Abstract
Background
To rethink the clinical significance of standardized uptake values (SUVs) of nasopharyngeal carcinoma (NPC) on 18F-fluorodeoxyglucose (18F-FDG) positron-emission tomography (PET).
Methods
We retrospectively reviewed 369 NPC patients who underwent pretreatment 18F-FDG PET. The predictive value of the SUVmax of the primary tumor (SUVmax-t) and regional lymph nodes (SUVmax-n) was evaluated using probability density functions. Receiver operating characteristic curves were used to determine optimal cutoffs for the SUVmax-n/SUVmax-t ratio (NTR). Kaplan–Meier and Cox regression analyses were used to assess survival.
Results
The optimal SUVmax-t and SUVmax-n cutoffs were 7.5 and 6.9, respectively. High SUVmax-t and SUVmax-n were related to local and regional recurrence, respectively. Patients with low SUVmax had better 3-year overall survival (OS). To avoid cross-sensitization of cutoff points, we stratified patients with high SUVmax into the low and high NTR groups. The 3-year distant metastasis-free survival (DMFS; 92.3 vs. 80.6%, P = 0.009), progression-free survival (PFS; 84.0 vs. 67.7%, P = 0.011), and OS (95.9 vs. 89.2%, P = 0.002) significantly differed between the high vs. low NTR groups for patients with high SUVmax. Multivariable analysis showed that NTR was an independent prognostic factor for DMFS (hazard ratio [HR]: 2.037, 95% CI: 1.039–3.992, P = 0.038), PFS (HR: 1.636, 95% CI: 1.021–2.621, P = 0.041), and OS (HR: 2.543, 95% CI: 1.214–5.325, P = 0.013).
Conclusion
High SUVmax was associated with NPC recurrence. NTR is a potential prognosticator for DMFS, suggesting that heterogeneity in the pretreatment 18F-FDG uptake between the primary tumor and lymph nodes is associated with high invasion and metastatic potential.
Similar content being viewed by others
Introduction
Nasopharyngeal carcinoma (NPC) is an epithelial malignant tumor prevalent in East and Southeast Asia [1]. According to Global Cancer Statistics, an estimated 133,000 new cases of NPC were diagnosed in 2020 worldwide [2]. Radiotherapy or concurrent chemoradiotherapy is widely used as the standard treatment for NPC [3].
The American Joint Committee on Cancer (AJCC) TNM staging system is used globally to predict the prognosis and guide the treatment of NPC [4]. However, this staging system is largely based on anatomic imaging, which has limitations in terms of evaluating the aggressiveness of tumors. Owing to this, NPC patients with the same TNM stage can have substantial differences in prognosis yet receive similar treatments; hence, solely relying on the current anatomic imaging-based staging system is insufficient to accurately predict the prognosis of NPC patients [5]. Optimizing the conventional staging system and quantifying the recurrence risk are required to enable individualized therapy for NPC.
Some retrospective studies have indicated that the standardized uptake value (SUV) of 18F-fluorodeoxyglucose positron-emission tomography (18F-FDG PET) is useful for risk stratification and prognostication in NPC [6,7,8,9,10,11] Table 1. Although the optimal SUV cutoff points for NPC are still debated, their prognostic value cannot be denied. Hence, adopting the advantages of the previous research, the present study aimed to determine the association between SUVs on pretreatment 18F-FDG PET and prognosis in patients with NPC.
Materials and methods
Patients
In this retrospective study, we enrolled 369 NPC patients who had been newly diagnosed with NPC and underwent complete treatment in our cancer center between January 2012 and June 2017. Patients were consecutively recruited if they met the following inclusion criteria: (i) biopsy-proven primary NPC, (ii) pretreatment whole-body 18F-FDG PET/CT, (iii) radical treatment, (iv) age between 18 and 70 years, (v) complete medical history and clinical information, including physical examination, adequate clinical examination, and laboratory data, (vi) absence of distant metastasis before or during treatment, and (vii) no evidence of another primary carcinoma or other concomitant fatal disease. Patients who did not fulfill all the listed criteria were excluded from the study. All patients were restaged according to the 8th edition of the AJCC staging system. This study was approved by the ethics committee of Fujian Cancer Hospital (No. YKT2020-011-01).
18F-FDG PET/CT imaging
PET/CT scanning was performed using a Gemini TF 64 PET/CT scanner (Philips, The Netherlands) and the 18F-FDG was manufactured by HM-10 cyclotron with >95% radiochemical purity [12]. Before 18F-FDG PET/CT scanning, all patients fasted ≥6 hours to maintain serum blood glucose level of 3.9 ~ 6.5 mmol/L. Then 18F-FDG was intravenously administered at a dose of 148 to 296 MBq. Patients rested for 40 to 60 minutes in a dimly lit room before PET/CT scan. The CT scanning was from head to proximal thigh with the following acquisition parameters: 140 kV; 2.5 mA; matrix 512 ×512; and scan slice thickness 4 mm. The reconstructed PET images were obtained after applying the CT images for attenuation correction.
The 18F-FDG SUV was based on the region of interest (ROI) of tumor lesions. It was calculated as the decay-corrected tissue activity (nCi/mL) divided by the injected dose of FDG (nCi) and the patient’s body weight (g) [12]. SUVmax-t was defined as the maximum SUV of the primary tumor, and SUVmax-n was defined as the highest SUV of the regional lymph nodes. The lymph node-to-primary tumor SUV ratio (NTR), which was the ratio of SUVmax-n/SUVmax-t, was also assessed in this study.
Chemotherapy
All chemotherapy regimens were administered according to a previously described protocol [13]. Patients with stage I disease received radiotherapy alone. Patients with stage II disease were administered radiotherapy along with 2–3 cycles of concurrent chemotherapy using a cisplatin-based regimen. Patients with stage III–IVA disease underwent 2–3 cycles of neoadjuvant chemotherapy prior to radiotherapy.
Radiotherapy
All patients received intensity-modulated radiotherapy (IMRT), and the target volume and dose of radiotherapy were calculated using a previously described treatment protocol [13, 14]. In brief, the planning target volumes obtained for the primary gross tumor volume or gross tumor volume in the involved lymph nodes were exposed to a total dose of 70 Gy in 31–35 fractions. A total dose of 60 Gy was administered to the planning target volume for high-risk clinical target volumes. The corresponding dose to the planning target volume for potentially involved low-risk clinical target volumes and the clinical target volume of the neck nodal regions was 54 Gy, in total.
Follow-up and clinical endpoints
The patients were examined every 3 months in the first 2 years, every 6 months in the following 3–5 years, and annually thereafter until death. The following endpoints were evaluated: local recurrence-free survival (LRFS, defined as time from diagnosis to local recurrence), regional recurrence-free survival (RRFS, time from diagnosis to regional recurrence), distant metastasis-free survival (DMFS, time from diagnosis to first distant metastasis), progression-free survival (PFS, time from diagnosis to disease progression or death from any cause), and overall survival (OS, time from diagnosis to death for any cause).
Statistical analysis
All statistical analyses were performed using IBM SPSS statistical software, version 26.0 and R software, version 4.1.1. The best cutoff values were determined using receiver operating characteristic (ROC) curve analysis. Violin plots, Kaplan–Meier curves, and correlation plots were created using Hiplot (https://hiplot.com.cn). Multivariate analysis was carried out to identify the prognostic factors influencing PFS, OS, LRFS, RRFS, and DMFS. The Cox proportional hazards regression model was used for the multivariate analysis, and the results were presented as estimated hazard ratios (HRs) with 95% confidence intervals (CIs). Tests were two-sided, and P values < 0.05 were regarded as statistically significant.
Results
Patients’ characteristics and outcomes
The characteristics of the patients are summarized in Supplementary Table S1. This study included a total of 369 patients with a median age of 47 years (range, 19–70 years). The median follow-up time was 51 months (range, 4–105 months). At the end of follow-up, 33 patients (8.9%) had died, and 48 patients (13%) had experienced disease relapse in the form of local recurrence (34 patients, 9.2%), regional failure (20 patients, 5.4%), and distant metastasis (47 patients, 12.7%). The 3-year LRFS, RRFS, DMFS, PFS, and OS rates were 93.2%, 96.2%, 88.9%, 81.3%, and 94.6%, respectively.
SUVmax and clinical stage
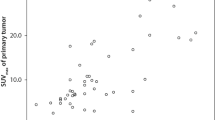
The SUVmax-t and SUVmax-n increased with the T and N stage, respectively (Fig. 1). When the values were distributed according to the disease stage, we found that the SUVmax-t data were more concentrated than the SUVmax-n data. The median SUVmax-t for the T1, T2, T3, and T4 stages were 6.2, 7.7, 9.4, and 11.2, respectively. The median SUVmax-n for the N0, N1, N2, and N3 stages were 1.6, 5.6, 7.1, and 9.1, respectively. The SUVmax-t and SUVmax-n significantly differed between different T stages and between different N stages, respectively (P < 0.001, Fig. 1). However, the SUVmax-t was not significantly correlated with the SUVmax-n (r = 0.13, P < 0.001, Supplementary Figure S1).
Optimal cutoff points for SUVmax-t and SUVmax-n
The mean SUVmax-t of the primary tumor and the mean SUVmax-n of cervical lymph node metastases were 9.2 ± 5.2 (range, 1.4–49.6) and 6.8 ± 5.8 (range, 0–36.6), respectively. The optimal cutoff SUVmax-t for predicting local recurrence was 7.5 (area under the curve [AUC] = 0.627, P = 0.015; Fig. 2). The optimal cutoff SUVmax-n for predicting regional recurrence was 6.9 (AUC = 0.757, P < 0.001). With an SUVmax-t threshold of 7.5, we could correctly identify approximately 82% of the patients with local recurrence. Moreover, at this cutoff, we could also identify approximately 44% of the patients with no risk of local recurrence (P < 0.001, Fig. 2A). With an SUVmax-n threshold of 6.9, we could correctly identify approximately 85% of the patients with regional recurrence and approximately 59% of the patients with no risk of regional recurrence (P < 0.001, Fig. 2B).
To control for potential confounders, we built a multivariable Cox proportional hazards model including all relevant variables. The results showed that SUVmax-t was an independent predictor of LRFS (HR = 3.741, 95% CI: 1.489–9.396, P = 0.005), while SUVmax-n was a risk factor for RRFS (HR = 3.238, 95% CI: 1.103–9.505, P = 0.033; Supplementary Table S2).
SUVmax and prognosis
Considering the above results, we stratified patients into 4 groups: (a) LL group, low SUVmax-n (≤6.9) and low SUVmax-t (≤7.5), (b) LH group, low SUVmax-n (≤6.9) and high SUVmax-t (>7.5), (c) HL group, high SUVmax-n (>6.9) and low SUVmax-t (≤7.5), and (d) HH group, high SUVmax-n (>6.9) and high SUVmax-t (>7.5). To better explain the results, we demonstrated several representative 18F-FDG PET images for each subgroup (Supplementary Figure S2, S3, S4 and S5).
The cumulative survival curves for the LH, HL, and HH groups were very close, but these curves were clearly separated from the survival curve for the LL group (P = 0.044; Fig. 3). The OS rate of the LL group significantly differed from those of the other 3 groups. Therefore, we reorganized all patients into 2 groups: a high-risk group consisting of the patients in the LH, HL, and HH groups, and a low-risk group consisting of the LL group patients. We found that the high-risk group had worse RRFS, LRFS, PFS, and OS rates than the low-risk group (all P < 0.05; Supplementary Figure S6).
NTR and prognosis in high-risk group
We further analyzed the high-risk group patients. ROC curve analysis revealed that the optimal cutoff value of the NTR for predicting DMFS was 0.23 in the LH group, 2.35 in the HL group, and 1.29 in the HH group. The high-risk group patients were again reorganized into a low NTR group (NTR ≤ 0.23, ≤ 2.35, and ≤ 1.29 in the LH, HL, and HH groups, respectively) and a high NTR group (NTR > 0.23, > 2.35, and > 1.29 in the LH, HL, and HH groups, respectively).
The 3-year LRFS, RRFS, DMFS, PFS, and OS rates in the low NTR vs. high NTR group were 94.1% vs. 86.0% (P = 0.082), 95.9% vs. 92.5% (P = 0.160), 92.3% vs. 80.6% (P = 0.009), 84.0% vs. 67.7% (P = 0.011), and 95.9 vs. 89.2% (P = 0.002), respectively. The OS, PFS, and DMFS rates were significantly worse in the high NTR group than in the low NTR group (all P < 0.005; Fig. 4).
Multivariate analysis with other known prognostic factors, including tumor stage (T stage), nodal stage (N stage), and age, revealed that the NTR was an independent prognostic factor for DMFS (HR = 2.037, 95% CI: 1.039–3.992, P = 0.038), PFS (HR = 1.636, 95% CI: 1.021–2.621, P = 0.041), and OS (HR = 2.543, 95% CI: 1.214–5.325, P = 0.013). The results of the multivariate analysis are summarized in Table 2.
Discussion
NPC has a better prognosis than other head and neck cancers. However, NPC patients with the same clinical stage and mode of clinical treatment may have different prognoses. The main causes of treatment failure in NPC are distant metastasis and local recurrence. Hence, the identification of predictors of metastasis or recurrence is of great interest because these could allow treatment to be tailored to the individual characteristics of the patient.
As a functional imaging technology, PET is a relatively new interdisciplinary technique for displaying the anatomy and morphology of lesions. 18F-FDG PET is based on the metabolic activity of the tumor and its parameters can reflect the biologic aggressiveness. SUVmax is the highest standardized uptake value within a volume of interest which reflects the part with the highest metabolic activity [15]. Recently, other functional and volumetric parameters, such as termed total lesion glycolysis (TLG), metabolic tumor volume (MTV) and SUVpeak also showed potential prognostic value [16, 17]. For instance, a prospective study proved that TLG was an independent prognosticator of OS in stage III–IVb NPC [18]. However, these volume-based parameters have not been sufficiently evaluated because the results were varied and controversial. Some researches declared that TLG and MTV did not show significant prognostic value for NPC [10, 11, 19]. Thus, SUVmax has been the most widely used parameter because the advantages of high accuracy, convenient measurement, and good repeatability.
As early as 2008, Lee et al. reported that SUVmax may predict disease-free survival, and that higher SUVmax may be useful for identifying patients requiring more aggressive treatment [20]. In 2015, Xiao et al. suggested that SUVmax at the primary site is a useful biomarker to predict distant metastasis in NPC patients treated with IMRT [21]. Subsequent studies by Jeong et al. and Cho et al. came to similar conclusions that the SUVs of the lymph nodes are important prognostic factors for distant metastasis [8, 11]. Consistent with the above studies, the present study showed that SUVmax reflects tumor aggressiveness and has prognostic importance in NPC. Additionally, our study investigated the potential link between the SUVs of the primary tumor and the lymph nodes by analyzing the NTR. The NTR has been reported to be strongly related to clinical outcomes and pathological characteristics in many tumor types such as esophageal carcinoma, endometrial carcinoma, and cervical carcinoma [22,23,24]. Chung et al. demonstrated that as the NTR increased, the risk of recurrence increased significantly in cervical carcinoma [24]. What’s more, there were strong correlations between NTR and lymphovascular space invasion, deep myometrial invasion, lymph node metastasis and high tumor grade (all P < 0.05) in gynecological oncology [23, 24]. That is to say, NTR may be a novel prognostic factor compensating the inherent limit of possible underestimation of SUV due to the partial volume effect. Hung et al. reported that the pretreatment NTR is a potential prognosticator for DMFS in NPC [9]. These findings suggest that the NTR is a novel marker for tumor aggressiveness, metastatic potential, and poor prognosis in NPC patients. We used different combinations of SUVmax-t and SUVmax-n to divide our total study population into 4 subgroups. The fact that the same cutoff of NTR be defined in different subgroups can be confusing even be incorrect in previous studies.
Considering the results of previous studies, we reviewed and analyzed the prognostic usefulness of SUVs in NPC. However, unlike previous studies, we took recurrence as an indicator of tumor aggressiveness. The 369 study patients were divided into 4 groups according to their SUVmax-t and SUVmax-n (LL, LH, HL, and HH groups). Comparative analysis of the groups showed that patients with a low SUVmax had better survival, suggesting that tumors with high 18F-FDG uptake could be more aggressive. Priority, more aggressive treatment or be followed up more closely, should be given to patients with a high SUVmax to improve their prognosis.
In the entire study cohort, we found no obvious correlation between SUVmax-t and SUVmax-n; however, both SUVmax-t and SUVmax-n increased with the T and N stage, respectively. This paper attempts to explore the reasons of tumor metastasis capacity from the aspects of tumor metastasis heterogeneity and the interaction between metastatic lymph node and primary lesion. The NTR is a good index that reflects the heterogeneity between metastatic lymph nodes and primary lesions. Moreover, this index has low inter-scanner variability. In our research study, a high NTR was associated with significantly worse DMFS and OS rates, suggesting that heterogeneity in the pretreatment 18F-FDG uptake between the lymph nodes and primary lesion is a strong indicator of tumor invasion and metastasis. This indicates that the NTR is a potential prognostic marker of tumor aggressiveness, metastatic potential, and poor prognosis in NPC patients with high SUVmax. By further analyzing the NTRs in different subgroups, we attempted to avoid cross-sensitization of cutoff points in different subgroups. The only cutoff points could create cross-risk and mix-ups in different subgroups. We hoped to ensure that clinicians could effectively differentiate prognostic risk between patients with different levels of heterogeneity in SUVmax between the metastatic lymph nodes and primary tumor.
High SUVmax-t was associated with local recurrence, while high SUVmax-n was associated with regional recurrence. Fei et al. found that in T4 NPC patients with a residual primary lesion after radical IMRT, a boost dose provides satisfactory tumor control with tolerable toxicities [25]. Yeh et al. recommended boosting irradiation to the neck for NPC patients with positive lymph nodes in order to achieve good regional control [26]. Further investigation is required to determine if local or regional control can be improved by increasing the irradiation dose to the target volume according to the SUV. Since our results showed that high NTRs are associated with significantly worse DMFS and OS rates, more aggressive systemic treatment is justified for such patients with high NTRs. Zong et al. demonstrated that in high-risk NPC patients, maintenance S1 chemotherapy following IMRT resulted in superior survival to that of patients treated without S1 chemotherapy [27]. A phase-2 multi-institutional trial (NCT00408694) reported that the addition of bevacizumab to standard chemoradiation treatment for patients with NPC is feasible, and might delay the progression of subclinical distant disease [28]. You et al. revealed that cetuximab or nimotuzumab in addition to concurrent chemoradiotherapy significantly improved 3-year OS (96.6% vs. 92.9%, P = 0.015) and 3-year DMFS (94.6% vs. 89.3%, P = 0.030) in patients with stage II–IVb NPC [29]. Xia et al. also supported that the addition of cetuximab to first-line chemoradiotherapy is associated with an improvement in DMFS in patients with locoregionally advanced NPC [30].
There were several limitations to the present study. First, as a retrospective study, treatment strategy and chemotherapy regimen among patients may be heterogeneous, which could be a selection bias and might influence the results. Second, SUV measurements may vary from different institutions depending on the differences in PET/CT protocols, scanners, and imaging analysis systems. This imposes limitations on reproducibility so the optimal cutoff value of 18F-FDG PET parameters in the present study may not consistently be the best in other researches. Well-designed prospective study is needed to confirm the present results and to determine the prognostic value of 18F-FDG PET in NPC.
Conclusion
In summary, high SUVmax of the primary tumor or lymph node lesions is associated with local or regional recurrence of NPC. Patients with higher SUVmax had significantly worse survival, and should receive more aggressive treatment to improve their prognosis. Higher NTRs were associated with significantly worse DMFS and OS rates, suggesting that heterogeneity in the pretreatment 18F-FDG uptake between the primary tumor and lymph nodes is associated with high invasion and metastatic potential. Patients in different subgroups (LH, HL, and HH groups) required different cutoffs of NTR to avoid cross-sensitization. The above findings might help to identify patients who require boost irradiation to reduce the risk of recurrence or those who require more aggressive systemic treatment to reduce the risk of distant metastasis.
Availability of data and materials
Data are available upon reasonable request. The data sets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- SUVs:
-
Standardized uptake values
- NPC:
-
Nasopharyngeal carcinoma
- 18F-FDG PET:
-
18F-fluorodeoxyglucose positron-emission tomography
- SUVmax-t:
-
SUVmax of the primary tumor
- SUVmax-n:
-
SUVmax of the regional lymph nodes
- NTR:
-
SUVmax-n/SUVmax-t ratio
- OS:
-
overall survival
- DMFS:
-
distant metastasis-free survival
- PFS:
-
progression-free survival
- HR:
-
hazard ratio
- AJCC:
-
The American Joint Committee on Cancer
- IMRT:
-
intensity-modulated radiotherapy
- LRFS:
-
local recurrence-free survival
- RRFS:
-
regional recurrence-free survival
- ROC:
-
receiver operating characteristic
- CIs:
-
confidence intervals
- AUC:
-
under the curve
References
Chen Y-P, Chan ATC, Le Q-T, Blanchard P, Sun Y, Ma J. Nasopharyngeal carcinoma. Lancet. 2019;394:64–80.
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209–49.
Wong KCW, Hui EP, Lo KW, Lam WKJ, Johnson D, Li L, et al. Nasopharyngeal carcinoma: an evolving paradigm. Nat Rev Clin Oncol. 2021;18:679–95.
Zhang CD, Li M, Hong YJ, Cai ZM, Huang KC, Lin ZX, et al. Development and Validation of Prognostic Nomograms Based on Gross Tumor Volume and Cervical Nodal Volume for Nasopharyngeal Carcinoma Patients With Concurrent Chemoradiotherapy. Front Oncol. 2021;11:682271.
Liu LT, Tang LQ, Chen QY, Zhang L, Guo SS, Guo L, et al. The Prognostic Value of Plasma Epstein-Barr Viral DNA and Tumor Response to Neoadjuvant Chemotherapy in Advanced-Stage Nasopharyngeal Carcinoma. Int J Radiat Oncol Biol Phys. 2015;93:862–9.
Chan SC, Chang JT, Wang HM, Lin CY, Ng SH, Fan KH, et al. Prediction for distant failure in patients with stage M0 nasopharyngeal carcinoma: the role of standardized uptake value. Oral Oncol. 2009;45:52–8.
Chan WK, Kwong DL, Yeung DW, Huang B, Khong PL. Prognostic impact of standardized uptake value of F-18 FDG PET/CT in nasopharyngeal carcinoma. Clin Nucl Med. 2011;36:1007–11.
Cho H, Kim SH, Kim H, Koh YW, Kim SH, Choi EC, et al. Lymph Node With the Highest FDG Uptake Predicts Distant Metastasis-Free Survival in Patients With Locally Advanced Nasopharyngeal Carcinoma. Clin Nucl Med. 2018;43:e220–e5.
Hung TM, Fan KH, Kang CJ, Huang SF, Lin CY, Ho AT, et al. Lymph node-to-primary tumor standardized uptake value ratio on PET predicts distant metastasis in nasopharyngeal carcinoma. Oral Oncol. 2020;110:104756.
Lee SJ, Kay CS, Kim YS, Son SH, Kim M, Lee SW, et al. Prognostic value of nodal SUVmax of 18F-FDG PET/CT in nasopharyngeal carcinoma treated with intensity-modulated radiotherapy. Radiat Oncol J. 2017;35:306–16.
Jeong Y, Baek S, Park JW, Joo JH, Kim JS, Lee S-w. Lymph node standardized uptake values at pre-treatment 18F-fluorodeoxyglucose positron emission tomography as a valuable prognostic factor for distant metastasis in nasopharyngeal carcinoma. Br J Radiol. 2017;90:20160239.
Fei Z, Chen C, Huang Y, Qiu X, Li Y, Li L, et al. Metabolic tumor volume and conformal radiotherapy based on prognostic PET/CT for treatment of nasopharyngeal carcinoma. Med (Baltimore). 2019;98:e16327.
Fei Z, Xu T, Li M, Chen T, Li L, Qiu X, et al. Effectiveness and cost-effectiveness analysis of nimotuzumab for the radiotherapy of locoregionally advanced nasopharyngeal carcinoma. Radiat Oncol. 2020;15:230.
Tang LL, Chen YP, Chen CB, Chen MY, Chen NY, Chen XZ, et al. The Chinese Society of Clinical Oncology (CSCO) clinical guidelines for the diagnosis and treatment of nasopharyngeal carcinoma. Cancer Commun (Lond). 2021. https://doi.org/10.1002/cac2.12218 Epub ahead of print.
Soret M, Bacharach SL, Buvat I. Partial-volume effect in PET tumor imaging. J Nucl Med. 2007;48(6):932–45.
Yang Z, Shi Q, Zhang Y, Pan H, Yao Z, Hu S, et al. Pretreatment (18)F-FDG uptake heterogeneity can predict survival in patients with locally advanced nasopharyngeal carcinoma--a retrospective study. Radiat Oncol. 2015;8(10):4.
Chang KP, Tsang NM, Liao CT, Hsu CL, Chung MJ, Lo CW, et al. Prognostic significance of 18F-FDG PET parameters and plasma Epstein-Barr virus DNA load in patients with nasopharyngeal carcinoma. J Nucl Med. 2012;53(1):21–8.
Chan SC, Yeh CH, Chang JT, Chang KP, Wang JH, Ng SH. Combing MRI Perfusion and 18F-FDG PET/CT Metabolic Biomarkers Helps Predict Survival in Advanced Nasopharyngeal Carcinoma: A Prospective Multimodal Imaging Study. Cancers (Basel). 2021;13(7):1550.
Ma G, Gu B, Hu J, Kong L, Zhang J, Li Z, et al. Pretreatment 18F-FDG uptake heterogeneity can predict treatment outcome of carbon ion radiotherapy in patients with locally recurrent nasopharyngeal carcinoma. Ann Nucl Med. 2021;35(7):834–42.
Lee SW, Nam SY, Im KC, Kim JS, Choi EK, Ahn SD, et al. Prediction of prognosis using standardized uptake value of 2-[(18)F] fluoro-2-deoxy-d-glucose positron emission tomography for nasopharyngeal carcinomas. Radiother Oncol. 2008;87:211–6.
Xiao W, Xu A, Han F, Lin X, Lu L, Shen G, et al. Positron emission tomography-computed tomography before treatment is highly prognostic of distant metastasis in nasopharyngeal carcinoma patients after intensity-modulated radiotherapy treatment: a prospective study with long-term follow-up. Oral Oncol. 2015;51:363–9.
Lin CH, Hung TM, Chang YC, Hsieh CH, Shih MC, Huang SM, et al. Prognostic Value of Lymph Node-To-Primary Tumor Standardized Uptake Value Ratio in Esophageal Squamous Cell Carcinoma Treated with Definitive Chemoradiotherapy. Cancers (Basel). 2020;12:607.
Chung HH, Cheon GJ, Kim JW, Park NH, Song YS. Prognostic value of lymph node-to-primary tumor standardized uptake value ratio in endometrioid endometrial carcinoma. Eur J Nucl Med Mol Imaging. 2018;45:47–55.
Chung HH, Cheon GJ, Kim JW, Park NH, Song YS. Prognostic importance of lymph node-to-primary tumor standardized uptake value ratio in invasive squamous cell carcinoma of uterine cervix. Eur J Nucl Med Mol Imaging. 2017;44:1862–9.
Fei Z, Xu T, Qiu X, Li M, Chen T, Li L, et al. Significance of boost dose for T4 nasopharyngeal carcinoma with residual primary lesion after intensity-modulated radiotherapy. J Cancer Res Clin Oncol. 2021;147:2047–55.
Yeh SA, Tang Y, Lui CC, Huang YJ, Huang EY. Treatment outcomes and late complications of 849 patients with nasopharyngeal carcinoma treated with radiotherapy alone. Int J Radiat Oncol Biol Phys. 2005;62:672–9.
Zong J, Xu H, Chen B, Guo Q, Xu Y, Chen C, et al. Maintenance chemotherapy using S-1 following definitive chemoradiotherapy in patients with N3 nasopharyngeal carcinoma. Radiat Oncol. 2019;14:182.
Lee NY, Zhang Q, Pfister DG, Kim J, Garden AS, Mechalakos J, et al. Addition of bevacizumab to standard chemoradiation for locoregionally advanced nasopharyngeal carcinoma (RTOG 0615): a phase 2 multi-institutional trial. Lancet Oncol. 2012;13:172–80.
You R, Hua YJ, Liu YP, Yang Q, Zhang YN, Li JB, et al. Concurrent Chemoradiotherapy with or without Anti-EGFR-Targeted Treatment for Stage II-IVb Nasopharyngeal Carcinoma: Retrospective Analysis with a Large Cohort and Long Follow-up. Theranostics. 2017;7:2314–24.
Xia WX, Liang H, Lv X, Wang L, Qian CN, Ye YF, et al. Combining cetuximab with chemoradiotherapy in patients with locally advanced nasopharyngeal carcinoma: A propensity score analysis. Oral Oncol. 2017;67:167–74.
Acknowledgments
None
Funding
This work was supported by research projects for the Natural Science Foundation of Fujian Province (2020J011124) and Bethune-Translational Medicine Research Fund for Oncology radiotherapy (flzh202126).
Author information
Authors and Affiliations
Contributions
Study concept and design: CB C, ZD F, XF Q. Acquisition, analysis, or interpretation of data: All authors. Drafting of the manuscript: ZD F, XF Q. Critical revision of the manuscript for important intellectual content: All authors. Statistical analysis: ZD F, HX W, T X. Study supervision: CB C. The authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Ethical Committee of Fujian Cancer Hospital (YKT2020-011-01) and was in accordance with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Patient identifiers such as names were not collected, instead patients were given a numerical identifier. Informed consent was obtained from all participants and for those under 18 years, from a parent or legal guardian. For confidentiality, the patients’ charts were used only within the confines of the records department and only the investigators and study assistant had access to the files.
Consent for publication
Not required.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
: Supplementary Table 1, Supplementary Table 2, Supplementary Figures S1-S6
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Qiu, X., Wu, H., Xu, T. et al. Reflecting on the utility of standardized uptake values on 18F-FDG PET in nasopharyngeal carcinoma. BMC Cancer 22, 495 (2022). https://doi.org/10.1186/s12885-022-09626-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-022-09626-w