer HM. Management of axillary disease. Surg Oncol Clin N Am. 2014;23(3):473–86. https://doi.org/10.1016/j.soc.2014.03.007.
Ecanow JS, Abe H, Newstead GM, Ecanow DB, Jeske JM. Axillary Staging of Breast Cancer: What the Radiologist Should Know. Radiographics: a review publication of the Radiological Society of North America, Inc. 2013;33(6):1589–612. https://doi.org/10.1148/rg.336125060
Liang X, Yu J, Wen B, Xie J, Cai Q, Yang Q. Mri and Fdg-Pet/Ct based assessment of axillary lymph node metastasis in early breast cancer: a meta-analysis. Clin Radiol. 2017;72(4):295–301. https://doi.org/10.1016/j.crad.2016.12.001.
Buus TW, Sandahl M, Thorup KS, Rasmussen F, Redsted S, Christiansen P, et al. Breast cancer: comparison of quantitative dual-layer spectral CT and axillary ultrasonography for preoperative diagnosis of metastatic axillary lymph nodes. Europ Radiol Experiment. 2021;5(1):16. https://doi.org/10.1186/s41747-021-00212-6.
Wu PQ, Liu CL, Liu ZY, Ye WT, Liang CH. Value of Mamography, Ct and Dce-Mri in detecting axillary lymph node metastasis of breast cancer. J Southern Medic University. 2016;36(4):493–9.
Kim WH, Lee SW, Kim HJ, Chae YS, Jeong SY, Jung JH, et al. Prediction of advanced axillary lymph node metastases (Ypn2-3) using breast Mr imaging and Pet/Ct after neoadjuvant chemotherapy in invasive ductal carcinoma patients. Sci Rep. 2018;8(1):3181. https://doi.org/10.1038/s41598-018-21554-z.
Zhu Y, Zhou W, Jia XH, Huang O, Zhan WW. Preoperative axillary ultrasound in the selection of patients with a heavy axillary tumor burden in early-stage breast cancer: what leads to false-positive results? J Ultrasound Medicine : Official J American Instit Ultrasound Medicine. 2018;37(6):1357–65. https://doi.org/10.1002/jum.14545.
Hieken TJ, Trull BC, Boughey JC, Jones KN, Reynolds CA, Shah SS, et al. Preoperative axillary imaging with percutaneous lymph node biopsy is valuable in the contemporary management of patients with breast cancer. Surg. 2013;154(4):831–8. https://doi.org/10.1016/j.surg.2013.07.017. (discussion 8-40).
Yi CB, Ding ZY, Deng J, Ye XH, Chen L, Zong M, et al. Combining the ultrasound features of primary tumor and axillary lymph nodes can reduce false-negative rate during the prediction of high axillary node burden in bi-rads category 4 or 5 breast cancer lesions. Ultrasound Med Biol. 2020;46(8):1941–8. https://doi.org/10.1016/j.ultrasmedbio.2020.04.003.
den Bakker MA. Is histopathology still the gold standard? Ned Tijdschr Geneeskd. 2017;160:D981.
Chen X, He Y, Wang J, Huo L, Fan Z, Li J, et al. Feasibility of using negative ultrasonography results of axillary lymph nodes to predict sentinel lymph node metastasis in breast cancer patients. Cancer Med. 2018;7(7):3066–72. https://doi.org/10.1002/cam4.1606.
Chue KM, Yong WS, Thike AA, Ahmed SS, Li HH, Wong CY, et al. Predicting the likelihood of additional lymph node metastasis in sentinel lymph node positive breast cancer: validation of the memorial Sloan-Kettering cancer Centre (Mskcc) nomogram. J Clin Pathol. 2014;67(2):112–9. https://doi.org/10.1136/jclinpath-2013-201524.
Kim GR, Choi JS, Han BK, Lee JE, Nam SJ, Ko EY, et al. Preoperative axillary us in early-stage breast cancer: potential to prevent unnecessary axillary lymph node dissection. Radiology. 2018;288(1):55–63. https://doi.org/10.1148/radiol.2018171987.
Qiu SQ, Wei XL, Huang WH, Wu MY, Qin YS, Li YK, et al. Diagnostic and therapeutic strategy and the most efficient prognostic factors of breast malignant fibrous histiocytoma. Sci Rep. 2013;3:2529. https://doi.org/10.1038/srep02529.
Xie F, Yang H, Wang S, Zhou B, Tong F, Yang D, et al. A logistic regression model for predicting axillary lymph node metastases in early breast carcinoma patients. Sensors (Basel, Switzerland). 2012;12(7):9936–50. https://doi.org/10.3390/s120709936.
Hanna WM, Slodkowska E, Lu FI, Nafisi H, Nofech-Mozes S. Comparative analysis of human epidermal growth factor receptor 2 testing in breast cancer according to 2007 and 2013 American society of clinical oncology/college of American pathologists guideline recommendations. J Clinic Oncol: Official J American Soc Clinic Oncol. 2017;35(26):3039–45. https://doi.org/10.1200/jco.2016.70.5319.
Marino MA, Avendano D, Zapata P, Riedl CC, Pinker K. Lymph node imaging in patients with primary breast cancer: concurrent diagnostic tools. Oncolog. 2020;25(2):e231–42. https://doi.org/10.1634/theoncologist.2019-0427.
Bedi DG, Krishnamurthy R, Krishnamurthy S, Edeiken BS, Le-Petross H, Fornage BD, et al. Cortical Morphologic Features of Axillary Lymph Nodes as a Predictor of Metastasis in Breast Cancer: In Vitro Sonographic Study. AJR American journal of roentgenology. 2008;191(3):646–52. https://doi.org/10.2214/ajr.07.2460.
Kim WH, Kim HJ, Lee SM, Cho SH, Shin KM, Lee SY, et al. Prediction of high nodal burden with ultrasound and magnetic resonance imaging in clinically node-negative breast cancer patients. Cancer Imaging: Official Public Int Cancer Imag Soc. 2019;19(1):4. https://doi.org/10.1186/s40644-019-0191-y.
Torstenson T, Shah-Khan MG, Hoskin TL, Morton MJ, Adamczyk DL, Jones KN, et al. Novel factors to improve prediction of nodal positivity in patients with clinical t1/t2 breast cancers. Ann Surg Oncol. 2013;20(10):3286–93. https://doi.org/10.1245/s10434-013-3110-7.
Ansari B, Morton MJ, Adamczyk DL, Jones KN, Brodt JK, Degnim AC, et al. Distance of breast cancer from the skin and nipple impacts axillary nodal metastases. Ann Surg Oncol. 2011;18(11):3174–80. https://doi.org/10.1245/s10434-011-1957-z.
Acknowledgements
We are indebted to all the teams and patients who participated in this trail and made it possible.
Funding
We greatly acknowledged the China Postdoctoral Science Foundation (Grant No. 2022M711721), the National Science Foundation for Young Scientists of China (Grant No. 82302208), the Social Development Program of Zhenjiang City (Grant No. SH2022066), and Medical Education Collaborative Innovation Fund of Jiangsu University (Grant No. JDY2022002).
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: LY, YFG and YFY. Performed the experiments: BW and MS. Contributed reagents/materials/analysis tools: LZ, LS and YFW. Wrote the paper: LY, ZZ and YFY. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study design followed the international regulations according to the Declaration of Helsinki. Our research was approved by the Ethical Committee of the Affiliated Hospital of Nantong University (2022-K108-01) and Affiliated Hospital of Jiangsu University (KY2021K1213), and written informed consent was obtained from participants.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yang, L., Gu, Y., Wang, B. et al. A multivariable model of ultrasound and clinicopathological features for predicting axillary nodal burden of breast cancer: potential to prevent unnecessary axillary lymph node dissection. BMC Cancer 23, 1264 (2023). https://doi.org/10.1186/s12885-023-11751-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-023-11751-z