Abstract
Owing to non-responsiveness of a high number of patients to the common melanoma therapies, seeking novel approaches seem as an unmet requirement. Chimeric antigen receptor (CAR) T cells were initially employed against recurrent or refractory B cell malignancies. However, advanced stages or pretreated patients have insufficient T cells (lymphopenia) amount for collection and clinical application. Additionally, this process is time-consuming and logistically cumbersome. Another limitation of this approach is toxicity and cytokine release syndrome (CRS) progress and neurotoxicity syndrome (NS). Natural killer (NK) cells are a versatile component of the innate immunity and have several advantages over T cells in the application for therapies such as availability, unique biological features, safety profile, cost effectiveness and higher tissue residence. Additionally, CAR NK cells do not develop Graft-versus-host disease (GvHD) and are independent of host HLA genotype. Notably, the NK cells number and activity is affected in the tumor microenvironment (TME), paving the way for developing novel approaches by enhancing their maturation and functionality. The CAR NK cells short lifespan is a double edge sword declining toxicity and reducing their persistence. Bispecific and Trispecific Killer Cell Engagers (BiKE and Trike, respectively) are emerging and promising immunotherapies for efficient antibody dependent cell cytotoxicity (ADCC). CAR NK cells have some limitations in terms of expanding and transducing NK cells from donors to achieve clinical response. Clinical trials are in scarcity regarding the CAR NK cell-based cancer therapies. The CAR NK cells short life span following irradiation before infusion limits their efficiency inhibiting their in vivo expansion. The CAR NK cells efficacy enhancement in terms of lifespan TME preparation and stability is a goal for melanoma treatment. Combination therapies using CAR NK cells and chemotherapy can also overcome therapy limitations.
Similar content being viewed by others
Background
Melanoma is among three skin cancers (squamous and basal cell carcinoma) with the highest metastatic potential and mortality rate [1, 2]. Although several drugs such as Ipilimumab (anti-CTLA-4) and Nivolumab (anti-PD1) checkpoint antibodies are applied, a high number of patients still do not response to these agents [3, 4]. Chimeric antigen receptors (CARs) T cells have been applied for relapsed malignancies [5, 6]. Nonetheless, some limitations in autologous and allogeneic settings are remained to be solved [7, 8]. Natural killer (NK) cells have several advantages over T cells in the application for therapies such as availability, unique biological features, safety profile, cost effectiveness and more prevalent existence in tissues [9,10,11,12]. In this method, personal cells are taken, engineered to synthesize specific receptors and expanded ex vivo and infused into the same patient as immunotherapy. The extracellular part of CAR includes single chain antigen-specific variable light and heavy chain antibodies and the intracellular domain comprises a signaling molecule from the cell receptor. More advanced CAR molecules also contain co-stimulatory molecules (nanobodies, CD28, designed ankyrin repeat proteins or DARPins, 4-1BB or CD137, CD3ζ or cytokines) in the intracellular domain (Fig. 1). CAR T cells were initially employed against recurrent or refractory B cell malignancies [13, 14]. However, advanced stages or pretreated patients have insufficient T cells (lymphopenia) amount for collection and clinically application.
Additionally, this process is time-consuming and logistically cumbersome. Another limitation of this approach is toxicity and cytokine release syndrome (CRS) progress and neurotoxicity syndrome (NS) [8, 15, 16]. Considering these, circumventing of limitations can be accomplished using alternative CAR NK cells. As CD3-CD56+ innate lymphoid cells, NK cells play a substantial antimicrobial and anticancer role. Inadvertently, NK cells killing effect against transformed cells is independent of antigen priming, major histocompatibility complex molecules and target cells expression [17,18,19]. After insertion of CAR construct into the T or NK cell genome, it is expressed onto the cell surface. The cells are expanded and injected into the cancer patient. CAR can recognize the tumor cells and destruct them. Despite advantages of alternative CAR NK cells versus CAR T cells, few clinical trials have been performed with this regard. As the innate immune part, NK cells intrinsically recognize absence of human leukocyte antigen (HLA)-proteins which overcome escape mechanisms by cancer cells. Several studies have deciphered that CAR NK cell based anticancer therapies have been highly efficient and rapid with lower costs than those of CAR T cells against melanoma [20,21,22,23,24].
NK cells and cancer
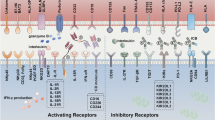
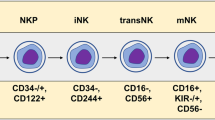
NK cells are a versatile component of the innate immunity, divided into two cytokine-producing CD56bright and CD56dimCD16+ cells [25]. These cells constitute only 5–15% of total NK cells and contain innate ability to recognize transformed cells [26,27,28]. NK cells have various stimulatory and inhibitory receptors playing a critical role in the elimination of infected, stressed, foreign and cancer cells by activating other immune cells such as dendritic cells, B cells and T cells and production of pro-inflammatory cytokines [26, 29,30,31]. These cells have various inhibitory and activator receptors. NK cells produce indispensable levels of INFγ and in NK cells deficient conditions, cure from several tumor cell lines has been difficult [32, 33]. Moreover, pre-activated murine NK cells combined with radiotherapy has more efficiently decreased cancer cells [34]. In melanoma patients, NK cells have been existed in peripheral blood as well as in tissues with variable results. However, CD56bright and CD56dim NK cells subsets may remain without alterations in metastatic melanoma patients blood, but their function may be impaired such as in degranulation, and production of IFNγ and NKG2D [35]. The clinical outcome of several tumors has been associated with NK cells primary infiltration and activation. CD57 + KIR+CD56dim NK cells as entirely mature and effector cells play an important role in melanoma cells combating [36, 37]. In lymph nodes, NK cells with high level expression of NKp46, CD16, NKG2D, NKp44, DNAM-1 and NKp30 has been found [38,39,40]. The efficacy of NK cells based therapies against solid tumors in controversial [41, 42]. One strategy was to electroporation of these cells with two receptor-specific mRNA constructs (CXCR-1 and NKG2D) into the tumor microenvironment (TME) of peritoneal ovarian cancer xenografts in mice [43, 44]. As antibody-dependent cytotoxic cells, NK cells interact with α-CTLA-4 antibody which in turn activate these cells [45, 46]. A recent clinical trial on 29 late stage (III/IV) melanoma patients unraveled that NK cells subsets were similar in rate among healthy and patients and demonstrated low rate of CD56bright NK cell subsets in treated melanoma patients [35]. Low CD56bright NK cell frequency in melanoma patients treated with Ipilimumab was considered as a good result of survival. These cells inhibit the T cell responses via CD38, perforin, CD11a and IFNγ [47, 48]. Importantly, immune cells such as NK cells may deplete in nutrients and exhaust for efficient anticancer cytotoxicity in the TME. Indeed, various melanoma cells lines response diversely to NK cell-mediated killing due to the diverse expression of various proteins [49]. Hence, providing required nutrients is necessary. In addition to the cytotoxicity against melanoma cells, NK cells promote their recruitment via High Mobility Group Box-1 (HMGB1) protein [50]. Melanoma cells mainly express NKG2D and DNAM-1 ligands but not ULBPs or nectin-2, which suggests that NK cells are activated via these receptors against melanoma [51, 52]. Other receptors such as MHC class I chain related-proteins A (MICA) and B (MICB) or MICA/B, NKp30, NKp44, and NKp46 have been also expressed in melanoma cell lines [53, 54]. Lower number of CD56bright NK cells in stage IV of melanoma can be a prognostic factor and a biomarker. Notably, the NK cells number and activity is affected in the TME, paving the way for developing novel approaches by enhancing their maturation and functionality.
Immune escape mechanisms by melanoma cells
In the primary stage of cancer, various immune cells participate in the elimination of melanoma cells, however, high plasticity of tumor cells leads to immune evasion [55, 56]. A mutation in v-raf murine sarcoma viral oncogene homolog (BRAF)-E600 gene occurred in about 40–50% of melanoma patients causes resistance to monotherapy [57, 58]. In spite of expression of several ligands for NK cells by various cancer cells, melanoma cells escape responses via cytokines production, immunosuppressive cells activation, lower MHC expression and creation of hypoxic tumor microenvironment [59,60,61]. Various inhibitory ligands such as HLA-E, galectin − 9, PD-L1, CD155 and CD 112 are expressed by melanoma cells [62,63,64,65,66]. Furthermore, immunosuppressive cytokines and molecules such as adenosine, indoleamine 2,3-dioxygenase (IDO), matrix metalloproteinases (MMPs), vascular endothelial growth factor (VEGF), arginase-1 (ARG-1), IL-10 and tumor growth factor-β (TGF-β) are employed to inactivate the NK cells [67,68,69]. The hypoxic conditions created by melanoma cells alter the immune cells activities by expression of HIF-1α. These conditions cause [70, 71] the autophagy induction in the NK cells and mitigates their responses to cytokines such as IL-2, IL-12, IL-15 and IL-21, thereby inhibiting natural cytotoxicity triggering receptors (NCRs) and NKG2D NK cells activating receptors. Hypoxia also alters the expression of cancer cells ligands.
Sources for NK cells expanding
Seeking and finding those suitable sources for NK cells obtainment and expansion is important. NK cells are derived from peripheral blood mononuclear cells (PBMCs), umbilical cord blood (UCB), bone marrow (BM) (healthy or patient-derived), induced pluripotent stem (iPS) cell and immortalized NK cell lines [72]. NK-92 cell line is a proper source for convenient and sufficient expanding of NK cells [73]. Additionally, high cytotoxicity and safety of NK-92 cells (irradiated before clinical use) has been verified in preclinical and clinical studies. These cells also lack NKp44 and NKp46 NK cells activating receptors [74, 75]. Other NK cell lines such as KHYG-1, NKL, NKG, NK-YS, NK-L, NK 3.3, EP3138905A1 and YT have been applied for expanding [76, 77]. K562 cell line with increased activity has also been developed [78, 79]. A wide anticancer cytotoxicity has been observed using CAR NK cells derived from KHYG-1 cells [80]. Due to the probability of activation and proliferation of regulatory cells, proper procedure for NK cells expanding seems essential. In addition, genetically modified NK cells to improve their function, persistence and capacity include introduction of genes into NK cells (IL-2 and IL-15 coding genes), CARs, NKG2A inhibitory receptor downregulation, viral transduction, transfection and mRNA electroporation [81, 82]. Regarding clinical trials, Anti-CD33 CAR NK cells for acute myeloid leukemia (NCT02944162, PMID: 28054442), Allogeneic anti-CD19 CAR NK cells for CD19 + Leukemia (NCT02892695, PMID: 28054442), ROBO1 CAR NK cells for Solid tumor expressing ROBO1 (NCT03940820) and allogeneic anti-MUC1 CAR pNK cells for MUC1-positive solid tumor (NCT02839954) have been performed.
Immunotherapies using NK cells
NK cells have infiltrated in various cancers differently. Targeting CTLA-4 and PD-1 checkpoint inhibitors (CPIs) have affected the function of T cells and NK cells in cancer treatment [83,84,85]. CD56dim NK cells express PD-1 as a differently expressed ligand on the NK cells [86]. Those NK cells expressing PD-1 can improve functionality (cytotoxicity and granzyme + perforin production) following PD-1 blockade [87, 88]. Interestingly, NK and CD8+ T cells cooperate in melanoma and tumors cells. Blockade of other inhibitory receptors such as KIR, NKG2A, T cell immunoglobulin and mucin domain-containing protein 3 (TIM-3) and T cell immunoreceptor with Ig and ITIM domains (TIGIT) have been also demonstrated [89, 90]. Hence, activated NK cells release granzyme B and perforin. Other therapies have included cytokine (IL-2, IL-15) therapy, oncolytic viruses and specific antibodies (Figs. 2 and 3) [91, 92]. CD16 receptor plays a substantial role in NK cells activity. However, variations in allotype of CD16 lead to different affinity of antibodies for appropriate antibody dependent cell cytotoxicity (ADCC) induction of NK cells [93,94,95]. Bispecific and Trispecific Killer Cell Engagers (BiKE and Trike, respectively) are emerging and promising immunotherapies for efficient ADCC by NK cells [96, 97]. BiKEs include two single chain variable fragments (scFvs) of antibody each specific for CD16 or antigen connected by a flexible linker. TriKEs have an additional antigen specific chain or cytokine (IL-15). IL-15 has higher efficiency and lower toxicity than IL-2 for NK cells activation [98, 99]. Furthermore, TriKEs are more efficient in NK cells activation and effector function (cytotoxicity and interferon gamma/INFγ and tumor necrosis factor alpha/TNFα production) [100]. However, not all cancer cells express antigens and therefore, expression of virus antigens by BiKE and Trike constructs will be promising for melanoma cells targeting. Talimogene laherparepvec (T-VEC) is a herpes simplex virus-1 (HSV-1) applied against melanoma cells [101, 102]. These antigens are not expressed by human cells, hence utilization of BiKE and Trike for their expression is promising as safer and more efficient immunotherapy. CAR NK cells recognize tumor antigens not only by CAR, but also through their own receptors. Indeed, a balance of inhibitory or provoking signals determines the NK cell function. The enhancement of safety and efficacy of CAR NK therapy can be achieved through some strategies such as integration of suicide genes and silencing NK inhibitory receptors (IL-4 and IL-7 receptor) [103, 104]. CAR NK cells have some limitations in terms of expanding and transducing NK cells from donors to achieve clinical response. Obtaining high number of NK cells can be achieved by feeder-free, bovine serum-free protocol [105]. CAR NK cells have shown proper anticancer effect by lower level production of INFγ and TNFα. Nevertheless, clinical efficacy and infusion persistence within TME is still low using CAR NK cells monotherapy against solid tumors despite sufficient safety.
Various therapeutic approaches to enhance the anticancer effects of NK cells; allogenic NK cells are developed for infusions, and genetically modified NK cells which include those for homing (CXCR4, CCR7), for activation (NKG2D-DAP10, scFV and endoplasmic NKG2A), for proliferation (mbIL-15) and for tumor retargeting (CAR-NK cells)
NK cells cancer immunotherapy enhancement via receptors modification; NKG2A: natural killer cell group 2 member A, KIR: Killer cell immunoglobulin-like receptors, TIGIT: T cell immunoreceptor with Ig and ITIM domains, PVRIG: PVR Related Immunoglobulin Domain Containing, TIM-3: T cell immunoglobulin and mucin domain-containing protein 3, LAG-3: Lymphocyte Activating 3, DNAM-1: DNAX Accessory Molecule-1, NCR: Natural Cytotoxicity Triggering Receptor 1
Combinatorial strategies against melanoma using NK cells
There is a scarcity of data regarding CAR NK cell-based combination therapies for the melanoma. CAR NK cell therapy has unraveled great breakthrough for the treatment of cancers, however, its efficacy is limited for solid tumors. Hence utilizing combination therapies with chemical anticancer drugs is warranted. Regarding melanoma, no previous combination therapies of CAR NK cells and chemical agents has been investigated. It was demonstrated that synergistic use of OV-IL15C plus epidermal growth factor (EGFR)-CAR NK cells was able to suppress glioblastoma (GBM) cells and also enhanced the infiltration of NK and CD8+ T cells [106]. Another study exhibited that EpCAM-CAR NK-92 cells and Regorafenib could synergistically prohibit the colorectal cancer cells in vitro and in vivo exhibiting a significantly higher efficacy than monotherapy using CAR NK cells. Additionally, Bortezomib- modified oncolytic viruses (OV) combined with the NK cell infusion therapy exhibited more efficient anticancer effects [107, 108].
Limitations of NK cells-based immunotherapies
The requirement for cytokine support for NK cells activation is an important limitation for successful clinical therapy despite their benefits for long-term low toxicity [26, 109]. In addition, the use of IL-2 and IL-15 cytokines for the proliferation of NK cells is associated with toxicity and neutropenia. Moreover, IL-2 activates the regulatory T (Treg) cells which inhibit the NK cells activation [110]. To overcome this problem, IL-2-diphteria toxin and lymphodepleting agents have been applied prior to the infusion of NK cells [111]. Furthermore, TME leads to inhibition of NK cells by some factors such as Tregs, MDSCs, tumor growth factor-β (TGF-β), CD20, HLA-E, galactin-9 and CD8 [31, 112,113,114]. It was exhibited that viral transduction leads to higher NK cells apoptosis compared to the CAR T cells.
CAR NK cells therapies against melanoma
There is a scarcity of data regarding the applications of CAR NK cells against melanoma (Fig. 3). Given the success of CAR NK cells in hematological cancer, their application in solid tumors is warranted. Solid tumors develop resistance against drugs and escape immune cells. CAR NK cells CD7-CAR NK-92MI and dCD7-CAR NK-92MI cells have outlined efficient anti-cancer effects in vitro and in vivo against T-leukaemia cell lines [115]. Moreover, CAR.CD19-CD28-zeta-2A-iC9-IL-15-transduced HLA-mismatched CB NK cells were applied for patients with CD19+ B-lymphoid malignancies [116]. CAR NK cells have been also employed against myeloma, lymphoma, ovarian cancer, glioblastoma, colorectal cancer, breast cancer, lung cancer, pancreatic cancer, glioma, prostatic and gastric cancer [31, 117,118,119]. Previous CAR NK cell therapies against melanoma have targeted GPA7 and CD276 (B7-H3) using NK-92 as the NK cell source [24, 28]. Additionally, the vectors included lentivirus and retrovirus. Additionally, the CAR construction has been His-tag and F (ab)2 and HLA-A2TM + CD3ζ. The CAR NK cell therapies are still in primary stages and combination therapies with adoptive cell therapies and immune checkpoint inhibitors (ICKs) will be promising. One limitation of CAR NK cell therapy is their short life span for irradiation before infusion which unable them for multiplication in vivo. Anti-CD19 car NK cells were applied for chronic lymphocytic leukemia and lymphoma in Phase I clinical trial. NK-92 cells targeting Her2 in solid tumors (NCT04050709), Indeed, NK cells need cytokine support for persistence in the body. Additionally, lack expression of natural cytotoxicity receptors (NCRs) and CD16, tumorigenesis of NK-92 cells and stroke risk include concerns about the CAR NK cells application [120]. CAR NK cells low penetration into the TME is also a limitation [121]. NK cells only develop memory cells against viruses not for cancer [9].
Future prospects
The improvement of CAR NK cells in terms of preparation, stability and lifespan and penetration into the TME is necessary for efficient application for approval of them in cancer therapy. Novel CAR macrophages which abundantly infiltrate into the TME is promising for solid tumor therapy. Combination therapies using CAR NK cells and chemotherapy can also overcome limitations.
Conclusion
The transforming of NK cells into potent tumor killing cells is crucial in melanoma treatment owing to high immunogenicity of melanoma cells. NK cells are versatile multifunctional immune cells which efficiently participate in anticancer activities in cooperation with CD8+ and CD4+ T cells. The therapeutic failure of CAR T cells in the TME is mainly due to the lack of CAR T cells specific tumor antigens which inhibit these cells. CAR NK cells have advantages over CAR T cells in terms of natural cytotoxic capacity, lack of off-target toxicity, low costs, ready availability and convenient antitumor activation by antigens both dependent and independent of MHC molecules. Short life span of CAR NK cells is advantageous for on-target/off-tumor toxicity. NK cells can be independent of the host specific HLA genotype. However, in solid tumors, their efficacy remains to be fully understood. Viral transduction, transfection, mRNA electroporation and feeder-free and bovine serum-free protocol can enhance the efficacy of NK cells anticancer effects. Combination therapy using immune checkpoints and CAR NK cells can be promising for more efficient cancer therapy. The anti-KIR (lirilumab) and anti- NKG2A (monalizumab) drugs are safer than anti-PD-1 antibody and thereby can be used in combination with the CAR NK cells for melanoma treatment.
Availability of data and materials
Not applicable. No data has been used in this study.
References
Carr S, Smith C, Wernberg J. Epidemiology and risk factors of melanoma. Surg Clin. 2020;100(1):1–12.
Davis LE, Shalin SC, Tackett AJ. Current state of melanoma diagnosis and treatment. Cancer Biol Ther. 2019;20(11):1366–79.
Domingues B, et al. Melanoma treatment in review. ImmunoTargets Ther. 2018;7:35.
Seth R, et al. Systemic therapy for melanoma: ASCO guideline. J Clin Oncol. 2020;38(33):3947–70.
Yu W-L, Hua Z-C. Chimeric antigen receptor T-cell (CAR T) therapy for hematologic and solid malignancies: efficacy and safety—a systematic review with meta-analysis. Cancers. 2019;11(1):47.
Roex G, et al. Chimeric antigen receptor-T-cell therapy for B-cell hematological malignancies: an update of the pivotal clinical trial data. Pharmaceutics. 2020;12(2):194.
Stoiber S, et al. Limitations in the design of chimeric antigen receptors for cancer therapy. Cells. 2019;8(5):472.
Beauvais D, et al. Clinical data, limitations and perspectives on chimeric antigen receptor T-cell therapy in multiple myeloma. Curr Opin Oncol. 2020;32(5):418–26.
Yilmaz A, et al. Chimeric antigen receptor-engineered natural killer cells for cancer immunotherapy. J Hematol Oncol. 2020;13(1):1–22.
Pfefferle A, Huntington ND. You have got a fast CAR: chimeric antigen receptor NK cells in cancer therapy. Cancers. 2020;12(3):706.
Li Y, et al. Human iPSC-derived natural killer cells engineered with chimeric antigen receptors enhance anti-tumor activity. Cell Stem Cell. 2018;23(2):181–92 e5.
Gong Y, et al. Chimeric antigen receptor natural killer (CAR-NK) cell design and engineering for cancer therapy. J Hematol Oncol. 2021;14(1):1–35.
Wang L, et al. Chimeric antigen receptor (CAR)-modified NK cells against cancer: opportunities and challenges. Int Immunopharmacol. 2019;74:105695.
Yu M, et al. Development of GPC3-specific chimeric antigen receptor-engineered natural killer cells for the treatment of hepatocellular carcinoma. Mol Ther. 2018;26(2):366–78.
Schubert ML, et al. Chimeric antigen receptor transduced T cells: tuning up for the next generation. Int J Cancer. 2018;142(9):1738–47.
Azoulay E, et al. Critical care management of chimeric antigen receptor T cell–related toxicity. Be aware and prepared. Am J Respir Crit Care Med. 2019;200(1):20–3.
Guo Y, et al. Mutant B2M-HLA-E and B2M-HLA-G fusion proteins protects universal chimeric antigen receptor-modified T cells from allogeneic NK cell-mediated lysis. Eur J Immunol. 2021;51(10):2513–21.
Hosseini M, et al. Preclinical studies of chimeric antigen receptor-modified natural killer cells in cancer immunotherapy: a review. Expert Opin Biol Ther. 2021;22:1–18.
Morgan MA, et al. Improved activity against acute myeloid leukemia with chimeric antigen receptor (CAR)-NK-92 cells designed to target CD123. Viruses. 2021;13(7):1365.
Parlar A, et al. Engineering antigen-specific NK cell lines against the melanoma-associated antigen tyrosinase via TCR gene transfer. Eur J Immunol. 2019;49(8):1278–90.
Tarazona R, Duran E, Solana R. Natural killer cell recognition of melanoma: new clues for a more effective immunotherapy. Front Immunol. 2016;6:649.
Lam BQ, et al. Accumulation and anti-tumor effect of chimeric antigen receptor (CAR) NK cells in metastasis uveal melanoma: AACR; 2019.
Soltantoyeh T, et al. Chimeric antigen receptor (CAR) T cell therapy for metastatic melanoma: challenges and road ahead. Cells. 2021;10(6):1450.
van Vliet AA, et al. Adoptive NK cell therapy: a promising treatment prospect for metastatic melanoma. Cancers. 2021;13(18):4722.
Braun M, et al. The CD6 scavenger receptor is differentially expressed on a CD56dim natural killer cell subpopulation and contributes to natural killer-derived cytokine and chemokine secretion. J Innate Immunity. 2011;3(4):420–34.
Bald T, et al. The NK cell–cancer cycle: advances and new challenges in NK cell–based immunotherapies. Nat Immunol. 2020;21(8):835–47.
Shimasaki N, Jain A, Campana D. NK cells for cancer immunotherapy. Nat Rev Drug Discov. 2020;19(3):200–18.
Lee H, et al. Targeting NK cells to enhance melanoma response to immunotherapies. Cancers. 2021;13(6):1363.
Sivori S, et al. Human NK cells: surface receptors, inhibitory checkpoints, and translational applications. Cell Mol Immunol. 2019;16(5):430–41.
Prager I, Watzl C. Mechanisms of natural killer cell-mediated cellular cytotoxicity. J Leukoc Biol. 2019;105(6):1319–29.
Xie G, et al. CAR-NK cells: a promising cellular immunotherapy for cancer. EBioMedicine. 2020;59:102975.
Zhang H, et al. The role of NK cells and CD39 in the immunological control of tumor metastases. Oncoimmunology. 2019;8(6):e1593809.
Chiossone L, et al. Natural killer cells and other innate lymphoid cells in cancer. Nat Rev Immunol. 2018;18(11):671–88.
Rückert M, et al. Immune modulatory effects of radiotherapy as basis for well-reasoned radioimmunotherapies. Strahlenther Onkol. 2018;194(6):509–19.
de Jonge K, et al. Circulating CD56bright NK cells inversely correlate with survival of melanoma patients. Sci Rep. 2019;9(1):1–10.
Ali TH, et al. Enrichment of CD56dimKIR+ CD57+ highly cytotoxic NK cells in tumour-infiltrated lymph nodes of melanoma patients. Nat Commun. 2014;5(1):1–9.
Vujanovic L, et al. CD56dim CD16− natural killer cell profiling in melanoma patients receiving a cancer vaccine and interferon-α. Front Immunol. 2019;10:14.
Messaoudene M, et al. Mature cytotoxic CD56bright/CD16+ natural killer cells can infiltrate lymph nodes adjacent to metastatic melanoma. Cancer Res. 2014;74(1):81–92.
Frazao A, et al. CD16+ NKG2Ahigh Natural Killer Cells Infiltrate Breast Cancer–Draining Lymph Nodes. Cancer Immunol Res. 2019;7(2):208–18.
Martinovic KMM, et al. Decreased expression of NKG2D, NKp46, DNAM-1 receptors, and intracellular perforin and STAT-1 effector molecules in NK cells and their dim and bright subsets in metastatic melanoma patients. Melanoma Res. 2014;24(4):295–304.
Stojanovic A, Cerwenka A. Natural killer cells and solid tumors. J Innate Immun. 2011;3(4):355–64.
Melaiu O, et al. Influence of the tumor microenvironment on NK cell function in solid tumors. Front Immunol. 2020:3038.
Ng YY, Tay JC, Wang S. CXCR1 expression to improve anti-cancer efficacy of intravenously injected CAR-NK cells in mice with peritoneal xenografts. Mol Ther Oncolytics. 2020;16:75–85.
Ang WX, et al. Intraperitoneal immunotherapy with T cells stably and transiently expressing anti-EpCAM CAR in xenograft models of peritoneal carcinomatosis. Oncotarget. 2017;8(8):13545.
Sottile R, et al. NK-and T-cell subsets in malignant mesothelioma patients: baseline pattern and changes in the context of anti-CTLA-4 therapy. Int J Cancer. 2019;145(8):2238–48.
Kohlhapp FJ, et al. NK cells and CD8+ T cells cooperate to improve therapeutic responses in melanoma treated with interleukin-2 (IL-2) and CTLA-4 blockade. J Immunother Cancer. 2015;3(1):1–13.
Crome SQ, et al. Natural killer cells regulate diverse T cell responses. Trends Immunol. 2013;34(7):342–9.
Morandi F, et al. CD56brightCD16− NK cells produce adenosine through a CD38-mediated pathway and act as regulatory cells inhibiting autologous CD4+ T cell proliferation. J Immunol. 2015;195(3):965–72.
Cappello S, et al. Protein signatures of NK cell–mediated melanoma killing predict response to immunotherapies. Cancer Res. 2021;81(21):5540–54.
Parodi M, et al. Natural killer (NK)/melanoma cell interaction induces NK-mediated release of chemotactic high mobility group Box-1 (HMGB1) capable of amplifying NK cell recruitment. Oncoimmunology. 2015;4(12):e1052353.
Morgado S, et al. NK cell recognition and killing of melanoma cells is controlled by multiple activating receptor-ligand interactions. J Innate Immun. 2011;3(4):365–73.
Sayitoglu EC, et al. Boosting natural killer cell-mediated targeting of sarcoma through DNAM-1 and NKG2D. Front Immunol. 2020;11:40.
Forsberg EM, et al. HER2 CAR-T cells eradicate uveal melanoma and T-cell therapy–resistant human melanoma in IL2 transgenic NOD/SCID IL2 receptor knockout mice. Cancer Res. 2019;79(5):899–904.
Kozar I, et al. Many ways to resistance: How melanoma cells evade targeted therapies. Biochimica et Biophysica Acta (BBA)-Reviews on Cancer. 2019;1871(2):313–22.
Marzagalli M, Ebelt ND, Manuel ER. Unraveling the crosstalk between melanoma and immune cells in the tumor microenvironment. Semin Cancer Biol. 2019;59:236–50. Elsevier.
Huang Z, et al. Targeted delivery of let-7b to reprogramme tumor-associated macrophages and tumor infiltrating dendritic cells for tumor rejection. Biomaterials. 2016;90:72–84.
Johansson CH, Brage SE. BRAF inhibitors in cancer therapy. Pharmacol Ther. 2014;142(2):176–82.
Grimaldi AM, et al. MEK inhibitors in the treatment of metastatic melanoma and solid tumors. Am J Clin Dermatol. 2017;18(6):745–54.
Simiczyjew A, et al. The influence of tumor microenvironment on immune escape of melanoma. Int J Mol Sci. 2020;21(21):8359.
Cerezo M, et al. Translational control of tumor immune escape via the eIF4F–STAT1–PD-L1 axis in melanoma. Nat Med. 2018;24(12):1877–86.
Passarelli A, et al. The metabolic milieu in melanoma: role of immune suppression by CD73/adenosine. Tumor Biol. 2019;41(4):1010428319837138.
Derré L, et al. Expression and release of HLA-E by melanoma cells and melanocytes: potential impact on the response of cytotoxic effector cells. J Immunol. 2006;177(5):3100–7.
Sabrina, R., et al., Autocrine signaling of NRP1 ligand Galectin-1 elicits resistance to BRAF-targeted therapy in melanoma cells. 2020.
Kim MH, et al. YAP-induced PD-L1 expression drives immune evasion in BRAFi-resistant melanoma. Cancer Immunol Res. 2018;6(3):255–66.
Chauvin J-M, et al. IL15 stimulation with TIGIT blockade reverses CD155-mediated NK-cell dysfunction in melanoma. Clin Cancer Res. 2020;26(20):5520–33.
Kawashima S, et al. TIGIT/CD155 axis mediates resistance to immunotherapy in patients with melanoma with the inflamed tumor microenvironment. J Immunother Cancer. 2021;9(11):e003134.
Ziani L, et al. Hypoxia increases melanoma-associated fibroblasts immunosuppressive potential and inhibitory effect on T cell-mediated cytotoxicity. OncoImmunology. 2021;10(1):1950953.
Peng Y-P, et al. Elevation of MMP-9 and IDO induced by pancreatic cancer cells mediates natural killer cell dysfunction. BMC Cancer. 2014;14(1):1–12.
Zhang F, et al. TGF-β induces M2-like macrophage polarization via SNAIL-mediated suppression of a pro-inflammatory phenotype. Oncotarget. 2016;7(32):52294.
Mandl M, et al. Hypoxia-inducible factor-1β (HIF-1β) is upregulated in a HIF-1α-dependent manner in 518A2 human melanoma cells under hypoxic conditions. Biochem Biophys Res Commun. 2013;434(1):166–72.
Park E-J, et al. Vanillin suppresses cell motility by inhibiting STAT3-mediated HIF-1α mRNA expression in malignant melanoma cells. Int J Mol Sci. 2017;18(3):532.
Fang F, et al. Advances in NK cell production. Cell Mol Immunol. 2022:1–22.
Klingemann H, Boissel L, Toneguzzo F. Natural killer cells for immunotherapy–advantages of the NK-92 cell line over blood NK cells. Front Immunol. 2016:91.
Lamas B, et al. Altered functions of natural killer cells in response to L-arginine availability. Cell Immunol. 2012;280(2):182–90.
Maki G, et al. Factors regulating the cytotoxic activity of the human natural killer cell line, NK-92. J Hematother Stem Cell Res. 2001;10(3):369–83.
Mallett CL, et al. Migration of iron-labeled KHYG-1 natural killer cells to subcutaneous tumors in nude mice, as detected by magnetic resonance imaging. Cytotherapy. 2012;14(6):743–51.
Cheng M, et al. Establishment, characterization, and successful adaptive therapy against human tumors of NKG cell, a new human NK cell line. Cell Transplant. 2011;20(11–12):1731–46.
Fujisaki H, et al. Expansion of highly cytotoxic human natural killer cells for cancer cell therapy. Cancer Res. 2009;69(9):4010–7.
Glienke W, et al. Advantages and applications of CAR-expressing natural killer cells. Front Pharmacol. 2015;6:21.
Stikvoort A, et al. CD38-specific chimeric antigen receptor expressing natural killer KHYG-1 cells: a proof of concept for an “off the shelf” therapy for multiple myeloma. HemaSphere. 2021;5(7).
Carlsten M, Childs RW. Genetic manipulation of NK cells for cancer immunotherapy: techniques and clinical implications. Front Immunol. 2015;6:266.
Kararoudi, M.N., et al. Genetic and epigenetic modification of human primary NK cells for enhanced antitumor activity. Semin Hematol. 2020. Elsevier.
Caudana P, et al. IL2/anti-IL2 complex combined with CTLA-4, but not PD-1, blockade rescues antitumor NK cell function by regulatory T-cell modulation. Cancer Immunol Res. 2019;7(3):443–57.
Subrahmanyam PB, et al. Distinct predictive biomarker candidates for response to anti-CTLA-4 and anti-PD-1 immunotherapy in melanoma patients. J Immunother Cancer. 2018;6(1):1–14.
Esen F, Deniz G, Aktas EC. PD-1, CTLA-4, LAG-3, and TIGIT: the roles of immune checkpoint receptors on the regulation of human NK cell phenotype and functions. Immunol Lett. 2021;240:15–23.
Della Chiesa M, et al. Features of memory-like and PD-1+ human NK cell subsets. Front Immunol. 2016;7:351.
Mariotti FR, et al. PD-1 in human NK cells: evidence of cytoplasmic mRNA and protein expression. Oncoimmunology. 2019;8(3):1557030.
Sakamoto Y, et al. Increased frequency of dysfunctional siglec-7− CD57+ PD-1+ natural killer cells in patients with non-alcoholic fatty liver disease. Front Immunol. 2021;12:603133.
Sanchez-Correa B, et al. Modulation of NK cells with checkpoint inhibitors in the context of cancer immunotherapy. Cancer Immunol Immunother. 2019;68(5):861–70.
Zhang Q, et al. Blockade of the checkpoint receptor TIGIT prevents NK cell exhaustion and elicits potent anti-tumor immunity. Nat Immunol. 2018;19(7):723–32.
Liu S, et al. NK cell-based cancer immunotherapy: from basic biology to clinical development. J Hematol Oncol. 2021;14(1):1–17.
Tarazona R, et al. Current progress in NK cell biology and NK cell-based cancer immunotherapy. Cancer Immunol Immunother. 2020;69(5):879–99.
Bachanova V, Miller JS. NK cells in therapy of cancer. Crit Rev Oncog. 2014;19(1-2).
Takahashi E, et al. Induction of CD16+ CD56bright NK cells with antitumour cytotoxicity not only from CD16− CD56bright NK cells but also from CD16− CD56dim NK cells. Scand J Immunol. 2007;65(2):126–38.
Srpan K, et al. Shedding of CD16 disassembles the NK cell immune synapse and boosts serial engagement of target cells. J Cell Biol. 2018;217(9):3267–83.
Cheng Y, et al. Trispecific killer engager 161519 enhances natural killer cell function and provides anti-tumor activity against CD19-positive cancers. Cancer Biol Med. 2020;17(4):1026.
Reusing SB, et al. CD16xCD33 bispecific killer cell engager (BiKE) as potential immunotherapeutic in pediatric patients with AML and biphenotypic ALL. Cancer Immunol Immunother. 2021;70(12):3701–8.
Yang Y, Lundqvist A. Immunomodulatory effects of IL-2 and IL-15; implications for cancer immunotherapy. Cancers. 2020;12(12):3586.
Guo J, et al. Tumor-conditional IL-15 pro-cytokine reactivates anti-tumor immunity with limited toxicity. Cell Res. 2021;31(11):1190–8.
Au KM, Park SI, Wang AZ. Trispecific natural killer cell nanoengagers for targeted chemoimmunotherapy. Sci Adv. 2020;6(27):eaba8564.
Larocca CA, et al. An update on the role of talimogene laherparepvec (T-VEC) in the treatment of melanoma: best practices and future directions. Am J Clin Dermatol. 2020;21(6):821–32.
Ferrucci PF, et al. Talimogene laherparepvec (T-VEC): an intralesional cancer immunotherapy for advanced melanoma. Cancers. 2021;13(6):1383.
Wang W, Jiang J, Wu C. CAR-NK for tumor immunotherapy: clinical transformation and future prospects. Cancer Lett. 2020;472:175–80.
Matosevic S. Viral and nonviral engineering of natural killer cells as emerging adoptive cancer immunotherapies. J Immunol Res. 2018.
Johnson C, et al. Feeder-cell-free and serum-free expansion of natural killer cells using Cloudz microspheres, G-Rex6M, and human platelet lysate. Front Immunol. 2022:13.
Ma R, et al. An oncolytic virus expressing IL15/IL15Rα combined with off-the-shelf EGFR-CAR NK cells targets glioblastoma. Cancer Res. 2021;81(13):3635–48.
Zhang Q, et al. Combination therapy with EpCAM-CAR-NK-92 cells and regorafenib against human colorectal cancer models. J Immunol Res. 2018.
Laskowski TJ, Biederstädt A, Rezvani K. Natural killer cells in antitumour adoptive cell immunotherapy. Nat Rev Cancer. 2022;22(10):557–75.
Shin MH, et al. NK cell-based immunotherapies in cancer. Immune Network. 2020;20(2).
Gasteiger G, et al. IL-2–dependent adaptive control of NK cell homeostasis. J Exp Med. 2013;210(6):1179–87.
Bachanova V, et al. Clearance of acute myeloid leukemia by haploidentical natural killer cells is improved using IL-2 diphtheria toxin fusion protein. Blood. 2014;123(25):3855–63.
Xiong J, Wang H, Wang Q. Suppressive myeloid cells shape the tumor immune microenvironment. Adv Biol. 2021;5(3):1900311.
Grabowski MM, et al. Immune suppression in gliomas. J Neuro-Oncol. 2021;151(1):3–12.
Gong Y, et al. Rosuvastatin enhances VSV-G lentiviral transduction of NK cells via upregulation of the low-density lipoprotein receptor. Mol Ther Methods Clin Dev. 2020;17:634–46.
You F, et al. A novel CD7 chimeric antigen receptor-modified NK-92MI cell line targeting T-cell acute lymphoblastic leukemia. Am J Cancer Res. 2019;9(1):64.
Rafei H, Daher M, Rezvani K. Chimeric antigen receptor (CAR) natural killer (NK)-cell therapy: leveraging the power of innate immunity. Br J Haematol. 2021;193(2):216–30.
Marofi F, et al. CAR-NK cell in cancer immunotherapy; a promising frontier. Cancer Sci. 2021;112(9):3427.
Daher M, et al. CAR-NK cells: the next wave of cellular therapy for cancer. Clin Transl Immunol. 2021;10(4):e1274.
Siegler EL, et al. Off-the-shelf CAR-NK cells for cancer immunotherapy. Cell Stem Cell. 2018;23(2):160–1.
Marofi F, et al. Renaissance of armored immune effector cells, CAR-NK cells, brings the higher hope for successful cancer therapy. Stem Cell Res Ther. 2021;12(1):1–21.
Pan K, et al. CAR race to cancer immunotherapy: from CAR T, CAR NK to CAR macrophage therapy. J Exp Clin Cancer Res. 2022;41(1):1–21.
Acknowledgements
This study has been written by the authors.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
MB, MKV, GRLA, SAK, YM, BM, AM, AS and AGh has collected data, manuscript drafting, edition and final approval of scientific contents.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All the authors have consent of publication.
Competing interests
None.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Bahmanyar, M., Vakil, M.K., Al-Awsi, G.R.L. et al. Anticancer traits of chimeric antigen receptors (CARs)-Natural Killer (NK) cells as novel approaches for melanoma treatment. BMC Cancer 22, 1220 (2022). https://doi.org/10.1186/s12885-022-10320-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-022-10320-0