Abstract
Background
Pulmonary pleomorphic carcinoma (PPC) is a rare type of non-small cell lung cancer characterized by high malignancy and a poor prognosis. PPC is associated with a high frequency of postoperative relapse, and shows resistance to chemotherapy. The high malignancy of cancers is associated with genomic instability, which is related to mutations of tumor suppressor genes, such as tumor protein p53 (TP53) and ataxia-telangiectasia mutated (ATM). In addition, signaling pathways involving the oncogenes such as phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA) and epidermal growth factor receptor (EGFR) are associated with resistance to chemotherapy. However, the association of PPC with these gene mutations remains unknown. We investigated the impact of TP53, ATM, PIK3CA, and EGFR mutations on the postoperative prognosis of PPC.
Methods
Fifty-five patients with PPC who underwent complete resection were studied. A gene mutation analysis was performed using next-generation sequencing. Postoperative overall survival of patients with gene mutations was evaluated using a multivariable Cox proportional hazards model in which the explanatory variables were the presence of each gene mutation, and the confounding factors were pathological stage and age. The robustness of the results was evaluated by a sensitivity analysis.
Results
The frequencies of pathogenic mutations in TP53, ATM, PIK3CA, and EGFR were 47, 0, 7, and 9%, respectively. A multivariable analysis adjusted for pathological stage and age showed a significant difference for only PIK3CA mutations. The hazard ratio (HR) for overall survival in cases with pathogenic mutations of PIK3CA for wild type or non-pathogenic mutations was 4.5 (95% confidence interval [CI] 1.1–18.8). Likewise, sensitivity analyses adjusted for pathological stage and sex (HR, 7.5; 95% CI 1.7–32.4) and for age and sex (HR, 5.4; 95% CI 1.4–21.7) resulted in similar findings. Although three patients with pathogenic mutations of PIK3CA that recurred postoperatively were treated by chemotherapy or immunotherapy, they survived for less than 2 years.
Conclusions
The postoperative prognosis of PPC with PIK3CA pathogenic mutations is particularly poor. Pathogenic mutations of PIK3CA may be a postoperative prognostic marker. Inhibition of signaling pathways associated with PIK3CA mutations may also be a target for chemotherapy after relapse of PPC.
Similar content being viewed by others
Background
Pulmonary pleomorphic carcinoma (PPC) is a rare histological type of non-small cell lung cancer (NSCLC) that accounts for approximately 0.4% of all cases of lung cancer [1]. Pathologically, it is defined as adenocarcinoma, squamous cell carcinoma, or large cell carcinoma, containing a component of spindle or giant cells with a sarcomatoid tumor component of at least 10% [2]. Although surgical resection is the first-line treatment for PPC as well as other histological types of lung cancer, postoperative relapse and metastasis occur frequently because of the aggressive progression [3, 4]. Treatment for postoperative relapse is often difficult due to resistance to radiotherapy and chemotherapy, which are characteristics of PPC [5]. Therefore, the establishment of a treatment strategy for this disease is highly desirable.
Recently, the knowledge of cancer-related genes in lung cancer has been accumulated thorough the development of next-generation sequencing technology [6], and it is expected that the precision medicine will be established for future lung cancer treatment. The tumor protein p53 (TP53)—a tumor suppressor gene that regulates the cell cycle and apoptosis—is known to be the most mutated gene in human cancers [7, 8]. A high frequency of TP53 mutations has also been reported in PPC [9]. Ataxia-telangiectasia mutated gene (ATM) is known as a tumor suppressor gene that functions to regulate the cell cycle and repair damaged DNA. In recent years, many studies have reported that ATM mutations are involved in the development of lung cancer [10, 11]. The phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha gene (PIK3CA) encodes the major catalytic subunit of the target protein—the phosphatidylinositol 3-kinases (PI3K)—and is an oncogene that contributes to key signaling, which is required for a wide range of normal cellular functions [12]. Epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitors (TKIs), which inhibit the function of the mutated products of EGFR—a driver gene in NSCLC—have been demonstrated to improve the prognosis and are widely used for NSCLC in clinical practice [13]. There are also reports that PIK3CA mutations are associated with the acquisition of resistance to EGFR-TKIs [14, 15].
However, due to its rarity, little is known about how TP53, ATM, PIK3CA, and EGFR mutations in PPC affect the clinical prognosis after surgery. This information may provide one strategy for the effective treatment of PPC harboring therapeutic resistance. We performed a genetic analysis of surgically resected tissue of PPC using next-generation sequencing and identified TP53, ATM, PIK3CA, and EGFR mutations. We then used multivariable analyses to evaluate the association of these genetic mutations with the postoperative prognosis of patients with PPC.
Methods
Patients
We retrospectively studied patients who underwent complete resection of NSCLC at the National Hospital Organization Kinki-Chuo Chest Medical Center (KCMC) between February 2002 and January 2021, and selected 67 patients with a pathological diagnosis of PPC. We defined complete resection as a grossly or microscopically removed tumor, which corresponds to R0 in the residual tumor (R) classification. The histopathological diagnosis according to the current 2015 World Health Organization classification was performed by pathologists belonging to KCMC [16]. Medical information, including age, sex, smoking status, surgical procedure, pathological tumor-node-metastasis (TNM) classification (eighth edition), PD-L1 expression status, and the outcome after surgery were collected from medical records. The present study was approved by the Institutional Review Board of KCMC (Approval number: 2020–067). The cut-off date for the analysis was set at June 30, 2022. The Institutional Review Board of KCMC waived the requirement for informed consent from all research participants due to the retrospective and anonymous nature of this study. Information about opting out of the study was provided on the homepage of KCMC. All methods were in accordance with relevant guidelines and regulations.
PD-L1 immunohistochemistry
We evaluated all viable cancer cells in the entire pathological tissue section of each tumor sample. The PD-L1 clone 22C3 pharmDx kit and Dako Automated Link 48 platform (Agilent Technologies, Dako, Carpinteria, CA, USA) were used to examine the PD-L1 expression. We calculated the PD-L1 tumor proportion score (TPS) as the percentage of complete or partial membrane staining in a sample. The cut-off values for the expression of PD-L1 were set at 50 and 1% based on a previous clinical study. We categorized the tumor samples of each patient into 3 groups (< 1% [negative], 1–49% [low expression], and ≥ 50% [high expression]) based on the presence of positively stained cells in specimen.
Genomic DNA extraction and quality check
To investigate alterations in cancer-related genes, we obtained 67 lung tissues specimens from patients with PPC by surgical resection or trans-bronchial lung biopsy. All samples were fixed by formalin and embedded in paraffin (FFPE). Genomic DNA (gDNA) was extracted from FFPE samples using QIAamp DNA FFPE Tissue Kit (QIAGEN, Japan) according to manufacturer’s protocol. After extraction, the DNA concentration was quantified by Qubit® 3.0 Fluorometer (Thermo Fisher Scientific, USA). The gDNA quality was determined by the A260/A280 and A260/A230 ratios using NanoDrop 1000 (Thermo Fisher Scientific). We excluded 8 of the 67 samples due to failure to extract a sufficient amount of DNA.
Library preparation and sequencing with the Cancer HotSpot panel
DNA libraries were generated by PCR using AmpliSeq for Illumina Cancer HotSpot Panel v2 (Illimina, USA) in 59 samples. AmpliSeq for Illumina Cancer HotSpot Panel v2 targets 2800 mutations in 50 cancer-related genes, including the genes of interest—TP53, ATM, PIK3CA and EGFR—in our study. gDNA (20 ng) was used to amplify the panel range of genes. The libraries were amplified using AmpliSeq Library PLUS for Illumina (Illumina, USA) according to manufacturer’s instructions. Each library was quantified using an Agilent DNA 100 kit with an Agilent 2100 Bioanalyzer (Agilent Technologies, USA). Sequencing was performed using a MiSeq NGS system (Illumina, USA). The MiSeq sequence data was processed and analyzed using BaseSpace Sequence Hub. Of the 59 samples, we excluded one sample because it was impossible to analyze due to an error. In comparison to the UCSC hg19 reference genome, sequences with amino acid changes were identified as somatic mutations.
Overall survival and relapse-free survival
The primary outcome of this study was overall survival (OS) after surgery, defined as the time from the date of curative resection to the date of death by any cause or the date of the last follow-up examination. The secondary outcome was relapse-free survival (RFS), which was defined as the length of time for which the patient survived after curative resection (as the primary treatment) without any signs or symptoms of cancer or death from any cause.
Statistical analyses
Finally, 55 patients were included in the statistical analysis (three patients of 58 patients in whom a DNA analysis of PPC was performed were excluded because we were unable to obtain medical information). The probability of OS was assessed using the Kaplan–Meier method and log-rank tests. A multivariable Cox proportional hazards analysis was performed to estimate the hazard ratios (HRs), with adjustment by risk factors for mortality or relapse. In a multivariable Cox proportional hazards analysis, the number of covariates that be can analyzed is the number of cases with a particular outcome ÷ 10. In other words, a multivariable Cox proportional hazards model requires a minimum of 10 outcome events per predictor variable (EPV) [17, 18]. However, it also suggests that the rule of using ≥10 EPV in Cox models is not clearly defined and can be relaxed. That is, a simulation study suggested that, in comparison to a Cox model with 10–16 EPV, a Cox model with 5–9 EPV provided acceptable results [19]. Therefore, we accepted 5–9 EPV the in the Cox model. In this study, the number of covariates that could be included in the Cox proportional hazards analysis of OS was 15 (i.e., the number of patients who died) divided by 5–9 (result: 1–3) and the number of RFS was 28 (i.e., the number of patients who had a confirmed relapse) divided by 5–9 (result: 3–5). It has been reported that older age, male sex, and advanced pathological stage tend to be associated with a poor prognosis in patients with treated PPC [20, 21]. Therefore, in addition to the gene status variable of interest, we selected age, sex, and pathological stage as confounding factors. Since “elderly” is generally defined as age ≥ 65 years, we set 65 years as the cut-off value for age. We selected the following three factors in the multivariable Cox hazards analysis of OS: gene status (pathogenic mutation [reference: variants of unknown significance (VUSs) or wild type (WT)]) as the explanatory variable; and pathological stage (stage III–IV [reference: stage I–II]) and age (≥65 [reference: < 65]) as confounding factors. Sensitivity analyses were performed by the multivariable Cox hazards analyses of OS in which pathological stages (stage III–IV [reference: stage I–II]) and sex (female [reference: male]), and age (≥65 [reference: < 65]) and sex (female [reference: male]), respectively, were included as confounding factors were performed to assess the robustness of the results of the analysis. On the other hand, we selected the following four factors according to the multivariable Cox hazards analysis of RFS: gene status (pathogenic mutation [reference: VUSs or WT] as the explanatory variable; and pathological stage (stage III–IV [reference: stage I–II]), age (≥65 [reference: < 65] and sex (female [reference: male]) as confounding factors. All statistical analyses were conducted using Easy R (EZR) (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria). EZR is a modified version of R commander with added biostatistical functions [22]. P values of < 0.05 were considered to indicate statistical significance.
Results
Patients characteristics
The clinical characteristics of the 55 patients with PPC who underwent complete surgical resection are shown in Table 1. The median age was 65 years (range, 58–72). The majority of the patients were male (75%) and smokers (84%). The pathological stage was mostly advanced (stage II, 42%; stage III, 38%). One case of stage IV with a single brain metastasis was included. In this case, the metastatic brain lesion was treated by gamma knife as oligometastasis followed by complete resection of the primary lunge lesion. Segmentectomy was the least common surgical procedure (2%), while lobectomy was the most common (60%). Advanced cases were also common (pneumonectomy, 9%; lobectomy with complicated resections, 20%). Regarding histological types in PPC, the epithelial component was dominated by adenocarcinoma (71%) and squamous cell carcinoma (20%). The expression of PD-L1 was observed in 95% of cases, and high expression levels (≥50%) were confirmed in 73% of cases. Postoperative relapse was confirmed in 28 of 55 patients (51%) and death after surgery was identified in 15 of 55 patients (27%). The median OS after surgery, which was the median follow-up period in patients who were alive at the end of follow-up, was 893 days (range, 523–1856 days). The follow-up period for OS was identical to that for postoperative relapse. The median follow-up time of postoperative RFS was 562 days (range, 92–1682 days).
Genomic alterations in PPC
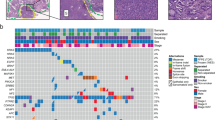
An overview of the mutations of TP53, ATM, PIK3CA and EGFR in the primary lesions of 55 patients with PPC is provided in Fig. 1 (detailed information is provided in Supplemental Table S1). TP53 was the most frequently mutated gene. Among cases with variants of unknown significance (VUSs), TP53 mutations were detected in 53 (98%) patients. Pathogenic mutations of TP53 were detected in 26 (47%) patients. The confirmed ATM mutations were only VUSs in 5 (9%) patients. PIK3CA mutations containing VUSs were detected in 10 (18%) patients. Pathogenic mutations of PIK3CA were confirmed in 4 (7%) patients. EGFR mutations, including VUSs, were detected in 21 (38%) patients, with 5 (9%) patients only showing pathogenic mutations.
Summary of four genomic mutations of 55 patients with pulmonary pleomorphic carcinoma. All mutations, including variants of unknown significance (VUSs) in TP53, ATM, PIK3CA and EGFR, were detected in 54 (98%), 5 (9%), 10 (18%) and 21 (38%) patients, respectively. Pathogenic mutations in TP53, ATM, PIK3CA and EGFR were detected in 25 (45%), 0 (0%), 4 (7%) and 5 (9%) patients, respectively
Overall survival and relapse-free survival after surgery
During a median follow-up period of 893 days after surgery, we confirmed that 15 (27%) patients died because of disease progression. The Kaplan-Meier curve for OS after surgery according to gene mutations—TP53, PIK3CA and EGFR which were detected pathogenic mutations—are shown in Fig. 2. OS after surgery in the PIK3CA mutation group was significantly shorter in comparison to the VUSs or WT groups, before adjustment for patient background (P < 0.02) (Fig. 2B). The Kaplan-Meier curves for OS after surgery according to the TP53 mutation and EGFR mutation status are shown in Fig. 2A, C. Before adjustment for patient background factors, OS after surgery did not differ among the TP53 (P = 0.63) and EGFR (P = 0.82) mutation status groups. The Kaplan-Meier curves of RFS after surgery according the TP53 mutation, PIK3CA mutation and EGFR mutation status are shown in the supplemental Fig. S1. Before adjusting for patient background factors, RFS after surgery did not differ among the TP53 (P = 0.61), PIK3CA (P = 0.24) and EGFR (P = 0.90) mutation status groups.
Multivariable analyses of factors associated with OS and RFS after surgery according to the gene mutation status
The results of the multivariable Cox proportional hazards analysis of pathogenic mutations associated with OS after surgery are shown in Table 2. A significant difference in OS was observed between the PIK3CA mutation group and the VUSs/WT group (adjusted hazard ratio [HR], 4.5; 95% confidence interval [CI] 1.1–18.8) after adjustment for patient background factors (pathological stage and age). On the other hand, OS after surgery did not differ to a statistically significant extent in the TP53 (adjusted HR, 0.9, 95% CI 0.3–3.1) and EGFR mutation groups (adjusted HR, 0.6, 95% CI 0.1–2.8). RFS after surgery did not differ to a statistically significant extent in the TP53 (adjusted HR, 1.1, 95% CI 0.5–2.5), PIK3CA (adjusted HR, 2.2, 95% CI 0.6–8.3) and EGFR mutation groups (adjusted HR, 0.8, 95% CI 0.2–2.7) (Supplemental Table S2).
Sensitivity analyses with different confounding factors in multivariable cox proportional hazards analyses of OS
Sensitivity analyses with modified confounding factors were performed for the PIK3CA mutation status, which showed significant differences in the multivariable Cox proportional hazards analysis. For the sensitivity analyses, we used multivariable Cox proportional hazards models with pathological stage and sex, and age and sex, respectively as confounding factors. Significant differences in OS after surgery were observed between the PIK3CA mutation group and the VUSs/WT group in both models. The HRs of postoperative OS in cases with PIK3CA mutation regarding VUSs/WT were 7.5 (95% CI 1.7–32.4) in the model adjusted for pathological stage and sex and 5.4 (95% CI 1.4–21.7) in the model adjusted for age and sex (Table 3).
Testing the proportional hazards assumption in cox models
We performed tests of proportional hazards assumption for the Cox models used in the multivariable analyses for OS and RFS. In the tests for proportional hazards assumption for each variable in each Cox model, the P values for all variables exceeded 0.05 (Supplemental Table S3 and S4); thus, the proportional hazards assumption was not rejected.
Efficacy of chemotherapy after postoperative relapse in cases of PPC harboring PIK3CA mutation
Of 4 patients with PPC harboring PIK3CA pathogenic mutation, one patient (No.24) had no postoperative relapse and was censored at 1114 days after surgery (Table 4). Although the remaining three patients received chemotherapy as an initial treatment, no patients achieved an objective response (Table 4). One patient (No.39) harboring an uncommon EGFR mutation was treated with gefitinib after postoperative relapse. However, the patient developed progressive disease in liver metastasis. Two patients (No.37 and No. 39) received immune checkpoint inhibitor (pembrolizumab) treatment after the determination of progressive disease with chemotherapy. Although a partial response was confirmed in patient No.37 2 months after the administration of pembrolizumab, he developed progressive disease with metastasis to the scapula at 4 months after showing a partial response. Patient No.39 showed progressive disease despite the administration of pembrolizumab and was then treated with best supportive care because of poor general condition.
Discussion
Two new points that emerged from our study are as follows. First, although TP53 mutations were most frequent in PPC, TP53 mutations were not significantly associated with postoperative OS. Second, although the frequency of PIK3CA mutations in PPC was only a few percent, the postoperative OS of patients with PIK3CA mutations was significantly shorter. To our knowledge, this is the first report of a relatively large (n = 55) study evaluating the association between genetic variants in PPC and the clinical prognosis after complete resection.
Regarding the first point (i.e., the association between PPC and TP53 mutations), we found a high frequency of TP53 mutations in our PPC cohort (26/55). TP53 mutation has been reported to be the most frequently mutated gene in lung cancers of all histological types [23]. Resistance to radiotherapy and chemotherapy due to TP53 mutations has also been suggested to be associated with a poor prognosis [24]. However, in our cohort, no significant association was found between TP53 mutations in PPC and the postoperative prognosis. Therefore, the prognostic impact of TP53 mutations in PPC may differ from that in other lung cancers; further studies are needed to clarify the association between TP53 mutations and the prognosis in patients with PPC. ATM, similarly to TP53, is a gene known to be involved in the repair pathway of damaged DNA. It has been reported that mutations in ATM are found in approximately 10% of lung cancers [25]. The frequency of ATM mutations in our PCC cohort was 9% (5/55), which is in line with previous studies [9]. On the other hand, no pathogenic mutations of ATM were detected in our cohort (0/55). This result is consistent with a previous report about PPC [23]. Pathogenic mutations of ATM may tend to be relatively rare in PPC.
Regarding the second point (i.e., the association between PPC and PIK3CA mutations), a previous study of PPC indicated that the frequency of PIK3CA mutations in PPC was 20% for variants including VUSs and 10% for pathogenic mutations alone [23]. On the other hand, the frequency of PIK3CA mutations in NSCLC is reported to be as low as 1.8–3% [26, 27]. In our PPC cohort, the frequency of PIK3CA mutations was 18% (10/55) for variants including VUSs and 7% (4/55) for pathogenic mutations alone, which was largely consistent with the previous reports [9, 23]. It is suggested that the frequency of PIK3CA mutations in PPC tends to be higher in comparison to that of PIK3CA mutations in NSCLC. Approximately 70% of the cases of PPC in our cohort contained adenocarcinoma as an epithelial component. Although it has been reported that 80% of lung adenocarcinoma with PIK3CA mutations is associated with EGFR mutations [28], no cases with pathogenic co-mutation of PIK3CA and EGFR were detected in our PPC cohort. That is, PIK3CA and EGFR mutations may be functionally independent in PPC. In addition, we found that EGFR mutations have no significant effect on the postoperative prognosis of PPC, whereas PIK3CA mutations were significantly associated with the postoperative OS. However, the association between PIK3CA mutations and RFS after surgery was not confirmed. These results suggest a significant contribution of PIK3CA mutations to the resistance of postoperative treatment in PPC.
Three of the four patients with pathogenic PIK3CA mutations relapsed after surgery and received chemotherapy; however, all of these patients died, which suggested resistance to chemotherapy. PPC has been reported to be associated with the high expression of PD-L1 [21], and 75% of the patients in our cohort showed high expression levels. The use of immune checkpoint inhibitors (ICIs) for PPC has been reported, and is suggested to be effective [29]. The outcomes of our PIK3CA mutation-positive PPC cases that were treated with chemotherapy and ICIs may be biologically explainable. No details are known about the mechanisms by which PPC develop resistance to chemotherapy. However, the mechanism of chemotherapy resistance in small cell lung cancer, which is characterized by many abnormalities in signaling pathways involving PIK3CA (PI3K/Protein kinase B [Akt]/the mammalian target of rapamycin [mTOR] pathway), may be informative. It has been reported that small cell lung cancer may be associated with chemotherapy resistance due to a phenotypic transition from suspension to an adhesion growth pattern caused by the activation of the PI3K/Akt/mTOR pathway [30]. PIK3CA mutation-positive PPC may have a similar mechanism. Our patient No.26, who was diagnosed before ICIs were covered by insurance, showed chemotherapy resistance. Inhibitors of the PI3K/Akt/mTOR pathway may be effective against PPC with PIK3CA mutations, because chemotherapy-resistant cell lines are reported to be sensitive to PI3K inhibitors [31]. Cases No.37 and 39 were resistant to 1st-line chemotherapy and subsequently received pembrolizumab. However, no significant immunotherapy response was observed. A mechanism of immunotherapy resistance in gastric cancer has been reported, in which activation of the PI3K/Akt/mTOR pathway increases the production of free fatty acids, which are taken up more efficiently by regulatory T cells than by effector T cells, resulting in an increase in regulatory T cells in the tumor [32, 33]. Similar changes in the immune environment occur in PPC with PIK3CA mutations, which may explain why ICIs are not fully effective for PPC harboring PIK3CA mutations. On the other hand, it has been reported that in NSCLC, patients with both TP53 and ATM mutations showed a higher response to treatment for ICIs due to having a significantly higher tumor mutation burden in comparison to groups with either mutation alone or without mutation [34]. Cases No.37 and 39 were TP53 mutation-positive and ATM mutation-negative, which may have limited the therapeutic effect of ICIs. Therefore, the use of PI3K/Akt/mTOR pathway inhibitors may be effective in PPC with PIK3CA mutations as a strategy to enhance the efficacy of ICIs. Precision medicine, in which combinations of PIK3CA, TP53 and ATM mutations in PPC are identified and PI3K/Akt/mTOR pathway inhibitors or ICIs are administered according to the mutations pattern, may become an effective treatment for PPC with PIK3CA mutations in the future.
The present study was associated several limitations. First, the number of confounders that could be included in the multivariable Cox proportional hazards model was limited by the small number of cases due to the rarity of PPC, making it impossible to include many factors in that model at one time. Therefore, the robustness of the results was assessed using a sensitivity analysis to vary the combination of factors included in the multivariable Cox proportional hazards model. Although ≥10 EPV is recommended for a Cox proportional hazards model, 5–9 EPV was accepted for our study based on reports that have demonstrated that it is possible to relax this constraint [19]. However, further studies are required to determine whether similar results can be obtained with sample sizes that satisfy the standard condition of ≥10 EPV. Second, because most cases of PPC are divided into epithelial and sarcomatoid components, it is strictly necessary to analyze the genomic DNAs from each component. However, the difference in the PPC component may not be very important, since it has been reported that approximately 70% of the epithelial and sarcomatoid components share the same driver gene mutation [9]. Third, the molecular mechanism underlying short postoperative overall survival and chemotherapy resistance, including ICIs of PPC harboring PIK3CA mutation is unclear. Further studies are needed in order to confirm this. Fourth, only four patients had PIK3CA mutations, and their survival probability was compared to that of 51 patients without PIK3CA mutations. Although the multivariable analysis confirmed a survival difference, the possibility that this difference occurred by chance cannot be ruled out. Therefore, further confirmatory studies are warranted.
Conclusion
We evaluated the relationship between gene mutations (TP53, ATM, PIK3CA, EGFR) and overall survival after surgery in PPC, and found that patients with PIK3CA mutations had significantly shorter postoperative overall survival. To our knowledge, this is the first report of this finding. PIK3CA mutations are prognostic markers for PPC, and inhibition of the PIK3CA-related pathway may contribute to improving the prognosis of patients with PPC, including improving the efficacy of ICI therapy.
Availability of data and materials
The datasets generated and analyzed during the current study are available in the DNA Data Bank of Japan (DDBJ) Sequenced Read Archive repository (http://www.ddbj.nig.ac.jp/index.html) under accession number DRA014590.
Abbreviations
- ATM :
-
Ataxia-telangiectasia mutated gene
- EGFR :
-
Epidermal Growth Factor Receptor gene
- NSCLC:
-
Non-Small Cell Lung Cancer
- HR:
-
Hazard Ratio
- TP53 :
-
tumor protein p53 gene
- PIK3CA :
-
phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha gene
- PPC:
-
Pulmonary Pleomorphic Carcinoma
- PD-L1:
-
programmed cell death-ligand 1
- OS:
-
Overall Survival
- VUSs:
-
variants of unknown significance
- WT:
-
Wild Type
References
Travis WD. Pathology of lung cancer. Clin Chest Med. 2002;23(1):65–81.
Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization classification of lung tumors. J Thorac Oncol. 2015;10(9):1243–60.
Mochizuki T, Ishii G, Nagai K, et al. Pleomorphic carcinoma of the lung: clinicopathologic characteristics of 70 cases. Am J Surg Pathol. 2008;32(11):1727–35.
Ito K, Oizumi S, Fukumoto S, et al. Clinical characteristics of pleomorphic carcinoma of the lung. Lung Cancer. 2010;68(2):204–10.
Bae HM, Min HS, Lee SH, et al. Palliative chemotherapy for pulmonary pleomorphic carcinoma. Lung Cancer. 2007;58(1):112–5.
Pestinger V, Smith M, Sillo T, et al. Use of an integrated pan-cancer oncology enrichment next-generation sequencing assay to measure tumour mutational burden and detect clinically actionable variants. Mol Diagnosis Ther. 2020;24(3):339–49.
Hollstein M, Sidransky D, Vogelstein B, Harris CC. p53 mutations in human cancers. Science. 1991;253(5015):49–53.
Kandoth C, McLellan MD, Vandin F, et al. Mutational landscape and significance across 12 major cancer types. Nature. 2013;502:333–9.
Nagano M, Kohsaka S, Hayashi T, et al. Comprehensive molecular profiling of pulmonary pleomorphic carcinoma. NPJ Precis Oncol. 2021;5(1):57.
Yang H, Spitz MR, Stewart DJ, et al. ATM sequence variants associate with susceptibility to non-small cell lung cancer. Int J Cancer. 2007;121(10):2254–9.
Lo YL, Hsiao CF, Jou YS, et al. ATM polymorphisms and risk of lung cancer among never smokers. Lung Cancer. 2010;69(2):148–54.
Li S, Li L, Zhu Y, et al. Coexistence of EGFR with KRAS, or BRAF, or PIK3CA somatic mutations in lung cancer: a comprehensive mutation profiling from 5125 Chinese cohorts. Br J Cancer. 2014;110:2812–20.
Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non–small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–39.
Camidge DR, Pao W, Sequist LV. Acquired resistance to TKIs in solid tumours: learning from lung cancer. Nat Rev Cli Oncol. 2014;11(8):473–81.
Thress KS, Paweletz CP, Felip E, et al. Acquired EGFR C797S mutation mediates resistance to AZD9291 in non-small cell lung cancer harboring EGFR T790M. Nat Med. 2015;21(6):560–2.
Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol. 2015;10:1243–60. https://doi.org/10.1097/JTO.0000000000000630.
Peduzzi P, Concato J, Feinstein AR, Holford TR. Importance of events per independent variable in proportional hazards regression analysis II. Accuracy and precision of regression estimates. J Clin Epidemiol. 1995;48(12):1503–10.
Peduzzi P, Concato J, Kemper E, Holford TR, Feinstem AR. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. 1996;49(12):1373–9.
Eric V, Charles EM. Relaxing the rule of ten events per variable in logistic and cox regression. Am J Epidemiol. 2007;165(6):710–8.
Chen Z, Liu J, Min L. Clinicopathological characteristics, survival outcomes and prognostic factors in pleomorphic carcinoma: a SEER population-based study. BMC Pulm Med. 2022;22:116.
Naito M, Tamiya A, Takeda M, et al. A high pd-l1 expression in pulmonary pleomorphic carcinoma correlates with parietal-pleural invasion and might predict a poor prognosis. Intern Med. 2019;58(7):921–7.
Kanda Y. Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transplant. 2013;48(3):452–8.
Manabe S, Kasajima R, Murakami S, et al. Analysis of targeted somatic mutations in pleomorphic carcinoma of the lung using next-generation sequencing technique. Thorac Cancer. 2020;11(8):2262–9.
Shetzer Y, Solomon H, Koifman G, Molchadsky A, Horesh S, Rotter V. The paradigm of mutant p53-expressing cancer stem cells and drug resistance. Carcinogenesis. 2014;35(6):1196–208.
Jette NR, Kumar M, Radhamani S, et al. ATM-deficient cancers provide new opportunities for precision oncology. Cancers (Basel). 2020;12(3):687.
Wu SG, Chang YL, Yu CJ, Yang PC, Shih JY. The role of PIK3CA mutations among lung adenocarcinoma patients with primary and acquired resistance to EGFR tyrosine kinase inhibition. Sci Rep. 2016;6:35249.
Wang Y, Wang Y, Li J, Li J, Che G. Clinical significance of PIK3CA gene in non-small-cell lung cancer: a systematic review and meta-analysis. Biomed Res Int. 2020;2020:3608241.
Zhu F, Li J, Li C, et al. Concealed driver in lung adenocarcinoma with single PIK3CA mutation: a case report and single-center genotyping review. Ann Transl Med. 2021;9(3):271.
Miyashita K, Karayama M, Inoue Y, et al. Efficacy of immune checkpoint inhibitors in non-small cell lung cancer with uncommon histology: a propensity-score-matched analysis. BMC Pulm Med. 2021;21:309.
Li X, Li C, Guo C, et al. PI3K/Akt/mTOR signaling orchestrates the phenotypic transition and chemo-resistance of small cell lung cancer. J Genet Genomics. 2021;48(7):640–51.
Jin Y, Chen Y, Tang H, et al. Activation of PI3K/AKT pathway is a potential mechanism of treatment resistance in small cell lung cancer. Clin Cancer Res. 2022;28(3):526–39.
Kumagai S, Togashi Y, Sakai C, et al. An oncogenic alteration creates a microenvironment that promotes tumor progression by conferring a metabolic advantage to regulatory T cells. Immunity. 2020;53(1):187–203.
Pan YH, Zhang JX, Chen X, et al. Predictive value of the TP53/PIK3CA/ATM mutation classifier for patients with bladder cancer responding to immune checkpoint inhibitor therapy. Front Immunol. 2021;12:643282.
Chen Y, Chen G, Li J, et al. Association of Tumor Protein p53 and Ataxia-telangiectasia mutated comutation with response to immune checkpoint inhibitors and mortality in patients with non-small cell lung cancer. JAMA Netw Open. 2019;2(9):e1911895.
Acknowledgements
Not applicable.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
KK conceived and designed the study. KK collected the patient data and performed the statistical analysis. SI performed a gene mutation analysis using next-generation sequencing. KK, HS, AF, TT, HY and KO interpreted the analyzed data. KK wrote the manuscript. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This retrospective cohort study was approved by the Institutional Review Board of the National Hospital Organization Kinki-Chuo Chest Medical Center (KCMC) (Approval number: 2020–067) and carried out in accordance with the Declaration of Helsinki. The Institutional Review Board of KCMC waived the requirement for informed consent from all research participants due to the retrospective and anonymous nature of this study. Information about opting out of the study was provided on the homepage of KCMC.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflicts of interest to in association with the present study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplemental Table S1.
NGS results for 55 patients with pulmonary pleomorphic carcinoma.
Additional file 2: Supplemental Fig. S1.
The Kaplan-Meier curves of RFS after surgery according the TP53 mutation (A), PIK3CA mutation (B) and EGFR mutation (C) status.
Additional file 3: Supplemental Table S2.
The Cox proportional hazards analysis of RFS according to the gene mutation status.
Additional file 4: Supplemental Table S3.
Testing the proportional hazards assumption in Cox models for OS.
Additional file 5: Supplemental Table S4.
Testing the proportional hazards assumption in Cox models for RFS.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Kojima, K., Imai, S., Samejima, H. et al. PIK3CA mutations associated with a poor postoperative prognosis in patients with pulmonary pleomorphic carcinoma: a retrospective cohort study. BMC Cancer 22, 1066 (2022). https://doi.org/10.1186/s12885-022-10176-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-022-10176-4