Abstract
Background
Immunosuppression is a significant factor contributing to the poor prognosis of cancer. S100P, a member of the S100 protein family, has been implicated in various cancers. However, its role in the tumor microenvironment (TME) of pancreatic cancer remains unclear. This study aimed to investigate the potential impact of S100P on TME characteristics in patients with pancreatic cancer.
Methods
Multiple data (including microarray, RNA-Seq, and scRNA-Seq) were obtained from public databases. The expression pattern of S100P was comprehensively evaluated in RNA-Seq data and validated in four different microarray datasets. Prognostic value was assessed through Kaplan-Meier plotter and Cox regression analyses. Immune infiltration levels were determined using the ESTIMATE and ssGSEA algorithms and validated at the single-cell level. Spearman correlation test was used to examine the correlation between S100P expression and immune checkpoint genes, and tumor mutation burden (TMB). DNA methylation analysis was performed to investigate the change in mRNA expression. Reverse transcription PCR (RT-PCR) and immunohistochemical (IHC) were utilized to validate the expression using five cell lines and 60 pancreatic cancer tissues.
Results
This study found that S100P was differentially expressed in pancreatic cancer and was associated with poor prognosis (P < 0.05). Notably, S100P exhibited a significant negative-correlation with immune cell infiltration, particularly CD8 + T cells. Furthermore, a close association between S100P and immunotherapy was observed, as it strongly correlated with TMB and the expression levels of TIGIT, HAVCR2, CTLA4, and BTLA (P < 0.05). Intriguingly, higher S100P expression demonstrated a negative correlation with methylation levels (cg14323984, cg27027375, cg14900031, cg14140379, cg25083732, cg07210669, cg26233331, and cg22266967), which were associated with CD8 + T cells. In vitro RT-PCR validated upregulated S100P expression across all five pancreatic cancer cell lines, and IHC confirmed high S100P levels in pancreatic cancer tissues (P < 0.05).
Conclusion
These findings suggest that S100P could serve as a promising biomarker for immunosuppressive microenvironment, which may provide a novel therapeutic way for pancreatic cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pancreatic cancer is a highly lethal disease worldwide with a mortality rate that closely parallels its incidence [1]. In the United States and Europe, it ranks as the third and fourth leading causes of cancer-related mortality, respectively. [2, 3]. Despite some improvement, the five-year relative survival rate remains low at only approximately 10% [4]. Early diagnosis and treatment have been shown to reduce surgical complications, disease recurrence, and clinical deterioration, and improve survival rates. Therefore, it is of paramount importance to determine the etiology mechanisms and explore more effective treatment strategies.
In recent years, immunotherapy and targeted therapies have brought revolutionary progress in the therapy of various cancer types, including melanoma [5], lung cancer [6], and colorectal cancer [7]. However, the results have not been promising in patients with pancreatic cancer. This difference may be correlated with the higher TME heterogeneity of pancreatic cancer, particularly its highly desmoplastic stroma and immunosuppressive cell populations [8, 9]. The TME has been shown to inactivate the ability of cytotoxic T cells to eliminate tumor cells [10], and CD8 + T cells, the central subpopulation of cytotoxic T lymphocytes, are primarily responsible for eliminating tumor cells [10]. Therefore, a comprehensive analysis of TME might reveal the mechanisms of resistance to immunotherapy, which may provide the basis for pancreatic cancer immunology and opportunities to improve survival [11]. Immunomodulatory factors are vital to immunotherapy, and more profound research on the TME of pancreatic cancer will provide a new theoretical basis for the development of immunotherapy.
Numerous studies have demonstrated that S100P is an oncogenic gene involved in many types of tumors, such as breast cancer [12], gastric cancer [13], and pancreatic cancer [14]. However, the current literature provides limited insights into the possible role of S100P in the regulation of the TME in pancreatic cancer. Therefore, this study was designed to explore the potential biological mechanisms and regulation of S100P in the pancreatic cancer microenvironment. The main objective of this study is to enhance our understanding of the immunomodulatory effects of S100P in pancreatic cancer, and potentially leverage its role in improving the effectiveness of immunotherapy.
Materials and methods
Data collection and pre-processing
In this study, multiple datasets were utilized to perform comprehensive analyses. Specifically, microarray, RNA-Seq, and scRNA-Seq datasets were selected to evaluate different aspects of the research question. The datasets were obtained from various public repositories, including the GEO (https://www.ncbi.nlm.nih.gov/gds/), the ArrayExpress (https://www.ebi.ac.uk/arrayexpress/), CCLE (https://sites.broadinstitute.org/ccle), and UCSC Xena (https://xenabrowser.net/datapages/). More specifically, GSE28735, GSE15471, GSE16515, GSE71729, and GSE155698 were retrieved from the GEO database, while E-MTAB-6134 was downloaded from ArrayExpress. RNA-Seq data were collected from cellular and tissue levels through CCLE and UCSC Xena, respectively. In line with a prior publication, the same procedures for microarray pre-processing were implemented [15].
Overview of S100P in pan-cancer and pancreatic cancer
The expression of S100P was initially examined in 33 solid tumor types using the GEPIA tool (http://gepia.cancer-pku.cn/) [16]. To assess S100P expression at the cellular level, we analyzed its expression in 31 cancer types using CCLE data. We then validated the expression levels of S100P in four different microarray datasets (GSE28735, GSE15471, GSE16515, and GSE71729) using the “limma” package. Clinical characteristics were subsequently analyzed in relation to S100P expression levels, and survival statistics for S100P were calculated using the “survival” package in multiple cancer cohorts and further validated in E-MTAB-6134. To explore the role of S100P in pancreatic cancer progression, we examined its expression levels in different TNM stages and KRAS mutation states.
Assessment of the correlation between S100P and tumor microenvironment
Firstly, the ESTIMATE algorithm [17] was employed to calculate the TumorPurity (proportion of cancer cells in the tumor tissue), StromalScore (proportion of stromal ingredient), ImmuneScore (proportion of immune ingredient), and ESTIMATEScore (sum of the ImmuneScore and StromalScore) for each pancreatic cancer sample. The correlation between these scores and S100P expression was subsequently evaluated. Next, single-sample gene set enrichment analysis (ssGSEA) was implemented to quantify the scores of 29 immune-related signatures in the datasets E-MTAB-6134 and GSE71729 via the “GSVA” package. The infiltration levels between the high- and low-expression groups were investigated based on the median of S100P expression. The correlation between S100P expression and CD8 + T cells was validated using five algorithms, including CIBERSORT, TIMER, MCP-counter, quanTIseq, and xCell. Additionally, the “ImmuneSubtypeClassifier” package was employed to calculate the six immune subtypes (C1: wound healing, C2: IFN-γ dominant, C3: inflammatory, C4: lymphocyte depleted, C5: immunologically quiet, and C6: TGF-β dominant) [18]. The correlation between these immune subtypes and S100P expression was evaluated using previously processed data [15].
Analysis of S100P in pancreatic cancer using scRNA-Seq data
S100P expression in the TME of pancreatic cancer was validated at the single-cell level using CRA001160 and GSE154778, with analysis conducted through the TISCH2 web (http://tisch.comp-genomics.org/) [19]. Then, we downloaded and reanalyzed the GSE155698 dataset, which includes seventeen primary pancreatic cancer samples. The dataset was subjected to quality control and processed using standardized protocols in the “Seurat” and “DoubletFinder” packages. The “harmony” package was also utilized to correct batch effect; the identities of cell types were characterized based on CellMarker 2.0 webserver (http://bio-bigdata.hrbmu.edu.cn/CellMarker/) [20]. Finally, to validate the relationship between the immune cell infiltration and S100P expression at the single-cell level, the proportions of these cells were calculated according to the S100P expression level.
Examining the relationship between S100P expression and immunotherapy
Previous results have indicated an inverse correlation between S100P and CD8 + T cells, highlighting the potential of S100P in immunotherapy. Hence, we used two datasets, E-MTAB-6134 and TCGA-PAAD, to explore the correlation between S100P expression and immune checkpoint molecules (PD-1/CD274, CTLA4, IDO1, BTLA, LAG3, TIM-3/HAVCR2, TIGIT). The RNA-Seq data from TCGA was also utilized to compute the tumor mutation burden (TMB), and the Spearman correlation coefficient was used to determine the correlation between S100P expression and TMB expression. Finally, patients with pancreatic cancer were divided into high and low S100P expression groups according to the median value. We utilized “maftools” packages to observe the difference in mutated genes between the two groups [21].
Exploring the correlation between S100P expression and DNA methylation levels
Investigating the potential causes of the increased S100P expression in patients with pancreatic cancer is of paramount importance. DNA methylation is acknowledged as a well-established mechanism for regulating gene expression [22]. Accordingly, we sought to investigate the relationship between DNA methylation and the expression of S100P by utilizing the MEXPRESS database (https://www.mexpress.be/). Furthermore, we aimed to explore the association between significant DNA methylation and infiltration levels of CD8 + T cells using Spearman correlation algorithm.
Cell culture, RNA extraction, and reverse transcription PCR (RT-PCR)
This study extracted total RNA from five types of pancreatic cancer cell lines (PANC-1, SW1990, BxPC-3, Capan 1, and AsPC-1), and its quality was assessed using an ND-1000 spectrophotometer (NanoDrop Technologies; Thermo Fisher Scientific, Inc). Next, cDNA was synthesized, and RT-PCR was performed using Applied Biosystems (Thermo Fisher Scientific, Inc). The primer sequences used were as follows: S100P, forward, 5’‑GCACCATGACGGAACTAGAGACA‑3’ and reverse, 5’‑CAGGTCCTTGAGCAATTTATCCAC‑3’; GAPDH, forward, 5’‑GCACCGTCAAGGCTGAGAAC‑3’ and reverse, 5’‑TGGTGAAGACGCCAGTGGA‑3’. Finally, the relative expression abundance was determined using the ΔCt(S100P-GAPDH) method.
Immunohistochemistry
Thirty patients with a pathological diagnosis of pancreatic cancer underwent radical surgery, and thirty adjacent tissues were carried out to assess the S100P expression at the protein level. First, the sections were deparaffinized and rehydrated. Next, it was incubated with polyclonal anti-S100P antibody (1:10000 dilution) (proteintech, 11283-1-AP), log2(H-score) was used to quantify S100P expression from immunohistochemistry images. Then, the expression of S100P protein was assessed in 137 primary tumors and 74 normal tissues using the UALCAN platform (http://ualcan.path.uab.edu/index.html) [23].
Results
Pan-cancer expression pattern, prognostic significance, and clinical correlation of S100P
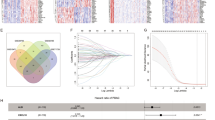
In pan-cancer, it was found that S100P was more highly expressed in breast cancer(BRCA), cervical cancer(CESC), colon cancer(COAD), liver cancer(LIHC), lung adenocarcinoma(LUAD), pancreatic cancer(PAAD), rectal cancer(READ), endometrioid cancer(UCEC), and uterine carcinosarcoma(UCS), but lower in large B-cell lymphoma(DLBC), prostate cancer(PRAD), melanoma(SKCM), thyroid cancer(THCA), and thymoma(THYM) (Fig. 1A), especially in PAAD (Figure S1). Four microarray datasets provided validation results that supported the notion that tumor tissues exhibited higher expressions of S100P as compared to non-tumor samples (Fig. 1B). Moreover, cellular analysis of S100P expression among thirty different types of cancer cells showed a significant trend of high expression in PAAD (Fig. 1C). Survival analysis identified that higher expression of S100P was statistically linked with poor survival in five cancers (Fig. 1D), including PAAD, glioma(GBMLGG), LUAD, LIHC, adrenocortical carcinoma(ACC) with 1.18(95% CI 1.08,1.28), 1.12(95% CI 1.04,1.20), 1.07(95% CI 1.02,1.12), 1.05(95% CI 1.00,1.09), 1.13(95% CI 1.01,1.28), significantly in PAAD, and this finding was validated in E-MTAB-6134 with identical results (Fig. 1E). In the clinical aspect, the expression of S100P was associated with KRAS mutation in TCGA-PAAD and E-MTAB-6134 datasets (Fig. 1F&G). Notably, the mRNA expression of S100P was significantly increased in patients with stage II-IV compared to patients with stage I, suggesting that S100P may potentially contribute to the progression of pancreatic cancer (Figure S2). Furthermore, according to the pathway analysis from TISIDB, S100P was involved in three pathways: immune system, innate immune system, and neutrophil degranulation (http://cis.hku.hk/TISIDB/browse.php?gene=S100P).
Overview of S100P in human cancers. (A) Assessment of S100P expression in tumor and adjacent tissues using the TCGA and GTEx databases. (B) Validation of S100P expression in GSE28735, GSE15471, GSE16515, and GSE71729. (C) Evaluation of S100P expression in pan-cancer cells using the CCLE dataset. (D) Estimation of the prognostic value of S100P in different cancer types using cox regression analysis. (E) Validation of the prognostic value of S100P using the E-MTAB-6134 dataset. (F-G) Comparison of S1000P expression in pancreatic cancer samples with different KRAS mutation statuses using the TCGA-PAAD and E-MTAB-6134 databases. **: P < 0.01, ***: P < 0.001
S100P expression in pancreatic cancer negatively correlates with immune infiltration
After calculating TumorPurity, StromalScore, ImmuneScore, and ESTIMATEScore, a subsequent analysis revealed a negative correlation between S100P and StromalScore, ImmuneScore, and ESTIMATEScore, whereas a positive correlation was observed with TumorPurity in E-MTAB-6134 dataset (Fig. 2A). This pattern was similarly observed in GSE71729 dataset (Fig. 2B). Notably, immune scores indicated that samples with high levels of S100P expression had significantly lower immune-infiltrating values. This trend was particularly notable in CD8 + T cells in E-MTAB-6134 and GSE71729 datasets (Fig. 3A&B). Five methods were used to validate the above findings, all of which revealed a significant negative association between S100P expression in pancreatic cancer tissues and CD8 + T cell infiltration. The correlation coefficients obtained were − 0.307, -0.193, -0.282, -0.419, and − 0.334, respectively (Fig. 3C). Furthermore, patients with pancreatic cancer were predominantly enriched in the C3 subtype, in which low expression of S100P was a noteworthy trend (Fig. 3D). Finally, we employed RNA-Seq data from TCGA to verify these findings and found consistent results through TISIDB (Figure S3).
The correlation between S100P expression and Tumor Purity, Stromal Score, Immune Score, and ESTIMATE Score, as well as differential analysis based on the median of S100P expression. (A) E-MTAB-6134 dataset. (B) GSE71729 dataset. TumorPurity: proportion of cancer cells in the tumor tissue, StromalScore: proportion of stromal ingredient, ImmuneScore: proportion of immune ingredient, ESTIMATEScore: sum of the ImmuneScore and StromalScore. *: P < 0.05, **: P < 0.01, ***: P < 0.001
The landscape of the TME in pancreatic cancer and the characteristics of different S100P subgroups. (A-B) Proportions of various TME cells were analyzed using E-MTAB-6134 and GSE71729 datasets, and the immune score of the two subgroups is illustrated by scattered dots. (C) The correlation between S100P expression level and CD8 + T cells in pancreatic cancer using five different algorithms. (D) The analysis of immune subtypes was conducted using E-MTAB-6134 dataset. *: P < 0.05, **: P < 0.01, ***: P < 0.001
S100P expression in pancreatic cancer associated with tumor microenvironment at the single cell level
At the single-cell level, S100P has been found to be closely associated with the TME of pancreatic cancer, as demonstrated by the results of CRA001160 and GSE154778 datasets. The expression of S100P was significantly higher in malignant cells compared to non-malignant cells in these datasets (Fig. 4). Further analysis of the GSE155698 dataset, which included 41,378 cells from 17 tumor samples, revealed that high expression of S100P was also predominantly observed in malignant cells (Fig. 5A). Interestingly, the percentage of immune-related cells, such as B cells, CD4 + T cells, CD8 + T cells, dendritic cells, endothelial cells, macrophages, mast cells, monocytes, NK cells, and plasma cells, was significantly lower in the group with higher expression of S100P compared to the group with low expression. Conversely, the proportion of malignant cells was higher in the group with high S100P expression (Fig. 5B).
Higher S100P expression correlates with reduced response to immunotherapy
The results of the TMB analysis revealed a significant positive correlation between TMB and S100P expression (R = 0.47, P < 0.05); notably, the high S100P expression group had a higher TMB value compared to the low expression group (Fig. 6A). Moreover, the mutation genes for the two groups were found to be distinct (Fig. 6C&D). Analysis of the expression of immunotherapy-related genes (IRGs) revealed a negative correlation between S100P expression and TIGIT, LAG3, IDO1, CTLA4, PD-1/CD274, and BTLA in the E-MTAB-6134 dataset. In the TCGA-PAAD dataset, S100P expression was negatively correlated with TIGIT, BTLA, TIM-3/HAVCR2, CTLA4, and BTLA (Fig. 6B). These findings suggest that patients with higher S100P expression may not benefit from immunotherapy due to the down-regulation of immune checkpoints and reduced CD8 + T cell infiltration in this group.
The association between S100P and immunotherapy. (A) The correction between S100P and TMB in pancreatic cancer. (B) The heatmap of S100P and immune checkpoint molecules expression level in E-MTAB-6134 and TCGA-PAAD datasets. (C) Waterfall plot of the top 20 most frequently mutated genes in S100P high- and low-expression groups. ***: P < 0.001
Overexpression of S100P negatively correlates with DNA methylation level
The expression of mRNA for the gene S100P was found to have a negative correlation with the methylation levels of eight different probes: cg14323984, cg27027375, cg14900031, cg14140379, cg25083732, cg07210669, cg26233331, and cg22266967 (P < 0.001, r = -0.583; P < 0.001, r = -0.728; P < 0.001, r = -0.737; P < 0.001, r = -0.734; P < 0.001, r = -0.551; P < 0.001, r = -0.689; P < 0.001, r = -0.387; P < 0.001, r = -0.436; respectively; as shown in Fig. 7). Additionally, these eight probes were found to have an association with CD8 + T cell infiltration (Fig. 8).
S100P overexpression at cellular and protein levels in pancreatic cancer
After conducting experiments and analyzing data, it was found that S100P expression is elevated in pancreatic cancer at both cellular and protein levels. RT-PCR results indicated the upregulation of S100P in five pancreatic cancer cell lines (Fig. 9), and immunohistochemistry test images revealed higher expression of S100P in pancreatic cancer tissues than in adjacent tissues (Fig. 10). Additionally, the UALCAN dataset also demonstrated a significant increase in S100P expression in pancreatic cancer tissues, as compared to normal tissues (P < 0.05) (Figure S4).
Discussion
Pancreatic cancer is widely regarded as one of the most malignant tumors, and the study of the TME is of paramount importance for uncovering the regulatory factors of pancreatic cancer therapy. Thus, this study aims to examine the possible role of S100P in the TME of pancreatic cancer in a systematic manner. Our findings demonstrate that S100P is highly expressed in pancreatic cancer and significantly correlates with CD8 + T cells. The expression of S100P is assessed at single-cell levels and validated in vitro RT-PCR and IHC experiments. Our findings indicated that S100P can potentially contribute to the immunosuppressive microenvironment associated with pancreatic cancer.
S100P, a 95-amino-acid member of the S100 protein family, has been previously established to promote pancreatic cancer growth, metastasis, and invasion [24]. Literature has shown that S100P is involved in the processes of angiogenesis, immune evasion, and can inactivate p53 [25, 26]. Recently, Zou et al. demonstrated that S100P was a novel immune-related biomarker and explored its prognostic significance [27]. Other members of the S100 family, such as S100A2 [28], S100A5 [29] and S100A14 [30] has been confirmed to be involved in the regulation of immune microenvironment. In addition, Camara et al. proved that S100P inhibitors might provide a novel approach to treating pancreatic cancer [31]. However, the molecular mechanisms underlying the role of S100P in the TME remain poorly understood. We argue that the in-depth study of S100P can provide crucial insights and serve as the basis for the development of pancreatic cancer therapy.
Firstly, we comprehensively analyzed the S100P landscape in cancer, including its expression, survival, and clinical associations. Our results revealed diverse expression patterns of S100P across different cancer types, with high expression observed in pancreatic cancer and a correlation with poor prognosis. The credibility of these findings was strengthened through validation using multiple microarrays and CCLE datasets. Additionally, our analyses indicated that S100P expression is upregulated in patients with KRAS mutation and II-IV stage, suggesting its involvement in fundamental tumorigenic pathways in the pancreas. Enrichment analysis suggested a connection between S100P and immune-related signaling pathways, with S100P being listed in the ImmPort database as a relevant gene. Given the impact of tumor-infiltrating immune cells on cancer progression and therapy [32], further investigation into the role of S100P in the tumor microenvironment and its potential for immunotherapy is imperative.
Next, our study aimed to investigate the potential role of S100P in the immune microenvironment of pancreatic cancer. Notably, our results revealed a negative correlation between S100P expression levels and ImmuneScore, StromalScore, and ESTIMATEScore. Furthermore, we observed a significant association between increased S100P expression and reduced immune cell infiltration in two datasets. These findings suggest the involvement of S100P in immunosuppression-related pathways, indicating its potential relevance as an immunologic marker in pancreatic cancer. We further employed five different algorithms to quantify CD8 + T cell proportion, which supported our earlier findings of increased S100P expression being associated with lower CD8 + T cell infiltration. Using three scRNA-Seq datasets, we consistently observed a reduction in immune cell populations, including CD8 + T cells, CD4 + T cells, NK cells, B cells, and others, in patients with higher S100P expression. Previous studies have indicated that pancreatic cancer patients often exhibit low levels of tumor-infiltrating T cells, leading to poor response rates to immunotherapy [33]. Our analysis resonates with these findings, as we observed a high proportion of pancreatic cancer samples in the C3 subtype, consistent with reports that inflammation is likely to be involved in regulating the immunosuppressive microenvironment of pancreatic cancer [34]. Our data provide compelling evidence that S100P may play a crucial role in the remodeling of the TME and its association with immune “cold” tumors. Hence, targeting S100P may offer a promising strategy to convert the tumor immunologically “hot” and trigger an anticancer immune response.
The tumor immune microenvironment (TIME) has been demonstrated to be imperative for tumor development and clinical outcomes [35,36,37]. Thus, there is a possibility that therapeutic interventions targeting the TIME could serve as a promising immunotherapeutic approach for tumors [38]. To elucidate the potential of S100P as a pancreatic cancer immunotherapy, we analyzed the association between S100P expression and seven classic immune checkpoints in the TCGA-PAAD and E-MTAB-6134 cohorts. Our findings indicated that S100P was negatively linked with the expression of PD-1, CTLA-4, IDO1, and LAG-3. Moreover, the usefulness of TMB has already been established in predicting the response to cancer immunotherapy in numerous cancer types [39]. Our results showed that the correlation between S100P and TMB was 0.47, highlighting the potential of S100P as a complementary biomarker for predicting immunotherapy efficacy. Although further studies are necessary to validate these findings, emerging evidence portrays S100P as a probable player in the immune microenvironment and response to pancreatic cancer immunotherapy.
The present discussion aims to elucidate the relationship between S100P and the TME, which may be associated with Ca2+ signaling. Previous investigations have highlighted that Ca2+ can trigger crucial pathways for tumorigenesis, such as cellular motility, proliferation, and apoptosis [40]. Correspondingly, S100P, a Ca2+ binding protein, has been implicated in TME remodeling [41]. Moreover, Ca2+ signaling has regulatory effects on T cells as identified by Schlunck et al., who discovered an inverse correlation between Ca2+ mobilization and cell proliferation in T cells [42]. Hence, we postulate that S100P may impede immune cell infiltration in the peritumoral compartment by exerting influence on Ca2+ signaling. Recent research has demonstrated that Ca2+ signaling may display anti-tumor efficacy via modulation of the immune microenvironment [43, 44]. For instance, Rong et al. discovered that MGP promotes CD8 + T cell exhaustion in colorectal cancer by enriching intracellular free Ca2+ levels [45]. Furthermore, the mechanism of action of S100P may involve its interaction with the receptor for advanced glycation end products (RAGE) [46]. The interaction between S100P and RAGE can activate downstream signaling pathways, including Ca2+ signaling [47]. Additionally, RAGE has been reported to modulate the immune microenvironment by influencing the activation and function of immune cells, including T cells [48, 49]. Various studies have shown that inhibitors targeting S100P can improve the effectiveness of pancreatic cancer therapy [46, 50, 51]. Thus, regulating S100P expression through inhibitors could facilitate TME remodeling and provide potential therapeutic interventions for pancreatic cancer.
The above analysis provided evidence to support the hypothesis that higher S100P expression is linked to immune resistance in pancreatic cancer patients. Therefore, it is significant to investigate the underlying reasons for this expression pattern. Our findings indicated no mutations in the S100P gene in pancreatic cancer patients, but its expression was closely connected to methylation status. These results were consistent with previous research, suggesting that epigenetic mechanisms regulate S100P expression [52]. DNA methylation plays a critical role in the progression of cancer by inducing “exhaustion” in cytotoxic T cells, and may be used in combination with current immunotherapies [53, 54]. Moreover, we identified eight DNA methylation probes of S100P potentially associated with the TME in pancreatic cancer, as these methylation sites were positively correlated with CD8 + T cells. A recent study found that DNA methylation can be a predictive biomarker for response to immune checkpoint inhibitors [55]. Thus, downregulating the expression of S100P may enhance T cell immune activity and achieve anti-tumor immunotherapy objectives.
As far as we acknowledge, this study was the first comprehensive analysis to investigate the potential role of S100P in the immune microenvironment of pancreatic cancer. By incorporating various methodologies and in vitro validation, we obtained a multi-dimensional understanding of the involvement of S100P. However, some limitations of this study also warrant recognition. The relationship between S100P and the tumor environment is mainly based on observed correlations, and further mechanistic studies are needed to establish causation and fully elucidate the role of S100P in restructuring the tumor microenvironment in pancreatic cancer. Despite the lack of experimental validation and possible limitations, our study serves as a foundation for future investigations.
Conclusion
Our study suggested a potential association between S100P and the restructuring of the tumor microenvironment in pancreatic cancer. The upregulation of S100P has been implicated in the immune dysfunction of pancreatic cancer, particularly in CD8 + T cells. Additionally, there is a potential for S100P to impact the regulation of pancreatic cancer through DNA hypomethylation. Further exploration of the molecular mechanisms underlying these findings should be conducted in future studies.
Data availability
All data generated or analyzed in this study are available upon reasonable request from the corresponding author. The relevant web links for accessing datasets are as follows: TCGA (https://portal.gdc.cancer.gov), GEO (https://www.ncbi.nlm.nih.gov/gds/), ArrayExpress (https://www.ebi.ac.uk/arrayexpress/), CCLE (https://sites.broadinstitute.org/ccle), UCSC Xena (https://xenabrowser.net/datapages/), UALCAN platform (http://ualcan.path.uab.edu/index.html), TISCH2 (http://tisch.comp-genomics.org/), MEXPRESS (https://www.mexpress.be/).
Abbreviations
- TME:
-
tumor microenvironment
- ssGSEA:
-
single-sample gene set enrichment analysis
- RT-PCR:
-
Reverse transcription PCR
- IHC:
-
immunohistochemical
- TMB:
-
tumor mutation burden
- IRGs:
-
immunotherapy-related genes
- TIME:
-
tumor immune microenvironment
References
Klein AP. Pancreatic cancer epidemiology: understanding the role of lifestyle and inherited risk factors. Nat Rev Gastroenterol Hepatol. 2021;18:493–502.
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7–33.
Dyba T, Randi G, Bray F, Martos C, Giusti F, Nicholson N, et al. The European cancer burden in 2020: incidence and mortality estimates for 40 countries and 25 major cancers. Eur J Cancer. 2021;157:308–47.
Park W, Chawla A, O’Reilly EM. Pancreat Cancer: Rev JAMA. 2021;326:851.
Mao L, Qi Z, Zhang L, Guo J, Si L. Immunotherapy in Acral and Mucosal Melanoma: current status and future directions. Front Immunol. 2021;12:680407.
Brozos-Vázquez EM, Díaz-Peña R, García-González J, León-Mateos L, Mondelo-Macía P, Peña-Chilet M, et al. Immunotherapy in nonsmall-cell Lung cancer: current status and future prospects for liquid biopsy. Cancer Immunol Immunother. 2021;70:1177–88.
Yap TA, Parkes EE, Peng W, Moyers JT, Curran MA, Tawbi HA. Development of Immunotherapy combination strategies in Cancer. Cancer Discov. 2021;11:1368–97.
Jia Q, Wang A, Yuan Y, Zhu B, Long H. Heterogeneity of the Tumor immune microenvironment and its clinical relevance. Exp Hematol Oncol. 2022;11:24.
Luo Y, Yin S, Lu J, Zhou S, Shao Y, Bao X, et al. Tumor microenvironment: a prospective target of natural alkaloids for cancer treatment. Cancer Cell Int. 2021;21:386.
Menares E, Gálvez-Cancino F, Cáceres-Morgado P, Ghorani E, López E, Díaz X, et al. Tissue-resident memory CD8 + T cells amplify anti-tumor immunity by triggering antigen spreading through dendritic cells. Nat Commun. 2019;10:4401.
Liu X, Wang W, Liu X, Zhang Z, Yu L, Li R et al. Multi-omics analysis of intra‐tumoural and inter‐tumoural heterogeneity in pancreatic ductal adenocarcinoma. Clin Transl Med. 2022;12.
Cong Y, Cui Y, Wang S, Jiang L, Cao J, Zhu S, et al. Calcium-binding protein S100P promotes Tumor Progression but enhances chemosensitivity in Breast Cancer. Front Oncol. 2020;10:566302.
Carneiro P, Moreira AM, Figueiredo J, Barros R, Oliveira P, Fernandes MS, et al. S100P is a molecular determinant of E-cadherin function in gastric cancer. Cell Commun Signal. 2019;17:155.
Nakayama H, Ohuchida K, Yonenaga A, Sagara A, Ando Y, Kibe S, et al. S100P regulates the collective invasion of Pancreatic cancer cells into the lymphatic endothelial monolayer. Int J Oncol. 2019. https://doi.org/10.3892/ijo.2019.4812.
Ye H, Li T, Wang H, Wu J, Yi C, Shi J, et al. TSPAN1, TMPRSS4, SDR16C5, and CTSE as Novel Panel for Pancreatic Cancer: a Bioinformatics analysis and experiments validation. Front Immunol. 2021;12:649551.
Tang Z, Li C, Kang B, Gao G, Li C, Zhang Z. GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017;45:W98–102.
Yoshihara K, Shahmoradgoli M, Martínez E, Vegesna R, Kim H, Torres-Garcia W, et al. Inferring tumour purity and stromal and immune cell admixture from expression data. Nat Commun. 2013;4:2612.
Thorsson V, Gibbs DL, Brown SD, Wolf D, Bortone DS, Ou Yang T-H, et al. The Immune Landscape of Cancer. Immunity. 2018;48:812–830e14.
Sun D, Wang J, Han Y, Dong X, Ge J, Zheng R, et al. TISCH: a comprehensive web resource enabling interactive single-cell transcriptome visualization of Tumor microenvironment. Nucleic Acids Res. 2021;49:D1420–30.
Hu C, Li T, Xu Y, Zhang X, Li F, Bai J, et al. CellMarker 2.0: an updated database of manually curated cell markers in human/mouse and web tools based on scRNA-seq data. Nucleic Acids Res. 2023;51:D870–6.
Mayakonda A, Lin D-C, Assenov Y, Plass C, Koeffler HP. Maftools: efficient and comprehensive analysis of somatic variants in cancer. Genome Res. 2018;28:1747–56.
Zhang W, Xu J. DNA methyltransferases and their roles in tumorigenesis. Biomark Res. 2017;5:1.
Chandrashekar DS, Karthikeyan SK, Korla PK, Patel H, Shovon AR, Athar M, et al. UALCAN: an update to the integrated cancer data analysis platform. Neoplasia. 2022;25:18–27.
Arumugam T, Simeone DM, Van Golen K, Logsdon CD. S100P promotes Pancreatic Cancer Growth, Survival, and Invasion. Clin Cancer Res. 2005;11:5356–64.
Bresnick AR, Weber DJ, Zimmer DB. S100 proteins in cancer. Nat Rev Cancer. 2015;15:96–109.
Gibadulinova A, Pastorek M, Filipcik P, Radvak P, Csaderova L, Vojtesek B, et al. Cancer-associated S100P protein binds and inactivates p53, permits therapy-induced senescence and supports chemoresistance. Oncotarget. 2016;7:22508–22.
Zou W, Li L, Wang Z, Jiang N, Wang F, Hu M, et al. Up-regulation of S100P predicts the poor long-term survival and construction of prognostic signature for survival and immunotherapy in patients with Pancreatic cancer. Bioengineered. 2021;12:9006–20.
Chen Y, Wang C, Song J, Xu R, Ruze R, Zhao Y. S100A2 is a prognostic biomarker involved in Immune Infiltration and Predict Immunotherapy Response in Pancreatic Cancer. Front Immunol. 2021;12:758004.
Li H, Chen J, Li Z, Chen M, Ou Z, Mo M, et al. S100A5 attenuates efficiency of Anti-PD-L1/PD-1 immunotherapy by inhibiting CD8 + T cell-mediated anti-cancer immunity in bladder carcinoma. Adv Sci Weinh Baden-Wurtt Ger. 2023;10:e2300110.
Wang C, Chen Y, Xinpeng Y, Xu R, Song J, Ruze R, et al. Construction of immune-related signature and identification of S100A14 determining immune-suppressive microenvironment in Pancreatic cancer. BMC Cancer. 2022;22:879.
Camara R, Ogbeni D, Gerstmann L, Ostovar M, Hurer E, Scott M, et al. Discovery of novel small molecule inhibitors of S100P with in vitro anti-metastatic effects on Pancreatic cancer cells. Eur J Med Chem. 2020;203:112621.
Liu C, Zhang M, Yan X, Ni Y, Gong Y, Wang C, et al. Single-cell dissection of cellular and molecular features underlying human cervical squamous cell carcinoma initiation and progression. Sci Adv. 2023;9:eadd8977.
Wu J, Cai J. Dilemma and challenge of Immunotherapy for Pancreatic Cancer. Dig Dis Sci. 2021;66:359–68.
Sacdalan DB, Lucero JA. The Association between Inflammation and immunosuppression: implications for ICI Biomarker Development. OncoTargets Ther. 2021;14:2053–64.
Wu J, Wang L, Xu J. The role of pyroptosis in modulating the Tumor immune microenvironment. Biomark Res. 2022;10:45.
Bruni D, Angell HK, Galon J. The immune contexture and immunoscore in cancer prognosis and therapeutic efficacy. Nat Rev Cancer. 2020;20:662–80.
Li X, Zhang M, Lei T, Zou W, Huang R, Wang F, et al. Single-cell RNA-sequencing dissects cellular heterogeneity and identifies two tumor-suppressing immune cell subclusters in HPV-related cervical adenosquamous carcinoma. J Med Virol. 2022;94:6047–59.
Jiang K-Y, Qi L-L, Kang F-B, Wang L. The intriguing roles of Siglec family members in the Tumor microenvironment. Biomark Res. 2022;10:22.
Chan TA, Yarchoan M, Jaffee E, Swanton C, Quezada SA, Stenzinger A, et al. Development of Tumor mutation burden as an immunotherapy biomarker: utility for the oncology clinic. Ann Oncol. 2019;30:44–56.
Feng M, Grice DM, Faddy HM, Nguyen N, Leitch S, Wang Y, et al. Store-Independent activation of Orai1 by SPCA2 in mammary tumors. Cell. 2010;143:84–98.
Sang L, Ju H, Liu G, Tian T, Ma G, Lu Y, et al. LncRNA CamK-A regulates Ca2+-Signaling-mediated Tumor Microenvironment Remodeling. Mol Cell. 2018;72:71–83e7.
Schlunck T, Schraut W, Riethmüller G, Ziegler-Heitbrock HWL. Inverse relationship of CA2 + mobilization and cell proliferation in CD8 + memory and virgin T cells. Eur J Immunol. 1990;20:1957–63.
Liu B, Bian Y, Liang S, Yuan M, Dong S, He F, et al. One-step integration of Tumor Microenvironment-Responsive Calcium and Copper Peroxides Nanocomposite for enhanced Chemodynamic/Ion-Interference therapy. ACS Nano. 2022;16:617–30.
Tan X, Huang J, Wang Y, He S, Jia L, Zhu Y, et al. Transformable nanosensitizer with Tumor Microenvironment-activated sonodynamic process and calcium release for enhanced Cancer Immunotherapy. Angew Chem Int Ed. 2021;60:14051–9.
Rong D, Sun G, Zheng Z, Liu L, Chen X, Wu F, et al. MGP promotes CD8 + T cell exhaustion by activating the NF-κB pathway leading to liver Metastasis of Colorectal cancer. Int J Biol Sci. 2022;18:2345–61.
Dakhel S, Padilla L, Adan J, Masa M, Martinez JM, Roque L, et al. S100P antibody-mediated therapy as a new promising strategy for the treatment of Pancreatic cancer. Oncogenesis. 2014;3:e92.
Kazakov AS, Deryusheva EI, Permyakova ME, Sokolov AS, Rastrygina VA, Uversky VN, et al. Calcium-bound S100P protein is a promiscuous binding Partner of the four-helical cytokines. Int J Mol Sci. 2022;23:12000.
Lin Z, Yu B, Yuan L, Tu J, Shao C, Tang Y. RAGE is a potential biomarker implicated in immune infiltrates and cellular senescence in lung adenocarcinoma. J Clin Lab Anal. 2022;36:e24382.
Mishra S, Charan M, Shukla RK, Agarwal P, Misri S, Verma AK, et al. cPLA2 blockade attenuates S100A7-mediated breast tumorigenicity by inhibiting the immunosuppressive Tumor microenvironment. J Exp Clin Cancer Res CR. 2022;41:54.
Arumugam T, Ramachandran V, Sun D, Peng Z, Pal A, Maxwell DS, et al. Designing and developing S100P inhibitor 5-methyl cromolyn for Pancreatic cancer therapy. Mol Cancer Ther. 2013;12:654–62.
Arumugam T, Ramachandran V, Gomez SB, Schmidt AM, Logsdon CD. S100P-derived RAGE antagonistic peptide reduces Tumor growth and Metastasis. Clin Cancer Res off J Am Assoc Cancer Res. 2012;18:4356–64.
Sato N, Fukushima N, Matsubayashi H, Goggins M. Identification of maspin and S100P as novel hypomethylation targets in Pancreatic cancer using global gene expression profiling. Oncogene. 2004;23:1531–8.
Cao J, Yan Q. Cancer epigenetics, Tumor Immunity, and Immunotherapy. Trends Cancer. 2020;6:580–92.
Yi M, Dong B, Chu Q, Wu K. Immune pressures drive the promoter hypermethylation of neoantigen genes. Exp Hematol Oncol. 2019;8:32, s40164-019-0156–7.
Klümper N, Ralser DJ, Bawden EG, Landsberg J, Zarbl R, Kristiansen G, et al. LAG3 (LAG-3, CD223) DNA methylation correlates with LAG3 expression by Tumor and immune cells, immune cell infiltration, and overall survival in clear cell renal cell carcinoma. J Immunother Cancer. 2020;8:e000552.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
ZJ and HW designed the study. HW and ZY analyzed the data. DJ and CP wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was conducted by the Institutional Review Board of the First Affiliated Hospital of Zhengzhou University. Informed consent was obtained from all individuals or individuals’ guardians.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Hao, W., Zhang, Y., Dou, J. et al. S100P as a potential biomarker for immunosuppressive microenvironment in pancreatic cancer: a bioinformatics analysis and in vitro study. BMC Cancer 23, 997 (2023). https://doi.org/10.1186/s12885-023-11490-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-023-11490-1