Abstract
Purpose
For the first-line treatment of KRAS mutant non-small cell lung cancer (NSCLC) patients, immunotherapy or platinum-based chemotherapy are the main treatment method. Here, we investigated the clinical efficacy and prognosis those two regimens as first-line treatment in real-world practice.
Methods
KRAS mutant NSCLC patients received chemotherapy or immunotherapy as first-line treatment from September 2014 to March 2022 were enrolled. Clinical characteristics, treatment scheme, clinical curative effect and follow-up data of enrolled patients were collected for analysis.
Results
Fifty patients received immunotherapy and 115 patients received chemotherapy were enrolled. Patients who received immunotherapy (HR = 0.350, 95%CI 0.156–0.781, P = 0.010), or pemetrexed-based regimen (HR = 0.486, 95%CI 0.255–0.928, P = 0.029), or antiangiogenic therapy (HR = 0.355, 95%CI 0.159–0.790, P = 0.011) were at a low risk of disease progression. And patients received antiangiogenic therapy had lower risk of death than those not (HR = 0.333, 95%CI 0.120–0.926, P = 0.035). Subgroup analysis revealed the immunotherapy compared to chemotherapy alone had lower risk of disease progression (HR = 0.377, 95%CI 0.166–0.856, P = 0.020) in PD-L1 expression ≥1% subgroup. And in non-G12C KRAS subgroup, but not in G12C KRAS subgroup, patients who received antiangiogenic therapy had lower risk of disease progression (HR = 0.254, 95%CI 0.098–0.656, P = 0.005) and death than those not (HR = 0.197, 95%CI 0.056–0.692, P = 0.011). In terms of different chemotherapy regimen, platinum-paclitaxel combined with antiangiogenic therapy achieved the highest ORR and DCR (P < 0.05), while the platinum-pemetrexed combined with antiangiogenic therapy had the longest PFS and OS (P < 0.001).
Conclusion
For the first-line treatment of KRAS mutant NSCLC patients, immunotherapy, antiangiogenic therapy, and pemetrexed-based regimen could obtain more benefits. Subgroup analysis revealed the benefits of immunotherapy compared to chemotherapy were applicable in PD-L1 expression≥1% subgroup, and antiangiogenic therapy could benefit non-G12C KRAS subgroup, but not G12C KRAS subgroup. In terms of different chemotherapy regimen, platinum-pemetrexed combined with antiangiogenic therapy may be the preferred chemotherapy regimen.
Similar content being viewed by others
Introduction
Lung cancer has the highest mortality among solid tumors worldwide, and non-small cell lung cancer (NSCLC) accounts for 80–85% of all lung cancer [1]. Kirsten rat sarcoma viral oncogene homolog (KRAS) mutation is one of the most common oncogenic mutation detected in patients with NSCLC [2, 3]. KRAS gene is a member of the RAS gene family which plays an important role in regulating cellular proliferation, differentiation, and apoptosis [4]. It has been recognized that oncogenic mutations in KRAS gene result in hyper-activation of downstream signaling cascades that lead to uncontrolled cell proliferation and survival, so as to tumorgenesis [5, 6]. However, due to the special structure of KRAS protein and the wide biological functions of the gene, the development of targeted therapy for KRAS mutant lung cancer has been frustrated for many years. Recently, clinical studies of targeted drugs for KRAS G12C have made some progress [7,8,9]. In 2021, the US Food and Drug Administration (FDA) approved the first KRAS-targeted drug, Sotorasib, for the treatment of NSCLC patients with KRAS G12C mutations who had received at least one previous systemic therapy [10]. As for the first-line treatment of KRAS mutant NSCLC patients, immunotherapy or platinum-based chemotherapy are still the the main treatment method. Studies have revealed that intratumoral PD-L1 expression, tumor mutation burden (TMB), the intensity of CD8+ T cell infiltrates could be biomarkers of response to immunotherapy [11,12,13]. And among these biomarkers, PD-L1 expression is the most widely used in NSCLC patients and help to guide the selection of immunotherapy regimens. Based on the results of clinical trials, immunotherapy monotherapy has been approved as the first-line treatment for advanced epidermal growth factor receptor (EGFR)/anaplastic lymphoma kinase (ALK) negative NSCLC patients with PD-L1 expression ≥50%, and for patients with PD-L1 expression < 50%, immunotherapy combined with chemotherapy and/or antiangiogenesis therapy can bring more survival benefits than chemotherapy [14,15,16]. However, the results of clinical trials are difficult to reproduce in the actual clinical environment due to the strict selection of subjects and control of clinical treatment regimen, and the efficacy of real-world patients needs to be answered with real-world data. In addition, studies have found that KRAS mutant NSCLC populations are highly heterogeneous, and different subtypes and co-mutations can affect the biological characteristics and treatment response of tumors [17,18,19,20]. Therefore, how to rationally select the existing immunotherapy or chemotherapy regimen to improve the survival and life quality of KRAS mutant NSCLC patients in different subgroups is particularly important. In this study, we retrospectively analyzed the real-world clinical data of advanced KRAS mutant NSCLC patients who received immunotherapy or chemotherapy regimen as first-line treatment, and explored the efficacy and prognosis of patients in different subgroups after diverse treatment regimen, so as to provide some clues for selecting appropriate treatment regimen as first-line therapy for advanced KRAS mutant NSCLC patients in real world.
Materials and methods
Patients and clinical data
We retrospectively studied NSCLC patients who diagnosed in Beijing Chest Hospital, Capital Medical University from September 2014 to March 2022, and included patients with KRAS mutation who received immunotherapy or chemotherapy regimen as first-line treatment for further analysis according to inclusion and exclusion criteria.
Inclusion criteria: (1) Patients with newly diagnosed metastatic or postoperative recurrence of NSCLC diagnosed by histology or cellular sediment embedding; (2) Cytological sediment or histological samples of patients underwent genomic testing and found at least one KRAS mutation; (3) Stage IIIB to IV according to the eighth edition of the American Joint Committee on Cancer/International Union Against Cancer TNM stage classification for lung cancer; (4) Patients receiving immunotherapy or chemotherapy as first-line treatment; (5) Patients with detailed clinical treatment and prognosis information.
Exclusion criteria: (1) Patients without clear pathological diagnosis information; (2) Patients with operable NSCLC; (3) Patients with serious dysfunction of important organs; (4) Patients with other tumors at the same time or patients with other tumors in the past 5 years; (5) Patients with incomplete clinical data.
Clinical and follow-up data of the patients were collected and recorded for analysis included age, sex, pathological type, smoking history, KRAS mutation status, mutation subtypes and other associated mutations (co-mutations), performance status (PS) score, TNM stage, treatment history, clinical efficacy and prognosis information.
Efficacy evaluation and prognosis
According to the Response Evaluation Criteria in Solid Tumors (RECIST) guideline version 1.1, the short-term efficacy was divided into complete Response (CR) and partial Response (PR), stable disease (SD) and progressive disease (PD). Objective response rate (ORR) = (CR + PR)/(CR + PR + SD + PD) × 100%; Disease control rate (DCR) = (CR + PR + SD)/(CR + PR + SD + PD) × 100%; Progression-free survival (PFS) was defined as the time from the initiation of the first-line chemotherapy until date of progression or last follow-up or death caused by any cause. Overall survival (OS) was defined as the time of postoperative disease recurrence in patients with early surgery or from the time of initial diagnosis in patients with advanced to the time of death or last follow-up.
Follow-up
Survival follow-up was conducted by telephone inquiry and medical record inquiry system. The cut-off date is June 30, 2022.
Statistical analysis
SPSS 23.0 and GraphPad Prism 7 were used for statistical analysis and mapping. Data were presented as frequencies and percentages for categorical variables and means or medians, with standard deviation or interquartile range for continuous variables. Whitney U test was used to compare PD-L1 expression in different subgroups. Chran’s and Mantel-Haenszel statistics and Chi-square or Fisher’s exact test were used for assessing the statistical significance of categorical variables and the odds ratio (OR) with 95% confidence intervals (95% CIs). Kapla-Meier method was used for univariate analysis of OS and PFS, whereas comparisons among the subgroups were analyzed using the log rank test. Cox proportional-hazards regression model was performed for multivariate analysis and computing the statistical significance and hazard ratio (HR) with 95% CIs. Statistical significance was set at P < 0.05.
Results
Epidemiological characteristics of KRAS mutations in NSCLC patients
A total of 5621 NSCLC patients underwent KRAS mutation gene testing in our hospital between September 2014 and March 2022, of which 3024 were detected by PCR and 2597 by NGS. And 554 (PCR:282; NGS:272) patients had positive KRAS mutation, the frequency of KRAS mutations was 9.86% (554/5621). Among the 272 KRAS mutant patients tested by NGS, the prevalent co-mutations include TP53 (102/272, 37.50%), SMAD4 (15/272, 5.51%), EGFR (14/272, 5.15%), BRAF (12/272, 4.41%), MET (8/272, 2.94%), DDR2 (8/272, 2.94%), STK11 (4/272, 1.47%). We also have obtained and analyzed the TCGA data using the cBioportal Tool (http://www.cbioportal.org/) and found that the prevalent co-mutations of KRAS mutant NSCLC patients are TTN(51.19%), RYR2(45.80%), MUC16(42.78%%), CSMD3(40.18%), LRP1B(39.55%), TP53(39.38%), USH2A(38.75%), ZFHX4(37.39%), SPTA1(32.89%), FLG(30.74%).
Clinical characteristics of enrolled patients with KRAS mutant NSCLC
According to the inclusion and exclusion criteria, 115 KRAS mutant NSCLC patients received chemotherapy regimen and 65 patients received immunotherapy as first-line treatment were included, the specific process is shown in Fig. 1. The median age of 165 enrolled patients was 64.5 years (range: 36–79 years). Due to the change of genomic testing methods and kit, 81 patients detected the TP53 co-mutations. And 5 patients could only detect whether there is a KRAS mutation, but not specific to KRAS subtypes. The most common mutation sites of KRAS are located at codon 12, accounting for 82.50% (132/160), among it, KRAS G12C (51/160, 31.88%), G12V (27/160, 8.13%) and G12D (40/160, 25.00%) were the three most frequent subtypes. In addition, codon 61 accounted for 8.75% (14/160) and codon 13 accounted for 2.50% (4/160), as shown in Fig. 2. Information about PD-L1 expression was available for 87 patients. It is found that immunotherapy group has higher median PD-L1 expression (5.0% vs 60.0%, P = 0.003, Fig. 3) than chemotherapy group. Chi-square test was used to compare the distribution of clinical characteristic in patients treated with immunotherapy and chemotherapy, and there were no statistical differences in gender, smoking history, PS score, pathological type, Stage, while there were statistical differences in age, KRAS mutant subtypes and PD-L1 expression, as shown in Table 1.
Treatment regimen
A total of 115 patients received chemotherapy regimen as first-line treatment were enrolled. According to whether combined antiangiogenic therapy (AT) or not, 58 (58/115, 27.1%) patients were treated with chemotherapy+antiangiogenic therapy (AT group), and 57 (57/115,72.9%) patients were treated with chemotherapy regimen alone (no-AT group). According to chemotherapy drugs, 60 (60/115, 49.5%) patients received pemetrexed combined with platinum (PEM group), 48 (48/115, 36.5%) patients received paclitaxel combined with platinum (TAX group), and 7 patients (7/115, 14.0%) received other chemotherapy drugs (OTHER group).
Fifty patients treated with either immunotherapy monotherapy (7/50, 14.0%, mono-IO group) or combination immunotherapy. According to the combined drugs, the immune combination group can be divided into immunotherapy+chemotherapy (28/50, 56.0%, IO+C group), immunotherapy+chemotherapy+antiangiogenesis therapy (14/50, 28.0%, IO+C + A group), and dual immunotherapy (1/50, 2.0%, dual-IO group). Details were shown in Fig. 4.
Treatment efficacy and prognosis
During the course of treatment, none of the 165 patients achieved CR, 57 patients achieved PR, and 140 patients achieved SD. The ORR and DCR of all KRAS mutant NSCLC patients were 34.55 and 84.85%, respectively. Among all KRAS mutant NSCLC, the immunotherapy group compared to chemotherapy group had higher ORR (44.00% vs 30.43%) and DCR (96.00% vs 80.00%). Considering the distribution differences of several clinical characteristics between the immunotherapy and the chemotherapy group, we used Chran’s and Mantel-Haenszel statistics to control for gender, KRAS mutation and PD-L1 expression categorical variables and found that there was no significant difference in ORR and DCR between immunotherapy and chemotherapy group. Next, we performed a subgroup analysis which also using Cochran’s and Mantel-Haenszel statistics to control for other variables, and found no statistically significant differences in ORR and DCR between the immunotherapy and the chemotherapy group in KRAS G12C subgroup, non-G12C KRAS subgroup, PD-L1 expressio≥1% subgroup, PD-L1 expression < 1% subgroup, as shown in Table 2.
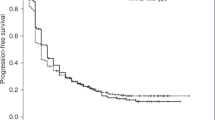
Up to the last follow-up time, a total of 126 patients had disease progression (126/165, 76.36%) and 100 patients had died (100/165, 60.61%). Median PFS and OS of all 165 KRAS mutant NSCLC were 9.0 months (95% CI 7.5–10.5) and 16.0 months (95% CI 13.3–18.7). Univariate analysis showed that age, gender, smoking history, PS score, pathological type and KRAS mutation subtype were not correlated with PFS and OS (P > 0.05), while various treatment scheme were correlated with PFS and OS (P < 0.05). Among all KRAS mutant NSCLC, the immunotherapy significantly improved PFS (11.7 vs 7.0 months, P < 0.001, Fig. 5A) and OS (23.8 vs 14.7 months, P = 0.013, Fig. 5D) compared to chemotherapy alone; Treatment containing pemetrexed had longer PFS (10.1 vs 6.2 months, P < 0.001, Fig. 5B) and OS (16.4 vs 14.1 months, P = 0.112, Fig. 5E) compared to treatment containing paclitaxel; And patients who received antiangiogenic therapy had significantly longer PFS (10.0 vs 6.5 months, P = 0.031, Fig. 5C) and OS (19.7 vs 13.7 months, P = 0.004, Fig. 5F) than those not. To control confounding factors, Cox multivariate analysis was performed on the factors with P < 0.2 in univariate analysis or factors considered to be related to prognosis, and the results showed that immunotherapy, chemotherapy drugs, antiangiogenic therapy were correlated with PFS and only antiangiogenic therapy was correlated with 0S. This result indicated that that patients who received immunotherapy (HR = 0.350, 95%CI 0.156–0.781, P = 0.010), or pemetrexed-based regimen (HR = 0.486, 95%CI 0.255–0.928, P = 0.029), or antiangiogenic therapy (HR = 0.355, 95%CI 0.159–0.790, P = 0.011) were at a low risk of disease progression. And patients received antiangiogenic therapy had lower risk of death than those not (HR = 0.333, 95%CI 0.120–0.926, P = 0.035), as shown in Table 3.
Survival curves of KRAS mutant NSCLC (A). The PFS survival curves between immunotherapy and chemotherapy group in KRAS mutant NSCLC patients (B). The PFS survival curves between treatment containing pemetrexed and containing paclitaxel in KRAS mutant NSCLC patients (C). The PFS survival curves between treatment with and without antiangiogenic therapy in KRAS mutant NSCLC patients (D). The OS survival curves between immunotherapy and chemotherapy group in KRAS mutant NSCLC patients (E). The OS survival curves between treatment containing pemetrexed and containing paclitaxel in KRAS mutant NSCLC patients (F). The OS survival curves between treatment with and without antiangiogenic therapy in KRAS mutant NSCLC patients (G). The PFS survival curves between immunotherapy and chemotherapy group in expression≥1% subgroup (H). The PFS survival curves between treatment with and without antiangiogenic therapy in non-G12C KRAS subgroup (I). The OS survival curves between treatment with and without antiangiogenic therapy in non-G12C KRAS subgroup
Subgroup analysis revealed the immunotherapy compared to chemotherapy alone had an improved PFS (12.9 vs 9.0 months, P = 0.011, Fig. 5G) and low risk of disease progression (HR = 0.377, 95%CI 0.166–0.856, P = 0.020) in PD-L1 expression ≥1% subgroup, And in non-G12C KRAS subgroup, but not in G12C KRAS subgroup, patients who received antiangiogenic therapy had significantly longer PFS (7.9 vs 5.8 months, P = 0.007, Fig. 5H) and OS (18.7 vs 13.7 months, P = 0.011, Fig. 5I) and low risk of disease progression (HR = 0.254, 95%CI 0.098–0.656, P = 0.005) and death than those not (HR = 0.197, 95%CI 0.056–0.692, P = 0.011)..
Treatment efficacy and prognosis of first-line chemotherapy
We further analyzed the treatment efficacy and prognosis of 115 KRAS mutant patients receiving different chemotherapy regimen. Univariate analysis showed that PEM group had longer PFS (9.8 vs 6.0 months, P = 0.041, Fig. 6A) and OS (16.0 vs 14.0 months, P = 0.276, Fig. 6B). compared to TAX group. Moreover, the AT group improved PFS (9.8 vs 5.0 months, P < 0.001, Fig. 6C) and OS (20.0 vs 10.0 months, P < 0.001, Fig. 6D) in KRAS mutant NSCLC patients. Multivariate analysis revealed that patients who received pemetrexed-based regimen (HR = 0.434, 95%CI (0.198–0.949, P = 0.036), or antiangiogenic therapy (HR = 0.307, 95%CI 0.117–0.805, P = 0.016) were at a low risk of disease progression. And patients received antiangiogenic therapy had lower risk of death than those not HR = 0.241, 95%CI 0.085–0.689, P = 0.008), as shown in Table 4.
Survival curves of KRAS mutant NSCLC treated with chemotherapy (A). The PFS survival curves between the PEM and TAX group (B). The OS survival curves between the PEM and TAX group (C). The PFS survival curves between the AT and non-AT group (D). The OS survival curves between the AT and non-AT group (E). The PFS survival curves of different chemotherapy scheme (F). The OS survival curves of different chemotherapy scheme
In terms of the combination of chemotherapy and antiangiogenic therapy, TAX+AT group achieved the highest ORR and DCR compared to PEM + AT group (ORR:59.09% vs 30.56%, OR = 3.283, 95% CI 1.085–9.930, P = 0.032; DCR: 95.45% vs 91.67%, OR = 1.909, 95% CI 0.186–19.589, P = 0.985), PEM (no-AT) group (ORR:59.09% vs 12.5%, OR = 10.111, 95% CI 2.305–44.348, P = 0.001; DCR: 95.45% vs 66.67%, OR = 10.500, 95% CI 1.189–92.727, P = 0.037), and TAX (no-AT) group (ORR:59.09% vs 26.92%, OR = 3.921, 95% CI 1.165–13.198, P = 0.024; DCR: 95.45% vs 73.08%, OR = 7.737, 95% CI 0.870–68.803, P = 0.092). However, PEM + AT group had the longest PFS and OS compared to PEM (no-AT) group (PFS:14.0 vs 4.0 months, HR = 0.487, 95% CI 0.264–0.900, P = 0.009; OS: 25.0 vs 10.0 months, HR = 0.419, 95% CI 0.214–0.822, P = 0.006), TAX+AT group (PFS:14.0 vs 8.0 months, HR = 0.474, 95% CI 0.253–0.889, P = 0.008; OS: 25.0 vs 19.0 months, HR = 0.793, 95% CI 0.385–1.594, P = 0.508), and TAX (no-AT) group (PFS:14.0 vs 5.0 months, HR = 0.303, 95% CI 0.153–0.601, P < 0.001; OS: 25.0 vs 11.0 months, HR = 0.336, 95% CI 0.174–0.648, P < 0.001), as shown in Table 5, Fig. 6E and F.
Treatment efficacy of first-line immunotherapy
We first analyzed the differences in PD-L1 expression among the immunotherapy groups, and found that mono-IO group has higher median PD-L1 expression compared to IO+C group, IO+C + A group (90.0% vs 40.0% vs 30.0%, P < 0.05, Fig. 7). Then we compared the treatment efficacy of above groups, no significant differences were found (ORR: 57.14% vs 42.86% vs 35.71%, P = 0.647; DCR: 85.71% vs 100.00% vs 92.86%, P = 0.152). Regarding the combined chemotherapy regimen, there were no statistical differences in ORR (50.00% vs 38.39%, P = 0.949) and DCR (100.00% vs 92.77.00%, P = 0.514) of immunotherapy combined paclitaxel-based or chemotherapy pemetrexed-based chemotherapy. In addition, patients with the common co-mutation TP53 did not show an efficacy difference in comparison to patients without TP53 (ORR: 53.33% vs 33.33%, P = 0.217; DCR: 100.00% vs 91.67%, P = 0.514).
Discussion
KRAS was one of the frequent genes found to be mutated in NSCLC. Studies have shown that KRAS mutations account for about 5–15% of lung cancer in Asian patients, and about 20–30% non-Asian patients [21,22,23]. In our retrospective study, 9.86% of Chinese NSCLC patients harbor KRAS mutation, which was consistent with the results of previous study studies. LCMC study revealed that up to third of KRAS mutant lung adenocarcinomas patients harbored another oncogenic driver, the most common of which are TP53 and STK11 [22]. Our study supported TP53 was the prevalent co-mutations in KRAS mutant NSCLC, but STK11 was much less common. Several studies suggested that KRAS/TP53 co-mutation generally present with a significant upregulation of PD-L1 expression and tumoricidal T-cell accumulation, which may help NSCLC patients respond to immunotherapy and get long-term survival [24,25,26,27]. However, our study did not observe the correlation of KRAS/TP53 co-mutation and PD-L1 expression or immunotherapy efficacy. Although EGFR, KRAS, and ALK mutations are generally considered mutually exclusive in NSCLC [21], recent studies have shown that above oncogenic driver mutations can co-exist in a certain amount of lung cancers. A French study which includes 17,664 patients, identified 0.93% of non-squamous NSCLC with multiple genetic alterations involving oncogenic drivers, and the frequent concomitant mutations associated with KRAS included EGFR (15%) mutations [28]. Another south Korean study found the proportion of KRAS and EGFR co-mutation was 1.5% in KRAS mutant NSCLC patients [29]. We found in NSCLC patients, the co-mutation rate of EGFR and KRAS was 5.15%. In addition, it was reported that KRAS mutations in NSCLC mainly occurred in codons 12,13 or 61, and the most frequent subtypes were G12C, G12V and G12D [29, 30]. In this study, a total of 160 patients underwent KRAS subtypes testing, and the most common mutation sites of KRAS are located at codon 12, accounting for 82.5%, and KRAS G12C, G12V and G12D were the three most frequent subtypes, which was consistent with previous reports.
Although KRAS mutation is the first driver mutation to be discovered in solid tumors, this target has long been considered “undruggable” [31]. So KRAS mutated NSCLC patients have been treated as patients with driver mutation-negative patients, which currently take immunotherapy or chemotherapy as an available first-line therapeutic options. Studies demonstrated that TMB, PD-L1 expression is related to KRAS status in NSCLC [32, 33]. It is generally believed that KRASKRAS mutant NSCLC patients can benefit from immunotherapy. The subgroup analysis of CheckMate 057 study and OAK study indicated that during the second-line treatment for KRAS mutant NSCLC patients, immune checkpoint inhibitors (ICIs) monotherapy had a higher OS benefit than docetaxel monotherapy [34, 35]. In the first-line treatment, clinical trials have also shown ICIs could benefit KRAS mutant NSCLC patients. Subgroup analysis of KEYNOTE-042 study [36] showed that in patients with KRAS mutant NSCLC and a PD-L1 tumour proportion score (TPS) of 1% or greater, pembrolizumab monotherapy could significantly improve ORR (56.7% vs 18.0%), PFS (12 months vs. 6 months; HR = 0.51), OS (28 months vs. 11 months; HR = 0.42) compared with platinum-containing chemotherapy; and in patients with KRAS G12C mutant NSCLC, pembrolizumab monotherapy could also improve ORR (66.7% vs 23.5%), PFS (15 months vs. 6 months; HR = 0.27), OS (NR months vs. 8 months; HR = 0.28) compared with platinum-containing chemotherapy. Subgroup analysis of KEYNOTE-189 study [37] showed that pembrolizumab combined with pemetrexed-platinum has improved ORR (40.7% vs 26.7%), PFS (9 months vs. 5 months; HR = 0.47), OS (21 months vs. 14 months; HR = 0.79) compared with pemetrexed-platinum chemotherapy in KRAS mutant non-squamous NSCLC; in patients with KRAS G12C mutant non squamous NSCLC, ORR (50.0% vs. 18.2%), PFS (11 months vs. 5 months; HR = 0.48) were improved, but not OS (18 months vs. 25 months; HR = 1.14). Subgroup analysis of IMpower150 study [38] revealed that both the atezolizumab/bevacizumab/carboplatin/paclitaxel (ABCP) (PFS: 8.1, HR 0.42; OS: 19.8, HR 0.50) and ACP arms (PFS: 4.8, HR 0.80; OS: 11.7 months, HR 0.63) demonstrated survival improvements compared with the BCP arm (PFS: 5.8 months; OS: 9.9 months) in KRAS mutant NSCLC; Across PD-L1 subgroups in mKRAS patients, in high PD-L1 expression (≥50%) subgroup, a similar prolonged OS was observed for patients treated with both ABCP (23.9 months; HR 0.40) and ACP (median 19.9 months; HR 0.35) compared with BCP (median, 9.9 months) ;In low PD-L1 expression (1- < 50%) and PD-L1-negative(< 1%) subgroups, the OS in ABCP arm (17.5 and 22.4 months) were longer than BCP arm (4.8 and 7.9 months), but OS in ACP arm (5.0 and 8.7 months) was similar to BCP arm. The exploratory analysis results of the above three clinical studies showed that for NSCLC patients with KRAS or KRAS G12C mutation, immunotherapy alone or combined chemotherapy ± antiangiogenic therapy can bring better clinical benefits to patients than chemotherapy ± antiangiogenic therapy. Of course, the KRAS mutant NSCLC sample size of the three clinical studies is small, and there are differences in patient characteristics, and all patients in Checkmate 057 study has a PD-L1 tumour proportion score (TPS) of 1% or greater. In addition, patients in IMpower150 were stratified by PD-L1 subgroups , and the analysis result suggested the survival benefit of immunotherapy combined with chemotherapy is similar to that of chemotherapy alone in patients with low (1- < 50%) and negative(< 1%) PD-L1 expression, while immunotherapy combined with chemotherapy plus antiangiogenic therapy can achieve significant survival benefit in these patients. Our real-world data also found that immunotherapy brought significant survival benefits compared to chemotherapy in PD-L1 expression ≥1% subgroup. And antiangiogenic therapy could benefit KRAS mutant NSCLC patients, especially non-G12C KRAS subgroup. Unfortunately, due to the small sample size and immature survival data, we did not compare survival benefits of different regimens in PD-L1 expression < 1% subgroup. More studies are needed in the future to observe the immunotherapeutic efficacy of KRAS mutant NSCLC patients, especially in patients with PD-L1 expression < 1%.
Despite recent advances in NSCLC treatment, a number of patients still receive platinum-based chemotherapy as first-line therapy in real world. KRAS mutated NSCLC patients was considered to have low ORR and poor prognosis as a whole when receiving chemotherapy [39,40,41], thus appropriate selection of chemotherapy regimen is vital to improve the prognosis of those patients. In our retrospective study, the platinum-paclitaxel regimen has significantly higher ORR than platinum-pemetrexed regimen, but its PFS and OS were far inferior. Moreover, the addition of antiangiogenic therapy can significantly improve the ORR, PFS and OS in KRAS mutant NSCLC received chemotherapy as first-line treatment. In terms of the combination of chemotherapy and antiangiogenic therapy, platinum-paclitaxel combined with antiangiogenic therapy achieved the highest ORR and DCR, while platinum-pemetrexed combined with antiangiogenic therapy had the longest PFS and OS. Renaud S et al. [42] analyzed 1190 KRAS mutated stage IV NSCLC patients receiving first-line platinum-based chemotherapy and found that compared with pemetrexed and vinorelbine group, the paclitaxel group had the highest ORR (P < 0.001) and significantly improved TTP (P < 0.001). The ORR of the paclitaxel combined with bevacizumab group was higher (P < 0.001), which was consistent with the results of this study. Mellema WW et al. [43] retrospectively analyzed 464 KRAS mutated NSCLC patients, and found patients treated with taxanes had a significant improved ORR (50%) compared to pemetrexed (21%) or gemcitabine (25%; P < 0.01). Patients treated with bevacizumab in addition to taxanes had the highest ORR (62%). The PFS was significantly improved in patients treated with taxanes compared to pemetrexed (P = 0.02), but not OS (P = 0.41). On the contrary, there was retrospective studies [44] containing 75 KRAS mutated NSCLC patients found pemetrexed-based chemotherapy had higher efficacy than taxane-based chemotherapy. Our study comprehensively analyzed the efficacy and prognosis of different chemotherapy regimens for KRAS mutant NSCLC and found that platinum-pemetrexed regimen was more likely to provide survival benefits to patients, although platinum-paclitaxel regimen increased response rate. Moreover, the addition of antiangiogenic therapy can significantly improve both response rate and survival benefits. Okada F et al. [45] found that RAS-gene mutations could increase KRAS-dependent VEGF expression, promoting tumor angiogenesis and growth. T Konishi et al. [46] confirmed the K-ras gene regulated VEGF expression in NSCLC. The association between KRAS and VEGF may contribute to the response of KRAS mutant patients to antiangiogenic therapy. Our data supported platinum-pemetrexed combined with antiangiogenic therapy as the most effective treatment in patients with KRAS mutation NSCLC.
However, our study has limitation of single centre and retrospective nature. Our study included patients from 2014 to 2022, the large time span of the study may affect the consistency of the treatment plan, including the choice of chemotherapy and antiangiogenic drugs, follow-up treatment after first-line progress. In conclusion, this study shows that immunotherapy compared to chemotherapy, treatment containing pemetrexed compared to treatment containing paclitaxel, treatment with antiangiogenic therapy compared to those without could obtain more benefits for the first-line treatment of KRAS mutant NSCLC patients,. Subgroup analysis revealed the benefits of immunotherapy compared to chemotherapy were applicable PD-L1 expressio≥1% subgroup, and antiangiogenic therapy could benefit non-G12C KRAS subgroup, but not G12C KRAS subgroup.. Antiangiogenic therapy should be considered in the non-G12C KRAS mutant NSCLC patients on larger and prospective clinical trials. Given the improved survival benefits, platinum-pemetrexed combined with antiangiogenic therapy may be the preferred chemotherapy regimen for KRAS mutant NSCLC patients.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- NSCLC:
-
Non-small cell lung cancer
- KRAS:
-
Kirsten rat sarcoma viral oncogene homolog
- FDA:
-
Food and drug administration
- PS:
-
Performance status
- RECIST:
-
Response evaluation criteria in solid tumors
- CR:
-
Complete response
- PR:
-
Partial response
- SD:
-
Stable disease
- PD:
-
Progressive disease
- ORR:
-
Objective response rate
- DCR:
-
Disease control rate
- PFS:
-
Progression-free survival
- OS:
-
Overall survival
- OR:
-
Odds ratio
- 95% CIs:
-
95% confidence intervals
- HR:
-
Hazard ratio
- ICIs:
-
Immune checkpoint inhibitors
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49.
Goldberg SB, Schlessinger J, Boyer JL, Herbst RS. A step towards treating KRAS-mutant NSCLC. The Lancet Oncology. 2013;14(1):3–5.
Jordan EJ, Kim HR, Arcila ME, Barron D, Chakravarty D, Gao J, et al. Prospective comprehensive molecular characterization of lung adenocarcinomas for efficient patient matching to approved and emerging therapies. Cancer discovery. 2017;7(6):596–609.
Kranenburg O. The KRAS oncogene: past, present, and future. Biochim Biophys Acta. 2005;1756(2):81–2.
Uras IZ, Moll HP, Casanova E. Targeting KRAS mutant non-small-cell lung Cancer: past, present and future. Int J Mol Sci. 2020;21(12).
Román M, Baraibar I, López I, Nadal E, Rolfo C, Vicent S, et al. KRAS oncogene in non-small cell lung cancer: clinical perspectives on the treatment of an old target. Mol Cancer. 2018;17(1):33.
Fell JB, Fischer JP, Baer BR, Blake JF, Bouhana K, Briere DM, et al. Identification of the clinical development candidate MRTX849, a covalent KRAS(G12C) inhibitor for the treatment of Cancer. J Med Chem. 2020;63(13):6679–93.
Skoulidis F, Li BT, Dy GK, Price TJ, Falchook GS, Wolf J, et al. Sotorasib for lung cancers with KRAS p.G12C mutation. N Engl J Med. 2021;384(25):2371–81.
Jänne PA, Riely GJ, Gadgeel SM, Heist RS, Ou SI, Pacheco JM, et al. Adagrasib in non-small-cell lung Cancer harboring a KRAS(G12C) mutation. N Engl J Med. 2022;387(2):120–31.
FDA Approves Sotorasib for KRAS G12C–Mutated NSCLC [https://www.onclive.com/view/fda-approves-sotorasib-for-kras-g12c-mutated-nsclc].
Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515(7528):563–7.
Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, Lee W, Yuan J, Wong P, Ho TS et al. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science (New York) 2015, 348(6230):124–128.
Tang H, Wang Y, Chlewicki LK, Zhang Y, Guo J, Liang W, et al. Facilitating T cell infiltration in tumor microenvironment overcomes resistance to PD-L1 blockade. Cancer Cell. 2016;30(3):500.
Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung Cancer. N Engl J Med. 2016;375(19):1823–33.
Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378(24):2288–301.
Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung Cancer. N Engl J Med. 2018;378(22):2078–92.
Cruz-Migoni A, Canning P, Quevedo CE, Bataille CJR, Bery N, Miller A, et al. Structure-based development of new RAS-effector inhibitors from a combination of active and inactive RAS-binding compounds. Proc Natl Acad Sci U S A. 2019;116(7):2545–50.
Ihle NT, Byers LA, Kim ES, Saintigny P, Lee JJ, Blumenschein GR, et al. Effect of KRAS oncogene substitutions on protein behavior: implications for signaling and clinical outcome. J Natl Cancer Inst. 2012;104(3):228–39.
Skoulidis F, Goldberg ME, Greenawalt DM, Hellmann MD, Awad MM, Gainor JF, et al. STK11/LKB1 mutations and PD-1 inhibitor resistance in KRAS-mutant lung adenocarcinoma. Cancer Discov. 2018;8(7):822–35.
Skoulidis F, Byers LA, Diao L, Papadimitrakopoulou VA, Tong P, Izzo J, et al. Co-occurring genomic alterations define major subsets of KRAS-mutant lung adenocarcinoma with distinct biology, immune profiles, and therapeutic vulnerabilities. Cancer Discov. 2015;5(8):860–77.
Zhuang X, Zhao C, Li J, Su C, Chen X, Ren S, et al. Clinical features and therapeutic options in non-small cell lung cancer patients with concomitant mutations of EGFR, ALK, ROS1, KRAS or BRAF. Cancer Med. 2019;8(6):2858–66.
El Osta B, Behera M, Kim S, Berry LD, Sica G, Pillai RN, et al. Characteristics and outcomes of patients with metastatic KRAS-mutant lung adenocarcinomas: the lung Cancer mutation consortium experience. J Thoracic Oncol. 2019;14(5):876–89.
Wang R, Zhang Y, Pan Y, Li Y, Hu H, Cai D, et al. Comprehensive investigation of oncogenic driver mutations in Chinese non-small cell lung cancer patients. Oncotarget. 2015;6(33):34300–8.
Bange E, Marmarelis ME, Hwang WT, Yang YX, Thompson JC, Rosenbaum J, et al. Impact of KRAS and TP53 co-mutations on outcomes after first-line systemic therapy among patients with STK11-mutated advanced non-small-cell lung Cancer. JCO Precis Oncol. 2019;3:PO.18.00326.
Dong ZY, Zhong WZ, Zhang XC, Su J, Xie Z, Liu SY, et al. Potential predictive value of TP53 and KRAS mutation status for response to PD-1 blockade immunotherapy in lung adenocarcinoma. Clin Cancer Res. 2017;23(12):3012–24.
Gu M, Xu T, Chang P. KRAS/LKB1 and KRAS/TP53 co-mutations create divergent immune signatures in lung adenocarcinomas. Ther Adv Med Oncol. 2021;13:17588359211006950.
Cha YJ, Kim HR, Lee CY, Cho BC, Shim HS. Clinicopathological and prognostic significance of programmed cell death ligand-1 expression in lung adenocarcinoma and its relationship with p53 status. Lung cancer (Amsterdam, Netherlands). 2016;97:73–80.
Guibert N, Barlesi F, Descourt R, Léna H, Besse B, Beau-Faller M, et al. Characteristics and outcomes of patients with lung Cancer harboring multiple molecular alterations: results from the IFCT study biomarkers France. J Thoracic Oncol. 2017;12(6):963–73.
Lee T, Lee B, Choi YL, Han J, Ahn MJ, Um SW. Non-small cell lung Cancer with concomitant EGFR, KRAS, and ALK mutation: Clinicopathologic features of 12 cases. J Pathol Transl Med. 2016;50(3):197–203.
Arbour KC, Jordan E, Kim HR, Dienstag J, Yu HA, Sanchez-Vega F, et al. Effects of co-occurring genomic alterations on outcomes in patients with KRAS-mutant non-small cell lung Cancer. Clin Cancer Res. 2018;24(2):334–40.
Uprety D, Adjei AA. KRAS: from undruggable to a druggable Cancer target. Cancer Treat Rev. 2020;89:102070.
Liu C, Zheng S, Jin R, Wang X, Wang F, Zang R, et al. The superior efficacy of anti-PD-1/PD-L1 immunotherapy in KRAS-mutant non-small cell lung cancer that correlates with an inflammatory phenotype and increased immunogenicity. Cancer Lett. 2020;470:95–105.
Calles A, Liao X, Sholl LM, Rodig SJ, Freeman GJ, Butaney M, et al. Expression of PD-1 and its ligands, PD-L1 and PD-L2, in smokers and never smokers with KRAS-mutant lung Cancer. J Thoracic Oncol. 2015;10(12):1726–35.
Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet (London, England). 2017;389(10066):255–65.
Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus Docetaxel in advanced nonsquamous non-small-cell lung Cancer. N Engl J Med. 2015;373(17):1627–39.
Herbst RS, Lopes G, Kowalski DM, Kasahara K, Wu YL, De Castro G, et al. Association of KRAS mutational status with response to pembrolizumab monotherapy given as first-line therapy for PD-L1-positive advanced non-squamous NSCLC in KEYNOTE-042. Ann Oncol. 2019;30.
Gadgeel S, Rodriguez-Abreu D, Felip E, Esteban E, Speranza G, Reck M, et al. KRAS mutational status and efficacy in KEYNOTE-189: Pembrolizumab (pembro) plus chemotherapy (chemo) vs placebo plus chemo as first-line therapy for metastatic non-squamous NSCLC. Ann Oncol. 2019;30:64–5.
West HJ, McCleland M, Cappuzzo F, Reck M, Mok TS, Jotte RM, et al. Clinical efficacy of atezolizumab plus bevacizumab and chemotherapy in KRAS-mutated non-small cell lung cancer with STK11, KEAP1, or TP53 comutations: subgroup results from the phase III IMpower150 trial. J Immunother Cancer. 2022;10(2):e003027.
Brady AK, McNeill JD, Judy B, Bauml J, Evans TL, Cohen RB, et al. Survival outcome according to KRAS mutation status in newly diagnosed patients with stage IV non-small cell lung cancer treated with platinum doublet chemotherapy. Oncotarget. 2015;6(30):30287–94.
Marabese M, Ganzinelli M, Garassino MC, Shepherd FA, Piva S, Caiola E, et al. KRAS mutations affect prognosis of non-small-cell lung cancer patients treated with first-line platinum containing chemotherapy. Oncotarget. 2015;6(32):34014–22.
Mellema WW, Dingemans AM, Thunnissen E, Snijders PJ, Derks J, Heideman DA, et al. KRAS mutations in advanced nonsquamous non-small-cell lung cancer patients treated with first-line platinum-based chemotherapy have no predictive value. J Thoracic Oncol. 2013;8(9):1190–5.
Renaud S, Guerrera F, Seitlinger J, Reeb J, Voegeli AC, Legrain M, et al. KRAS-specific amino acid substitutions are associated with different responses to chemotherapy in advanced non-small-cell lung Cancer. Clinical Lung Cancer. 2018;19(6):e919–31.
Mellema WW, Masen-Poos L, Smit EF, Hendriks LE, Aerts JG, Termeer A, et al. Comparison of clinical outcome after first-line platinum-based chemotherapy in different types of KRAS mutated advanced non-small-cell lung cancer. Lung cancer (Amsterdam, Netherlands). 2015;90(2):249–54.
Lei L, Wang WX, Yu ZY, Liang XB, Pan WW, Chen HF, et al. A real-world study in advanced non-small cell lung Cancer with KRAS mutations. Transl Oncol. 2020;13(2):329–35.
Okada F, Rak JW, Croix BS, Lieubeau B, Kaya M, Roncari L, et al. Impact of oncogenes in tumor angiogenesis: mutant K-ras up-regulation of vascular endothelial growth factor/vascular permeability factor is necessary, but not sufficient for tumorigenicity of human colorectal carcinoma cells. Proc Natl Acad Sci U S A. 1998;95(7):3609–14.
Konishi T, Huang CL, Adachi M, Taki T, Inufusa H, Kodama K, et al. The K-ras gene regulates vascular endothelial growth factor gene expression in non-small cell lung cancers. Int J Oncol. 2000;16(3):501–11.
Acknowledgements
Not applicable.
Funding
This study was supported by the grants of Beijing Municipal Science and Technology Commission (Z211100002921013) and Tongzhou Lianggao Talents Project (YH201920).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection were performed by YX, YG, YW, CZ and ZY. Data analysis and chart production was performed by YX, BL and TM. The first draft of the manuscript was written by YX and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of Beijing Chest Hospital, Capital Medical University. All methods were carried out in accordance with relevant guidelines and regulations. Due to the retrospective study design, the ethics committee of Ethics Committee of Beijing Chest Hospital, Capital Medical University approved a waiver of written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Liu, Y., Gao, Y., Wang, Y. et al. A single center analysis of first-line treatment in advanced KRAS mutant non-small cell lung cancer: real-world practice. BMC Cancer 22, 1175 (2022). https://doi.org/10.1186/s12885-022-10236-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-022-10236-9