Abstract
Background
Our review discuss (i) the findings from analyzed data that have examined KRAS, NRAS and BRAF mutations in patients with colorectal cancer (CRC) in North Africa and to compare its prevalence with that shown in other populations and (ii) the possible role of dietary and lifestyle factors with CRC risk.
Methods
Using electronic databases, a systematic literature search was performed for the KRAS, NRAS, and BRAF mutations in CRC patients from Morocco, Tunisia, Algeria and Lybia.
Results
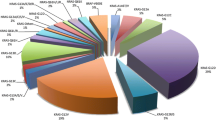
Seventeen studies were identified through electronic searches with six studies conducted in Morocco, eight in Tunisia, two in Algeria, and one in Libya. A total of 1843 CRC patients were included 576 (31.3%) in Morocco, 641 (34.8%) in Tunisia, 592 (32.1%) in Algeria, and 34 (1.8%) in Libya. Overall, the average age of patients was 52.7 years old. Patients were predominantly male (56.6%). The mutation rates of KRAS, NRAS and BRAF were 46.4%, 3.2% and 3.5% of all patients, respectively. A broad range of reported KRAS mutation frequencies have been reported in North Africa countries. The KRAS mutation frequency was 23.9% to 51% in Morocco, 23.1% to 68.2% in Tunisia, 31.4% to 50% in Algeria, and 38.2% in Libya. The G12D was the most frequently identified KRAS exon 2 mutations (31.6%), followed by G12V (25.4%), G13D (15.5%), G12C (10.2%), G12A (6.9%), and G12S (6.4%). G12R, G13V, G13C and G13R are less than 5%. There are important differences among North Africa countries. In Morocco and Tunisia, there is a higher prevalence of G12D mutation in KRAS exon 2 (≈50%). The most frequently mutation type in KRAS exon 3 was Q61L (40%). A59T and Q61E mutations were also found. In KRAS exon 4, the most common mutation was A146T (50%), followed by K117N (33.3%), A146P (8.3%) and A146V (8.3%).
Conclusion
KRAS mutated CRC patients in North Africa have been identified with incidence closer to the European figures. Beside established anti-CRC treatment, better understanding of the causality of CRC can be established by combining epidemiology and genetic/epigenetic on CRC etiology. This approach may be able to significantly reduce the burden of CRC in North Africa.
Similar content being viewed by others
Introduction
Colorectal cancer (CRC) is the third most commonly occurring cancer in men and the second most commonly occurring cancer in women. According to GLOBOCAN 2020 data, there are 1.15 million new cases of colon cancer, 0.7 million new cases of rectal cancers, and 50,000 new cases of anal cancer in 2020 globally [1]. With continuous progress, these numbers are predicted to increase to 1.92 million, 1.16 million, and 78,000 in 2040, respectively [2]. Globally it is one of the cancers whose incidence is increasing comprising 11% of all cancer diagnoses [3]. Over the world, the risk of CRC is highest and 3–4 times more common in countries with a very high human development index (HDI) than in countries with a low HDI [4]. CRC is considered one of the clearest markers of epidemiological and nutritional transition in societies undergoing socioeconomic development and transition to a lifestyle more typical of industrialized countries. Moreover, the data suggest the existence of a threshold at which CRC incidence stabilizes or declines according to the HDI [5].
Studies of CRC in the North of Africa (including Morocco, Algeria, Tunisia, and Libya) have shown rising trend in incidence rate. It was 7.1/100,000 [6]. When compared with sub-Saharan Africa (SSA), North Africa had the highest age-standardized incidence rates (ASR) of 8.66, while SSA had an ASR of 5.91 [7]. Libya reports the highest ASR for CRC with an incidence closer to the European figures. The ASR per 100,000 for CRC was 17.5 and 17.2 for males and females, respectively [8]. CRC ASR in Northern Tunisia was of 12.4 in 2009 (13.6 against 11.1, respectively, in men and women). This incidence increased during the period 1994–2009, from 6.4/100,000 in 1994 to 12.4/100,000 in 2009 [9]. CRC in young patients is more common in North Africa countries with a proportion of 11% in Morocco and much higher in the Middle East with 46% in UAE [10] and 25% in Egypt [11].
CRC is a complex and genetically heterogeneous disease with involving in oncogene and tumor suppressor genes, as well as genes involved in DNA damage recognition and repair. CRC includes also different categories of tumors based on their specific spectrum of mutations and molecular phenotype, driving various oncogenic signaling pathways [12]. The RAS/RAF/MEK/ERK/MAPK is the most well-known pathway in the pathogenesis of CRC. Mutations in genes of this pathway have been reported in about 50% of patients with CRC [13]. RAS and BRAF are members of the RAS/RAF/MEK/ERK/MAPK pathway which mediates cellular response to growth signals. Both RAS and RAF are members of multi-gene families and there are three RAS members (KRAS, NRAS and HRAS) and three RAF members (BRAF, RAF1 (a.k.a c-Raf) and ARAF). The KRAS proto-oncogene (locus 1p13.2) is the most commonly mutated RAS family member (75% of RAS mutations). The KRAS proto-oncogene encodes a protein (p21-ras) belonging to the family of GTP/GDP-binding proteins with GTPase activity and is involved in the transduction of mutagenic signals. KRAS gene mutations occur commonly in colon [14, 15]. The NRAS proto-oncogene (locus 1p13.2) is also a member of the RAS gene family which encodes proteins involved in signal transmission in cells and participates in the regulation of cell growth. NRAS gene mutations is associated with of CRC tumors [14, 15]. The BRAF gene on chromosome 7 (7q34) encodes for a serine/threonine protein kinase) that sends signals from outside of the cell to the nucleus that in turn drives the growth of a cell. BRAF is a downstream target of RAS, playing a pivotal role in the MAPK/ERK signaling pathway. Mutations of BRAF are found in CRCs [16] and are associated with a poor prognosis in stage II, III, and IV [17].
This systematic review aims to discuss (i) the findings from analyzed data that have examined KRAS, NRAS and BRAF mutations in patients with CRC in North Africa and to compare its prevalence with that shown in other populations and (ii) the possible role of dietary and lifestyle factors with CRC risk.
Materials and methods
We included articles that described the prevalence of KRAS, NRAS, or BRAF gene mutations in CRC patients in North Africa. Pub Med, Science Direct and Google Scholar were searched up to December 2021 for eligible studies using the following keywords: “colorectal cancer”, or “colon cancer”, or “rectum cancer”, or “RAS”, or “KRAS”, or “NRAS”, or “BRAF”, or “mutation”, or “oncogenic mutation”, or “oncogenic driver mutation”, or “activating mutation”, or “prevalence”, or “rate”, or “incidence”, or “frequency”.
An additional literature search was also conducted using North Africa and specific country names belonging to the considered region and any other variant names for any of North Africa countries (ex: Mediterranean countries, Maghreb, Arab population). We manually checked reference lists of the included studies and relevant reviews to identify additional studies. We also searched relevant abstracts reported in the most important multi-disciplinary societies of medical oncology such as the American Society of Clinical Oncology (ASCO) meetings to identify unpublished studies.
Eligible analytical study designs were prospective or retrospective cohort studies, cross-sectional, and case–control studies and were conducted in CRC patients. The study must give information on the prevalence of KRAS, NRAS, and BRAF mutations in a specific or selected coding region of KRAS, NRAS genes (exon 2: codon 12 and 13; exon 3: codon 59 and 61; exon 4: codon 117 and 146) and BRAF (exon 15). Studies published as original articles on human subjects, evaluating KRAS, NRAS, and BRAF mutation status and recording clinico-pathological features in CRC patients, and written in English, were included for analysis.
The following information data were abstracted from each of the eligible studies: 1) characteristics of the study (including publication year, country where the study was conducted, study type, sample size, number of subjects with mutation results); 2) characteristics of study participants (including gender, age); 3) characteristics of study outcomes (including mutation detection assay, type of mutation, mutation exon/codon numbers, mutation prevalence, whether mutation data were from primary or metastatic tumor, and whether mutation data were obtained from tissue or liquid biopsy using plasma); 4) clinicopathological characteristics (including disease stage, T stage, metastasis status, tumor site, tumor differentiation and tumor histology).
Letters, comments, and review articles or meta-analysis without original data, were excluded from analysis.
Results
The search of the databases yielded 17 relevant references which are closely related to defining the inclusion criteria and was included in this review. The retrieved articles describe studies conducted in Morocco (n = 6) [18,19,20,21,22,23], Tunisia (n = 8) [24,25,26,27,28,29,30,31], Algeria (n = 2) [32, 33], and Libya (n = 1) [34]. A total of 1843 patients with CRC were included for our analysis, including 576 (31.3%) in Morocco [18,19,20,21,22,23], 641 (34.8%) in Tunisia [24,25,26,27,28,29,30,31], 592 (32.1%) in Algeria [32, 33], and 34 (1.8%) in Libya [34]. The average age of patients was 52.7 years old [18,19,20, 22,23,24,25,26,27,28, 31,32,33], with 45.1% (260/577) [18, 20, 26, 29, 34] of patients diagnosed above the age of 50 years. Male patients were predominant in all of the considered studies, accounting for 56.6% (960/1697) [18,19,20, 22,23,24,25,26, 28, 29, 31,32,33,34].
From the specimens where tumor site information was available, 31.4% (344/1095) of them were in the rectum [22, 23, 26, 28, 29, 31,32,33,34], 30.1% (188/625) were in the right colon [18, 22, 24, 25, 28, 31], and 56.5% (353/625) in the left colon [18, 22, 24, 25, 28, 31]. The predominant histopathological type of CRC is adenocarcinoma (82.2%, 374/455) [18, 20, 25]. Almost half (58%, 302/521) of the tumors were reported to be in stage III and IV [18, 23, 24, 27, 33]. Baseline characteristics and clinico-pathologic of enrolled studies are summarized in Table 1.
Among the 17 studies, 13 (76.5%) [18,19,20,21,22,23,24,25, 27, 30,31,32,33] used FFPE tumor samples for KRAS, NRAS and BRAF mutation analysis. Both exon 2, 3, and 4 mutations of KRAS and NRAS genes were analyzed in 23.5% (4/17) [18, 19, 25, 32] of North Africa series, exon 2, 3 and 4 mutations of KRAS gene were evaluated in one study (5.9%, 1/17) [33], specific KRAS exons (exon 2 and/or exon3) were assessed in 58.8% (10/17) [20,21,22,23,24, 26,27,28,29,30] and NRAS exons (exon 2 and 3) in 11.8% (2/17) investigations [20, 24]. KRAS mutations in exon 2 codon 12 and 13 have been assessed in the most of the studies (94.1%, 16/17) [18,19,20,21,22,23,24,25,26,27,28,29,30, 32,33,34]. BRAF gene mutation was analyzed in 41.2% (7/17) studies [18, 19, 21,22,23, 31, 33].
Different molecular methods were used for KRAS, NRAS and BRAF mutations screening. Sequencing assay was broadly used, as it was used in 64.70% (11/17) studies [21, 23, 26,27,28,29,30,31,32,33,34]. Other methodologies described in the considered studies include Pyrosequencing [18, 20, 25], array based techniques Mass ARRAY [19, 22], amplification-refractory mutation system-PCR (ARMS-PCR) [24], Allele-specific PCR [32], End-point genotyping [22], High Resolution Melting [30] and Denaturating High Performance Liquid Chromatography [30]. The details of studies examining KRAS, NRAS and BRAF genes in North Africa are presented in Table 2.
The prevalence of KRAS, NRAS and BRAF mutations has been reported in 16 [18,19,20,21,22,23,24,25,26,27,28,29,30, 32,33,34], 6 [18,19,20, 24, 25, 32], and 7 [18, 19, 21,22,23, 31, 33] of the 17 included studies, respectively. In total, KRAS mutations were most frequently detected among North Africa patients with CRC, accounting for 41.7% (749/1795) [18,19,20,21,22,23,24,25,26,27,28,29,30, 32,33,34]. Tunisia highlights a wide range of KRAS mutations rates, ranging from 23.1% (12/52) [29] to 68.2% (88/129) [25], as compared with other countries from the region. Overall, KRAS Mutations were distributed among the different exons as follows: 95.9% (718/749) exon 2 [18,19,20,21,22,23,24,25,26,27, 29, 30, 32,33,34], 2.7% (13/481) exon 3 [18, 20, 25, 32], and 2.9% (14/481) exon 4 [18,19,20, 25] (Table3). Among mutations in exon 2, 79.7% (94/118) had single mutations in codon 12 [18, 22, 27, 34] and 20.3% (24/118) had point mutations in codon 13 [18, 22, 27, 34] (Table 4).
As seen in Table 5, the G12D was the most frequently identified exon 2 mutations (31.6%, 137/433) [18, 20,21,22,23,24,25,26,27, 29, 33, 34], followed by G12V (25.4%, 110/433) [18, 20,21,22,23,24,25,26, 29, 33, 34], G13D (15.5%, 67/433) [18, 20,21,22,23,24,25,26,27, 29, 33, 34], G12C (10.2%, 44/433) [18, 20,21,22,23,24,25,26,27, 29, 33, 34], G12A (6.9%, 30/433) [18, 20,21,22, 24, 27, 29, 33], G12S (6.4%, 28/433) [20, 23,24,25, 27, 33]. G12R, G13V, G13C and G13R are less than 5% [18, 20, 21, 24,25,26]. However, there are important differences among North Africa countries. In Morocco and Tunisia there is a higher prevalence of G12D mutation (50%) [22, 27]. The most frequently mutation type in exon 3 was Q61L (40%, 2/5) [18, 20]. A59T [20] and Q61E [25] mutations were also found in our review. In exon 4, the most common mutation was A146T (50%, 6/12) [18, 20, 25], followed by K117N (33.3%, 4/12) [18, 25], A146P (8.3%, 1/12) [18] and A146V (8.3%, 1/12) [18].
NRAS total mutations were identified in 3.2% (35/1090) [18,19,20, 24, 25, 32] tumor samples. Morocco highlights a wide range of NRAS mutations rates, ranging from 2% (1/51) to 5.3% (6/114) [19, 20]. A higher prevalence of NRAS mutations has been reported in Tunisia (7.3%, 7/96) [24]. Overall, the common mutation site of NRAS gene was located in exons 2 and 3, with 28.6% (10/35) [18, 24, 25] and 48.6% (17/35) [18, 20, 24, 25] of CRC patient (Table 3). NRAS mutations were more common in codon 61, accounting for 48.6% (17/35)[18, 20, 24, 25] (Table 4). The most common mutations were G12D in exons 2 and Q61K in exon 3 and accounted for 40% (4/10) [18, 25] and 35.3% (6/17) [18, 20, 25] of patients with CRC, respectively (Table 5).
The total mutation frequency in the BRAF gene was 2.8% (17/602) [18, 19, 21,22,23, 31, 33]. The highest frequency of BRAF mutations was found in Tunisia and reported in 8.3% (4/48) of patients with CRC [31]. The overall frequency of the BRAF V600E mutation was 2.8% (17/602) [18, 19, 21,22,23, 31, 33] (Tables 3 and 5).
Discussion
CRC is a pathologically and clinically heterogeneous malignancy. Phenotypic and molecular characteristics of CRC represents a significant key step in defining diagnosis, prognosis and treatment predictive value both in localized and in the setting of CRC [35]. Mutation detection in any of the genes involved in the RAS/RAF/MEK/ERK/MAPK pathway has been used to predict the outcomes of EGFR-targeted therapy for CRC. KRAS mutations were found in ≈33% of in the COSMIC dataset comprising 75,000 tested specimens samples. In other datasets based on small sample sizes (< 500), KRAS was found mutated in ≈40%-45% CRCs. Notably however, the private Foundation Medicine dataset comprising 13,336 colorectal samples reports a KRAS mutation frequency of ≈50% [36]. According to the COSMIC database (Catalogue of Somatic Mutations in Cancer, http://cancer.sanger.ac.uk/cosmic), mutations at codon 12 (G12A, G12V, G12S, G12R, G12C, G12D) are the most prominent (> 90%), followed by those affecting codon 13 (G13D, G13C), codon 61 (Q61L, Q61R, Q61H), codon 146 (A146T, A146V, A146P), and codon 117 (K117N). NRAS mutations are found in 5%-10% of CRC. NRAS is mutated in the same codon of KRAS particularly in exon 2 (3%-5%) and exon 3 (2%-6%). BRAF mutations are identified in about 8%-12% of CRC patients and 90% of all identified mutations are a T1799A transversion in exon 15, which results in a valine amino acid substitution (V600E) [37].
In our review, KRAS mutation was detected in the CRC tumor of 41.7% of the patients among whom 95.9% had a single mutation at exon 2, 2.7% at exon 3, and 2.9% at exon 4. Among mutations in KRAS exon 2, 79.7% of cases had single mutations in codon 12 and 20.3% of cases in codon 13. The G12D was the most frequently identified exon 2 mutations (31.6%), followed by G12V, G13D, G12C, G12A, and G12S. The most frequently mutation type in exon 3 was Q61L (40%). In exon 4, the most common mutation was A146T, K117N, A146P and A146V. NRAS total mutations were identified in 3.2% tumor samples. The common mutation site of NRAS gene was located in exons 2 and 3, with 28.6% and 48.6% of CRC patient. NRAS mutations were more common in codon 61, accounting for 48.6%. The most common mutations were G12D in exon 2 and Q61K in exon 3 and accounted for 40% and 35.3% of CRC patients, respectively. In BRAF gene, the overall frequency of the BRAF V600E mutation was 2.8%.
Our review of mutation prevalence among CRC patients in North Africa identified a significant difference in prevalence of both RAS (KRAS and NRAS) and BRAF mutations. Interestingly, our finding showed that the KRAS mutation rate was different with more investigation that therefore the geographic location and ethnicity/race can impact on the prevalence of KRAS mutation in CRC patients. The KRAS mutation frequency was 23.9% to 51% in a Moroccan population [18,19,20,21,22,23], 23.1% to 68.2% in a Tunisian population [24,25,26,27,28,29,30], 31.4% to 50% in an Algerian population [32, 33], and 38.2% in a Libyan population [34]. This broad range of reported KRAS mutation frequencies may be related with diverse ethnic background. Arabs and Berbers make up the overwhelming majority of the population throughout the North Africa today. However, throughout its history, North Africa has been the site of invasions and migratory waves of ethnic groups such as Phoenicians, Romans, Vandals, Arabs, Ottomans and Europeans.
Worldwide, a persistent finding in the literature is the substantial variation in KRAS mutation frequency between race/ethnic groups. In Nigerian, the frequency of CRC with KRAS mutations is 21% [38]. In Asian populations, KRAS mutations vary from 37.9% (Japan) to 52.7% (China) [39, 40], while Middle Eastern population reported frequencies of 32% (Saudi Arabia) and 48% (Iraq) [41, 42]. Data from USA showed KRAS mutation rate slightly higher among African American compared to Caucasians (47% versus 43%) [43]. Studies performed in Latin America report KRAS mutation prevalence ranging from 13% (Colombia and Venezuela) to 40% (Chile) [44]. The European data showed mutation rate in Greece (29%), Netherland (37%), Germany (39%) and Slovenia (46%), Spain (48%) [43, 45, 46]. In Turkey, the mutation frequency for the KRAS mutation gene has been reported to be in the range of 11% to 49.1% [47,48,49,50,51]. Turkey is known from the geographical location between Europe, the Middle East and the Caucasus region. Thus, Turkey is comprised of many ethnic groups with European, Middle Eastern, Caucasian or Asian origins. This difference can be primarily attributed to ethnicity [51]. The KRAS mutation prevalence may also vary in the same population. Studies from Japan with homogeneous ethnic groups found a wide range of KRAS mutation frequencies (9–71%) [52, 53]. Studies with heterogeneous ethnic groups, such as studies from USA, also reported various frequencies of KRAS mutation (14–83%) [54, 55]. The finding of novel KRAS and BRAF gene mutations in cancerous tissue obtained from Saudi CRC patients were typical of those observed elsewhere, and may be attributed to environmental factors and/or racial/ethnic variations due to genetic differences in Saudi Arabia [56, 57].
In agreement with COSMIC, G12D and G12V mutation frequencies were the most frequent mutations among all CRC patients with KRAS mutation in North Africa. However, there are important differences among North Africa populations. In Morocco and Tunisia there is a higher prevalence of G12D mutation (≈50%) [22, 27]. G12D mutation accounts for about 41% of all the G12 mutations [58, 59]. Generally, KRAS mutations are predominantly found in codon G12 and mutations in codon 12 diminish both inherent and GAP-mediated hydrolysis without affecting the rate of nucleotide exchange, except for G12C, which exhibits GTPase activity similar to that of wild type [60]. G12C has become a promising target for novel strategies to treat KRAS-mutant CRC [61], adding up a new role for routine KRAS testing. A series of novel inhibitors that act against G12C in its GDP-bound state have been developed (such as Sotorasib and Adagrasib) and provides new therapeutic strategies to improve patient outcomes.
The aspects that seem to play an important role in the incidence of KRAS gene mutations in CRC in North Africa populations are the change in dietary patterns and nutrient intakes. In Morocco, The major pattern of nutritional change includes a large increase in the consumption of high calorie diets and fatty foods. Consumption of high amounts of these foods was associated with increased body weight, BMI, and risk of overweight and obesity. Between 2001 and 2014, the consumption of high-fat diets increased following the intake of oils which increased on average by 5.4 L (22.4 L against 17.02 L per person per year) [62]. Significant associations were found between the highest intakes of red meats, cold meats, sausages and the risk of CRC in a case–control study on dietary risk factors for CRC in Morocco [63]. High levels of animal protein, acrylamide foods, and low levels of vitamin A consumption have been shown to be associated with increased risk of CRC tumors with KRAS mutations [64]. Polyunsaturated fatty acids (PUFA) may be positively associated with CRC risk by potentially generating G > A transitions in the KRAS oncogene in Moroccan population. El Asri et al., showed that an increase in the consumption of PUFA above 16.9 g/day was associated with an increase in the presence of KRAS mutations (Odds Ratio OR = 2.48, 95% Confidence Interval CI = 1.22–4.96) as compared to the reference group whose consumption was less than 16.9 g [65]. A high intake of PUFA, in particular linoleic acid, may be an important dietary risk factor for KRAS mutated colon tumors, possibly by generating G > A transitions or G > T or G > C transversions in the KRAS oncogene [66]. S Deoula et al., showed that consumption of red meat was positively associated with colon cancer (OR = 1.23, 95% CI = 1.05–1.44) and CRC risk in Moroccan patients (OR = 1.14, 95% CI = 1.02–1.27) [67]. In Tunisia, the CRC incidence has increased markedly from 1994 to 2009, and it is suggested that if no interventions are implemented it is going to double by 2024. It is relevant to highlight that an upward trend in obesity largely explains the increasing incidence of CRC in Tunisian population. The trend of the incidence of obesity had increased from 10.9% in 1998 to 26.9% in 2016 [68] and 80% of Tunisian aged over 15 years old did not consume enough fruits and vegetables per day [69]. A high total day meat consumption (> 100 g) was significantly associated with a high risk of CRC compared to low consumption (< 50 g) in Tunisian population [70]. The growth of meat consumption is likely to increase in Morocco and Tunisia. Between 1971 and 2020, total production of meat of Morocco grew substantially from 205,387 to 1.45 million tonnes rising at an increasing annual rate that reached a maximum of 23.12% in 1987 and then decreased to 2.70% in 2020. Production of meat of Tunisia increased from 19,400 thousand tonnes in 1971 to 41,600 thousand tonnes in 2020 growing at an average annual rate of 2.19%. In Algeria, occupational exposures showed a significant link with an increased risk of CRC, as did obesity, alcohol consumption, and passive smoking. Yogurt, cereals, sugar, butter, and margarine consumption were significant protective factors, while cheese, dried fruits, red meat, juice, and fizzy drink consumption was associated with increased CRC risk [71]. The World Health Organization (WHO) and International Agency for Research on Cancer (IARC) declared that over-intake of red meat and/or processed meat increases CRC risk [72]. A recent Global Health Data Exchange review indicated that red and processed meat intake accounts for 1.77% and 1.18%, respectively, of worldwide CRC mortality, respectively [73]. The World Cancer Research Fund International Continuous Update Project showed that red meat or processed meats and alcohol consumption increase CRC risk and that food containing dietary fibre and dairy products decrease the CRC risk [74].
Red meat consumption may increase colon cancer risk by inducing the endogenous production of N-nitroso compounds and their precursors, which may induce KRAS mutations [70, 71]. Analysis of 900 CRCs with whole exome sequencing and epidemiologic annotations revealed an alkylating mutational signature that was associated with red meat consumption and distal tumor location, as well as predicted to target KRAS G12D/G13D [72]. Heterocyclic amines (HCAs) and polycyclic aromatic hydrocarbons (PAHs) formed during high-temperature meat cooking are also an important pathway for environmental colon cancer [73, 74]. HCAs and PAHs have been found to be mutagenic after they are metabolized by specific enzymes in the body. Previous studies have identified that the activity of these enzymes may be relevant to the cancer risks associated with exposure to these compounds [75, 76].
Interestingly, the use of antibiotics such as Fluoroquinolone and the third-generation cephalosporins was increasing in North Africa [77]. An increasing of antibiotic consumption was associated with an increasing CRC risk, particularly when used commonly [78] and colon cancer pathogenesis across all age-groups in a large population-based case–control study of patients with early onset-CRC [79]. Antibiotics are known to influence the gut microbiome [80, 81], which has been implicated in CRC or tumor progression, possibly through bacterial involvement in nutrient metabolism and direct interaction with gut mucosa [82]. In the context of CRC, bacteria have been shown to play a role in cell signaling [83,84,85]. In a KRAS-specific context, the role of KRAS signaling on the tumor microbiome is still to be determined, even though, it is accepted that the exposure to bacterial can shape the development of CRC of which KRAS can be one major player [86, 87]. Nevertheless, in KRAS-driven CRCs, it has been shown that genotoxic stress and some other factors, including metabolites produced by the microbiota, can facilitate genetic and epigenetic changes leading to carcinogenesis [88]. A priori, computational modeling is a promising approach for studying genotype-to-metabolic-phenotype relations or microbe–microbe and host–microbe metabolic interactions in CRC [89]. Microbiome modulation is one of the most prospective new strategies in medicine to improve the gut health in individuals and is likely to play a key role in the outcome of colorectal surgery in North Africa.
Modifiable dietary and lifestyle risk factors could prevent most cases of CRC and. Up to 47% of CRC cases could be prevented by staying physically active, maintaining a healthy body weight and eating a healthy diet [90]. In North Africa, dietary and lifestyle factors are one of the most promising approaches to reduce the occurrence and progression of CRC. Dietary and lifestyle factors may not only play a role in causing mutations and epigenetic modifications, but also in enhancing tumor growth in tissues that have already acquired specific epigenetic aberrations. There may be direct causal associations between diet and lifestyle factors and molecular changes in CRC, and establishing this is important for prevention strategies, and increasing the ability to better predict disease progression and prognosis [91].
Beside established anti-CRC treatment, better understanding of the causality of CRC can be established by combining epidemiology and genetic/epigenetic on CRC etiology. This approach may be able to significantly reduce the burden of disease in North Africa population. Furthermore, the government should develop policy on CRC prevention and public health programs which may serve as a feasible setting to increase public awareness on lifestyle risk factors.
Availability of data and materials
The datasets generated and analyzed during the current study are available in the [Prevalence and Patterns of Mutations in RASRAFMEKERKMAPK Signaling in North Africa Tables] repository.Pub med (Open access): https://pubmed.ncbi.nlm.nih.gov/. Science Direct (Open access): https://www.sciencedirect.com/. Google Scholar (Open access): https://scholar.google.com/.
Abbreviations
- ASCO:
-
American Society of Clinical Oncology
- ASR:
-
Age-Standardized Incidence Rates
- BMI:
-
Body Mass Index
- CI:
-
Confidence Interval
- COSMIC:
-
Catalogue Of Somatic Mutations In Cancer
- CRC:
-
Colorectal Cancer
- EGFR:
-
Epidermal Growth Factor Receptor
- ERK:
-
Extracellular-signal-Regulated Kinase
- GDP:
-
Guanosine Bi-Phosphate
- GLOBOCAN:
-
Global Cancer Observatory: CANCER TODAY
- GTP:
-
Guanosine Tri-Phosphate
- HCAs:
-
Heterocyclic Amines
- HDI:
-
High Human Development Index
- HRAS:
-
Harvey Rat Sarcoma
- IARC:
-
International Agency for Research on Cancer
- KRAS:
-
Kirsten Rat Sarcoma
- MAPK:
-
Mitogen-Activated Protein kinase
- MEK:
-
Mitogen-Activated Protein kinase kinase
- NRAS:
-
Neuroblastoma Rat Sarcoma
- OR:
-
Odds Ratio
- PAHs:
-
Polycyclic Aromatic Hydrocarbons
- PUFA:
-
Polyunsaturated fatty acids
- RAF:
-
Rapidly Accelerated Fibrosarcoma
- RAS:
-
Rat Sarcoma
- SSA:
-
Sub-Saharan Africa
- UAE:
-
United Arab Emirates
- USA:
-
United States of America
- WHO:
-
World Health Organization
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209–49.
Xi Y, Xu P. Global colorectal cancer burden in 2020 and projections to 2040. Transl Oncol. 2021;14:101174.
Wong MCS, Huang J, Lok V, Wang J, Fung F, Ding H, et al. Differences in Incidence and Mortality Trends of Colorectal Cancer Worldwide Based on Sex, Age, and Anatomic Location. Clin Gastroenterol Hepatol. 2021;19:955-966.e61.
Rawla P, Sunkara T, Barsouk A. Epidemiology of colorectal cancer: incidence, mortality, survival, and risk factors. Prz Gastroenterol. 2019;14:89–103.
Fidler MM, Bray F, Vaccarella S, Soerjomataram I. Assessing global transitions in human development and colorectal cancer incidence. Int J Cancer. 2017;140:2709–15.
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424.
Arhin N, Ssentongo P, Taylor M, Olecki EJ, Pameijer C, Shen C, et al. Age-standardised incidence rate and epidemiology of colorectal cancer in Africa: a systematic review and meta-analysis. BMJ Open. 2022;12:e052376.
Bodalal Z, Azzuz R, Bendardaf R. Cancers in Eastern Libya: first results from Benghazi Medical Center. World J Gastroenterol. 2014;20:6293–301.
Khiari H, Ben Ayoub HW, Ben Khadhra H, Hsairi M. Colorectal Cancer Incidence Trend and Projections in Tunisia (1994–2024). Asian Pac J Cancer Prev. 2017;18:2733–9.
Gado A, Ebeid B, Abdelmohsen A, Axon A. Colorectal cancer in Egypt is commoner in young people: Is this cause for alarm? Alex J Med. 2014;50:197–201.
Fayadh MH, Sabih SA, Quadri HA. 8 years observational study on colorectal cancer in UAE. J Coloproctology (Rio de Janeiro). 2019;39:394–5.
Miele E, Abballe L, Spinelli GP, Besharat ZM, Catanzaro G, et al. BRAF mutant colorectal cancer: ErbB2 expression levels as predictive factor for the response to combined BRAF/ErbB inhibitors. BMC Cancer. 2020;20:129.
Gong S, Xu D, Zhu J, Zou F, Peng R. Efficacy of the MEK Inhibitor Cobimetinib and its Potential Application to Colorectal Cancer Cells. Cell PhysiolBiochem. 2018;47:680–93.
Cefalì M, Epistolio S, Palmarocchi MC, Frattini M, De Dosso S. Research progress on KRAS mutations in colorectal cancer. J Cancer Metastasis Treat. 2021;7:26.
Prior IA, Hood FE, Hartley JL. The Frequency of Ras Mutations in Cancer. Cancer Res. 2020;80:2969–74.
Luu LJ, and Price TJ. BRAF Mutation and Its Importance in Colorectal Cancer. In: Segelov E, editor. Advances in the Molecular Understanding of Colorectal Cancer. IntechOpen.2019.
Markowitz SD, Bertagnolli MM. Molecular origins of cancer: Molecular basis of colorectal cancer. N Engl J Med. 2009;361:2449–60.
El Agy F, El Bardai S, El Otmani I, Benbrahim Z, Karim MH, Mazaz K, et al. Mutation status and prognostic value of KRAS and NRAS mutations in Moroccan colon cancer patients: A first report. PLoS One. 2021;16:e0248522.
Houssaini MS, Damou M, Guessous F, Ismaili N. Mutational status in Ras/Raf/MAPK signaling pathway in Moroccan colorectal cancer patients. Ann Oncol. 2020;8(4):S206.
Dehbi H, Aitboujmia OK, Benayad S, El Idrissi HH, Karkouri M. KRAS and NRAS Mutations in Moroccan Patients with Colorectal Cancer. J Cancer Biol Therap. 2019;5(1):254–9.
Jadda H, El Fahime M, Kettani F, Bellaoui H. A simple method for detection of KRAS and BRAF hotspots mutations in patients with colorectal cancer. Int J Pharm Pharm Sci. 2014;2:448–52.
Marchoudi N, HassaniJoutei HA, Jouali F, Fekkak J, Rhaissi H. Distribution of KRAS and BRAF mutations in Moroccan patients with advanced colorectal cancer. Pathol Biol (Paris). 2013;61:273–6.
Bennani B, Gilles S, Fina F, Nanni I, Ibrahimi SA, Riffi AA, et al. Mutation analysis of BRAF exon 15 and KRAS codons 12 and 13 in Moroccan patients with colorectal cancer. Int J Biol Markers. 2010;25:179–84.
Ounissi D, Weslati M, Boughriba R, Hazgui M, Bouraoui S. Clinicopathological characteristics and mutational profile of KRAS and NRAS in Tunisian patients with sporadic colorectal cancer. Turk J Med Sci. 2021;51:148–58.
Jouini R, Ferchichi M, BenBrahim E, Ayari I, Khanchel F, Koubaa W, et al. KRAS and NRAS pyrosequencing screening in Tunisian colorectal cancer patients in 2015. Heliyon. 2019;5:e01330.
Chaar I, Ounissi D, Boughriba R, Ben Ammar A, Sameh A, Khalfallah T, et al. Implication of K-ras and p53 in colorectal cancer carcinogenesis in Tunisian population cohort. Tumour Biol. 2014;35:7163–75.
Aissi S, Buisine MP, Zerimech F, Kourda N, Moussa A, Manai M, et al. KRAS mutations in colorectal cancer from Tunisia: relationships with clinicopathologic variables and data on TP53 mutations and microsatellite instability. Mol Biol Rep. 2013;40:6107–12.
Ouerhani S, Bougatef K, Soltani I, Elgaaied AB, Abbes S, Menif S. The prevalence and prognostic significance of KRAS mutation in bladder cancer, chronic myeloid leukemia and colorectal cancer. Mol Biol Rep. 2013;40:4109–14.
Sammoud S, Khiari M, Semeh A, Amine L, Ines C, Amira A, et al. Relationship between expression of ras p21 oncoprotein and mutation status of the K-ras gene in sporadic colorectal cancer patients in Tunisia. Appl Immunohistochem Mol Morphol. 2012;20:146–52.
Bougatef K, Coulet F, Rouissi K, Kourda N, Omrane I, Marrakchi R, et al. KRAS mutation detection in Tunisian sporadic coloractal cancer patients with direct sequencing, high resolution melting and denaturating high performance liquid chromatography. Cancer Biomark. 2010;8:331–40.
Bougatef K, Ouerhani S, Moussa A, Kourda N, Coulet F, Colas C, et al. Prevalence of mutations in APC, CTNNB1, and BRAF in Tunisian patients with sporadic colorectal cancer. Cancer Genet Cytogenet. 2008;187:12–8.
Mazouzi K. Genomic study of KRAS/NRAS mutations of metastatic colorectal cancer in eastern Algeria [abstract]. In: Proceedings of the AACR International Conference: New Frontiers in Cancer Research; 2017 Jan 18–22; Cape Town, South Africa. Philadelphia (PA): AACR; Cancer Res. 2017;77 Suppl 22:Abstract nr A20.
Boudida-Berkane K, Benchaa H, AitYounes S, Ait Kaci H, Oukkal M, Mahfouf H, et al. Molecular Analysis and Clinicopathologic Features of Advanced Colorectal Cancer in Algerian Patients. Edorium J Tumor Bio. 2016;3:1–8.
Abudabous A, Drah M, Aldehmani M, Parker I, Alqawi O. KRAS mutations in patients with colorectal cancer in Libya. Mol ClinOncol. 2021;15:197.
Sepulveda AR, Hamilton SR, Allegra CJ, Grody W, Cushman-Vokoun AM, Funkhouser WK, et al. Molecular Biomarkers for the Evaluation of Colorectal Cancer: Guideline From the American Society for Clinical Pathology, College of American Pathologists, Association for Molecular Pathology, and American Society of Clinical Oncology. J Mol Diagn. 2017;19:187–225.
Serebriiskii IG, Connelly C, Frampton G, Newberg J, Cooke M, Miller V, et al. Comprehensive characterization of RAS mutations in colon and rectal cancers in old and young patients. Nat Commun. 2019;10:3722.
Cantwell-Dorris ER, O’Leary JJ, Sheils OM. BRAFV600E: implications for carcinogenesis and molecular therapy. Mol Cancer Ther. 2011;10:385–94.
Abdulkareem FB, Sanni LA, Richman SD, Chambers P, Hemmings G, Grabsch H, et al. KRAS and BRAF mutations in Nigerian colorectal cancers. West Afr J Med. 2012;31:198–203.
Kawazoe A, Shitara K, Fukuoka S, Kuboki Y, Bando H, Okamoto W, et al. A retrospective observational study of clinicopathological features of KRAS, NRAS, BRAF and PIK3CA mutations in Japanese patients with metastatic colorectal cancer. BMC Cancer. 2015;15(1):258.
Fang G, Gong H, Zhao H, Chen J, Zhang Y, Zhang L, et al. Mutation Status and Prognostic Values of KRAS, NRAS, BRAF and PIK3CA in 353 Chinese Colorectal Cancer Patients. Sci Rep. 2018;8(1):6076.
Zekri J, Rizvi A, Al-Maghrabi J, and bin Sadiq B. K-Ras in Colorectal Cancer Tumors From Saudi Patients: Frequency, Clinco-Pathological Association and Clinical Outcome. Open Colorectal Cancer J. 2012;5(1):22–27.
Al-Allawi NA, Ismaeel AT, Ahmed NY, Merza NS. The Frequency and Spectrum of K-Ras Mutations among Iraqi Patients with Sporadic Colorectal Carcinoma. Indian J Cancer. 2012;49(1):163–8.
Itrat M, Essam A, Al Bahrani BJ. KRAS Mutations: Does Ethnicity Play a Role? J Clin Oncol. 2014;32:e14628–e14628.
Sanchez-Ibarra HE, Jiang X, Gallegos-Gonzalez EY, Cavazos-González AC, Chen Y, Morcos F, et al. KRAS, NRAS, and BRAF mutation prevalence, clinicopathological association, and their application in a predictive model in Mexican patients with metastatic colorectal cancer: A retrospective cohort study. PLoS One. 2020;15(7):e0235490.
Symvoulakis EK, Zaravinos A, Panutsopulos D, Zoras O, Papalambros E, Sigala F, et al. Highly conserved sequence of exon 15 BRAF gene and KRAS codon 12 mutation among Greek patients with colorectal cancer. Int J Biol Markers. 2007;22:12–8.
Herreros-Villanueva M, Maximiliano R, Claver M, Muñiz P, Lastra E, García-Girón C, et al. KRAS, BRAF, EGFR and HER2 Gene Status in a Spanish Population of Colorectal Cancer. Mol Biol Rep. 2011;38:1315–20.
Selcukbiricik F, Erdamar S, Ozkurt CU, Molinas Mandel N, Demirelli F, Ozguroglu M, et al. The role of K-RAS and B-RAF mutations as biomarkers in metastatic colorectal cancer. J BUON. 2013;18:116–23.
Akkiprik M, Celikel CA, Düşünceli F, Sönmez O, Güllüoğlu BM, Sav A, et al. Relationship between overexpression of ras p21 oncoprotein and K-ras codon 12 and 13 mutations in Turkish colorectal cancer patients. Turk J Gastroenterol. 2008;19:22–7.
Demiralay E, Saglam Y, Altaca G, et al. The Frequency of K-ras Mutation in Colorectal Adenocarcinomas with Absence of Distant Metastasis at Diagnosis. Surg Sci. 2012;3:111–5.
Ozen F, Ozdemir S, Zemheri E, Hacimuto G, Silan F, Ozdemir O. The proto-oncogene KRAS and BRAF profiles and some clinical characteristics in colorectal cancer in the Turkish population. Genet Test Mol Biomarkers. 2013;17:135–9.
Baskin Y, Calibasi G, Amirfallah A, Dagdeviren YK, Canda AE, Sarioglu S, et al. KRAS and BRAF mutation frequencies in a series of Turkish colorectal cancer patients. Transl Cancer Res. 2014;3:160–6.
Mitomi H, Nakamura T, Ihara A, Otani Y, Sada M, Igarashi M, et al. Frequent Ki-ras mutations and transforming growth factor-alpha expression in adenocarcinomas of the small intestine: report of 7 cases. Dig Dis Sci. 2003;48:203–9.
Nishiyama K, Yao T, Yonemasu H, Yamaguchi K, Tanaka M, Tsuneyoshi M. Overexpression of p53 protein and point mutation of K-ras genes in primary carcinoma of the small intestine. Oncol Rep. 2002;9:293–300.
Staudacher JJ, Yazici C, Bul V, Zeidan J, Khalid A, Xia Y, et al. Increased Frequency of KRAS Mutations in African Americans Compared with Caucasians in sporadic colorectal cancer. Clin Transl. 2017;8:e124.
Fu T, Guzzetta AA, Jeschke J, Vatapalli R, Dave P, Hooker CM, et al. KRAS G˃A mutation favors poor tumor differentiation but may not be associated with prognosis in patients with curatively resected duodenal adenocarcinoma. Int J Cancer. 2013;132:2502–9.
Naser WM, Shawarby MA, Al-Tamimi DM, Seth A, Al-Quorain A, Nemer AM, et al. Novel KRAS gene mutations in sporadic colorectal cancer. PLoS One. 2014;9:e113350.
Rasool M, Natesan Pushparaj P, Buhmeida A, Karim S. Mutational spectrum of BRAF gene in colorectal cancer patients in Saudi Arabia. Saudi J Biol Sci. 2021;28:5906–12.
Hobbs GA, Wittinghofer A, Der CJ. Selective targeting of the KRAS G12C mutant: kicking KRAS when it’s down. Cancer Cell. 2016;29:251–3.
Roa I, Sánchez T, Majlis A, Schalper K. KRAS gene mutation in colorectal cancer. Rev Med Chile. 2013;141:1166–72.
Hunter JC, Manandhar A, Carrasco MA, Gurbani D, Gondi S, Westover KD. Biochemical and structural analysis of common Cancer-associated KRAS mutations. Mol Cancer Res. 2015;13:1325–35.
Hong DS, Fakih MG, Strickler JH, Desai J, Durm GA, Shapiro GI, et al. KRAS (G12C) inhibition with Sotorasib in advanced solid tumors. N Engl J Med. 2020;383:1207–17.
Maaroufi, Y. Enquête Nationale sur la Consommation et Les Dépenses des Ménages Site Institutionnel du Haut-Commissariat au Plan du Royaume du Maroc. 2014. https://www.hcp.ma/Enquete-nationale-sur-la-consommation-et-les-depensesdes-menages_a95.html. Accessed 29 Dec 2021.
Imad FE, Drissi H, Tawfiq N, Bendahhou K, Benider A, Radallah D. A case-control study on dietary risk factors for colorectal cancer in Morocco. Pan Afr Med J. 2020;35:59.
El Asri A, Zarrouq B, El Kinany K, Bouguenouch L, Ouldim K, El Rhazi K. Associations between nutritional factors and KRAS mutations in colorectal cancer: a systematic review. BMC Cancer. 2020;20:696.
El Asri A, Ouldim K, Bouguenouch L, Sekal M, Moufid FZ, Kampman E, et al. Dietary Fat Intake and KRAS Mutations in Colorectal Cancer in a Moroccan Population. Nutrients. 2022;14:318.
Brink M, de Goeij AFPM, Weijenberg MP, Guido MJM, Roemen GMJM, Lentjens MHFM, et al. K-ras oncogene mutations in sporadic colorectal cancer inThe Netherlands Cohort Study. Carcinogenesis. 2003;24:703–10.
Mint Sidi Douala M, El Kinany K, Hatime Z, Boudouaya HA, El Rhazi K. Meat and Colorectal Cancer in Middle Eastern and North African Countries: Update of Literature Review. Public Health Rev. 2020;41:7.
El Ati J, Traissac P, Beji C, Oueslati A, Gaigi S, Kolsteren, P, et al. Overweight and obesity in Tunisia (Tahina project): trends over the last 25 years. Ann Nutr Metab. 2007;51 Suppl 1:251–406.
Saidi O, Zoghlami N, Skhiri H, Hsairi M, Skhiri A, Ben Mansour N, et al. Tunisian health examination survey-2016. Ministère de la Santé, Institut National de la Santé: République Tunisienne; 2019.
Gharbi I, Letaief F, Dadaa Z, Yahyaoui Y, Gabsi A, Maghrebi H, et al. The Consumption of Red and Processed Meat and The Risk of Colorectal Cancer: A Case-Control Study among the Tunisian Population. Tunis Med. 2020;98:726–9.
Negrichi S, Taleb S. Hereditary, environmental, and dietary risk factors of colorectal cancer: a case-control study in the Algerian East. Environ Sci Pollut Res Int. 2021;28:12372–81.
IARC Working Group. Red Meat and Processed Meat. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Lyon (France): International Agency for Research on Cancer; 2018.
Mattiuzzi C, Lippi G. Epidemiologic Burden of Red and Processed Meat Intake on Colorectal Cancer Mortality. Nutr Cancer. 2020;73:562–7.
Vieira AR, Abar L, Chan DSM, Vingeliene S, Polemiti E, Stevens C, et al. Foods and beverages and colorectal cancer risk: a systematic review and meta-analysis of cohort studies, an update of the evidence of the WCRF-AICR Continuous Update Project. Ann Oncol. 2017;28:1788–802.
Cai T, Yao L, Turesky RJ. Bioactivation of heterocyclic aromatic amines by UDP glucuronosyltransferases. Chem Res Toxicol. 2016;29:879–91.
Melkonian SC, Daniel CR, Ye Y, Tannir NM, Karam JA, Matin SF, et al. Gene-environment interaction of genome-wide association study-identified susceptibility loci and meat-cooking mutagens in the etiology of renal cell carcinoma. Cancer. 2016;122:108–15.
Browne AJ, Chipeta MG, Haines-Woodhouse G, Kumaran EPA, Hamadani BHK, Zaraa S, et al. Global antibiotic consumption and usage in humans, 2000–18: a spatial modelling study. Lancet Planet Health. 2021;5:e893–904.
Dik VK, van Oijen MG, Smeets HM, Siersema PD. Frequent Use of Antibiotics Is Associated with Colorectal Cancer Risk: Results of a Nested Case-Control Study. Dig Dis Sci. 2016;61:255–64.
McDowell R, Perrott S, Murchie P, Cardwell C, Hughes C, Samuel L. Oral antibiotic use and early-onset colorectal cancer: findings from a case-control study using a national clinical database. Br J Cancer. 2022;126:957–67.
Falony G, Joossens M, Vieira-Silva S, Wang J, Darzi Y, Faust K, et al. Population-level analysis of gut microbiome variation. Science. 2016;352:560–4.
Francino MP. Antibiotics and the human gut microbiome: dysbioses and accumulation of resistances. Front Microbiol. 2016;6:1543.
Zhu Q, Gao R, Wu W, Qin H. The role of gut microbiota in the pathogenesis of colorectal cancer. Tumour Biol. 2013;34:1285–300.
Burns MB, Lynch J, Starr TK, Knights D, Blekhman R. Virulence genes are a signature of the microbiome in the colorectal tumor microenvironment. Genome Med. 2015;7(1):55.
Flemer B, Lynch DB, Brown JM, Jeffery IB, Ryan FJ, Claesson MJ, et al. Tumour-associated and non-tumour-associated microbiota in colorectal cancer. Gut. 2017;66:633–43.
Rubinstein MR, Wang X, Liu W, Hao Y, Cai G, Han YW. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/beta-catenin signaling via its FadA adhesin. Cell Host Microbe. 2013;14:195–206.
Mima K, Sukawa Y, Nishihara R, Qian ZR, Yamauchi M, Inamura K, et al. Fusobacterium nucleatum and T cells in colorectal carcinoma. JAMA Oncol. 2015;1:653–61.
Zou S, Fang L, Lee MH. Dysbiosis of gut microbiota in promoting the development of colorectal cancer. Gastroenterol Rep (Oxf). 2018;6:1–12.
Guerriero JL. Macrophages: their untold story in T cell activation and function. Int Rev Cell Mol Biol. 2019;342:73–93.
Ternes D, Karta J, Tsenkova M, Wilmes P, Haan S, Letellier E. Microbiome in Colorectal Cancer: How to Get from Meta-omics to Mechanism? Trends Microbiol. 2020;28(8):698.
World Cancer Research Fund/American Institute for Cancer Research. Continuous Update Project Report: Diet. Physical Activity and Colorectal Cancer: Nutrition; 2017.
Hughes LAE, Simons CCJM, van den Brandt PA, van Engeland M, Weijenberg MP. Lifestyle, Diet, and Colorectal Cancer Risk According to (Epi)genetic Instability: Current Evidence and Future Directions of Molecular Pathological Epidemiology. Curr Colorectal Cancer Rep. 2017;13:455–69.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
MJ, AL, and WB have conceived the study, exploited data, coordinated and drafted the paper. SB and SEZ participated in the designed. TM, HEA, RA, RT, HEH, LB and, SE generated data and involved in data analyses. AAA, AB, MM, MRT, YS, IAL, MI, AEN, SEK, and AB have read and agreed to the published version of the manuscript. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Jafari, M., Laraqui, A., Baba, W. et al. Prevalence and patterns of mutations in RAS/RAF/MEK/ERK/MAPK signaling pathway in colorectal cancer in North Africa. BMC Cancer 22, 1142 (2022). https://doi.org/10.1186/s12885-022-10235-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-022-10235-w