Abstract
Background
This study aimed to assess the effectiveness and cost-effectiveness of nimotuzumab in patients with locoregionally advanced nasopharyngeal carcinoma (LA-NPC).
Methods
LA-NPC patients treated between October 2013 and December 2016 were retrospectively reviewed. A well-balanced cohort of patients who received nimotuzumab in addition to standard treatment (n = 50) and patients who did not receive nimotuzumab (n = 100) was selected using propensity score-matching method (1:2 ratio) for the cost-effectiveness analysis.
Results
Compared with concurrent chemoradiotherapy (CCRT) alone, addition of nimotuzumab to CCRT significantly improved the 3-year overall survival (OS) (98.00% vs. 91.00%, P = 0.032). On multivariate analysis, nimotuzumab (hazard ratio = 0.124, 95% confidence interval: 0.017–0.902, P = 0.039) showed prognostic significance for OS. No serious treatment-related adverse events were observed in the nimotuzumab group (P > 0.05). Cost-effectiveness analysis revealed that addition of nimotuzumab increased the average treatment costs by $14,364.63. The additional cost for every one percent increase in OS rate was $ 2,052.09.
Conclusion
Addition of nimotuzumab to CCRT for LA-NPC confers significant survival benefits; however, it is not cost-effective.
Similar content being viewed by others
Introduction
Nasopharyngeal carcinoma (NPC) is a highly aggressive malignant tumor derived from nasopharyngeal epithelial cells. The disease is endemic in South China, Southeastern Asia, Middle East and North Africa [1, 2]. For anatomic constrain, up to 70% of newly diagnosed patients with nasopharyngeal cancer have locoregionally advanced disease [3] with an unfavorable prognosis. Platinum-based concurrent chemoradiotherapy (CCRT) with or without induction chemotherapy (IC) is the standard treatment for patients with locoregionally advanced nasopharyngeal carcinoma (LA-NPC) [4]. Application of intensity-modulated radiotherapy (IMRT) has helped improved locoregional control and survival [5, 6]. However, approximately 20% of patients with locoregionally advanced disease tend to develop recurrent disease and/or metastasis [7]. Therefore, optimizing the systemic treatment strategies is a key imperative to improve the survival of patients with locoregionally advanced disease.
Epidermal growth factor receptor (EGFR) overexpression is observed in more than 80% of NPC [8], and is associated with tumor invasiveness, treatment resistance, and poor prognosis [9]. Nimotuzumab (NTZ) is a blocking monoclonal antibody against EGFR, which is highly humanized and has a higher effective dose concentration compared with other anti-EGFR drugs [10]. Nimotuzumab is designed to reduce immunoreactivity and to enhance radio sensitivity with few serious complications and acceptable safety. Furthermore, several studies have demonstrated the favorable efficacy and safety profile of nimotuzumab administered in combination with CCRT in patients with LA-NPC [11]. In a multicenter randomized controlled study, induction therapy administered in combination with nimotuzumab showed better response than chemotherapy alone (77.8% vs 63.0%, P = 0.033) in patients with stage III-IVa NPC with EGFR expression [12]. Currently, nimotuzumab is approved only for the treatment of EGFR-positive stage III–IV NPC in combination with radiotherapy or chemoradiotherapy.
NPC has a high incidence in southeast China’s Fujian province (population 39.41 million). Our cancer center is the only specialist cancer hospital in Fujian. NPC is the dominant disease in our hospital, and it is representative in the epidemic area. The high cost of nimotuzumab has prevented its wider use for the treatment of NPC. The cost-effectiveness of addition of nimotuzumab to standard treatment for NPC is not known. In this study, the propensity score-matching (PSM) method was used to retrospectively analyze the efficacy of nimotuzumab in LA-NPC. Based on the analyses, we assessed the cost-effectiveness of nimotuzumab in combination with standard treatment for LA-NPC.
Materials and methods
Patients
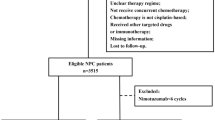
We reviewed the medical records of 1753 consecutive patients with newly confirmed NPC who under-went complete treatment at our center between October 2013 and December 2016. The inclusion criteria were: (1) stage III-IV disease in accordance to the American Joint Committee on Cancer (AJCC) staging system (7th edition, 2010); (2) confirmed by pathology; (3) positive EGFR expression; (4) Karnofsky performance score (KPS) ≥ 80; (5) treatment regimen: IC followed by CCRT with or without nimotuzumab; (6) radical IMRT. The exclusion criteria were as follows: (1) diagnosed with a previous malignancy or other concomitant malignant disease; (2) pregnancy or lactation; (3) received other anti-EGFR targeting therapy; (4) metastatic disease at diagnosis; (5) patients > 70 years old. Based on these criteria, 394 patients were selected for the matched study.
Treatment
Chemotherapy
IC followed by CCRT was recommended for patients with stage III–IV disease at our institution during the study reference period. The chemotherapy regimen comprised of 2–3 cycles of IC prior to CCRT. The IC regimen consisted of gemcitabine (1000 mg/m2, on days 1 and 8) plus cisplatin (80 mg/m2, on day 2); or paclitaxel (135 mg/m2, on day 1) plus cisplatin (80 mg/m2, on day 2). CCRT based on cisplatin (80 mg/m2) was administered intravenously for 2 cycles until the completion of RT. Chemotherapy, including CCRT and IC, were repeated every 3 weeks. Radiotherapy was administered simultaneously with the first cycle of concurrent chemotherapy [13].
Radiotherapy
All patients were treated with Volumetric Modulated Arc Therapy (VMAT), which is a novel form of IMRT. The target volume and radiotherapy dose were delineated using an institutional treatment protocol as previously reported [14]. The primary gross tumor volume (GTV-P) and the cervical metastatic lymph nodes (GTV-N), included all gross disease was determined by imaging (CT and MRI fusion), clinical, and endoscopic results. The dose to the GTV-P and GTV-N was 69.7–70.0 Gy administered in 31–35 fractions; the corresponding dose to the high-risk region (CTV1) was 62–62.7 Gy, while dose to the subclinical prophylactic low-risk region (CTV2) was 54.4–56.2 Gy administered in the same number of fractions. The planning target volume (PTV) was created on the basis of each volume with an additional 3 mm margin. Organ at risk (OAR) include the brain stem, spinal cord, optic nerve, optic chiasm, temporal lobe, crystal, and parotid, pituitary and mandibular glands.
Nimotuzumab
Owing to the high cost of nimotuzumab, its use depended on the patient’s preference, affordability, and the physician’s experience. Nimotuzumab was administered concomitantly with radiotherapy at a dose of 200 mg once weekly for 8 weeks, commencing on a day before IMRT. A total of 50 patients received full doses of nimotuzumab.
Follow-up and clinical endpoints
After completing standard treatment, the therapeutic effects were evaluated every 3 months for the first 2 years, then every 6 months up to the first 5 years, and once a year thereafter. Nasopharyngoscopy, enhanced MRI of the head and neck, chest computed tomography (CT), and abdominal ultrasound were routinely performed. In consideration of the value of 18F-Fluorodeoxyglucose positron emission tomography and CT (PET-CT) in the diagnosis and treatment of nasopharyngeal cancer, the PET-CT should be performed when the physician considers it necessary [15]. The final date of follow-up was January 2020.
The primary clinical endpoints were overall survival (OS, defined as time from diagnosis to death for any cause) and progression-free survival (PFS, time from diagnosis to disease progression or death from any cause), other outcome variables included locoregional relapse-free survival (LRRFS, time from diagnosis to local or regional recurrence or both) and distant metastasis-free survival (DMFS, time from diagnosis to first distant metastasis). The duration was calculated from the date of diagnosis to the date of each event or last follow-up. Acute toxicity was graded according to the Common Terminology Criteria for Adverse Events (version 3.0).
Statistical analysis
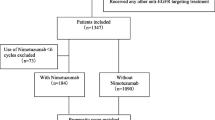
The baseline demographic and clinical characteristics of the study population were summarized (Table 1) and the differences between the nimotuzumab group and non-nimotuzumab group were compared using the Chi-squared test. Logistic regression analysis was used to identify confounders between the treatment groups. Propensity score-matching method was used in a 1:2 ratio to balance various factors, including sex, age, tumor stage (T stage), and node stage (N stage). Using a caliper width of 0.1, 1:2 matching was performed between patients in the nimotuzumab group and non-nimotuzumab group based on the propensity scores. After propensity score matching, a total of 150 patients were selected (50 patients in the nimotuzumab arm and 100 patients in the non-nimotuzumab arm) (Fig. 1). OS, PFS, LRRFS, and DMFS were calculated using the Kaplan–Meier method. Between-group differences in survival outcomes were assessed using the log-rank test. Multivariate analysis was performed using the Cox proportional hazards models. All statistical analyses were performed using the SPSS 22.0 (IBM Corporation, Chicago, IL, USA) and R 2.15.3 software. All tests were two-tailed and P values < 0.05 were considered indicative of statistical significance.
Based on the retrospectively analysis using PSM method, we performed a cost-effectiveness analysis from the perspective of traditional payers to measure the therapeutic value of nimotuzumab in LA-NPC [16, 17]. In this study, the direct costs in each patient’s statement of accounts during the in-hospital period were calculated as total costs, irrespective of the mode of payment (insurance or no insurance). The total costs include cost of anti-tumor drugs, radiotherapy, supportive drugs, hospitalization, therapies related to 3–4 AEs, and imaging or biochemical investigations [18, 19]. Indirect and implicit costs were ignored as a consequence of individual differences [20]. All costs are expressed in U.S. Dollars (USD), and the exchange rate in December 2016 was used [1 USD = 6.88 China Yuan (CNY)] [21].
The 3-year OS and 3-year PFS rates were used as measures of effectiveness. Cost-effectiveness ratio (C/E%) and the incremental cost-effectiveness ratio (ICER) were used as outcome measures for the cost-effectiveness analysis. The ICER was calculated by dividing the total cost difference between the nimotuzumab group and non-nimotuzumab group by the difference in effectiveness between the two groups [21]. In view of the recommendations of the 2015 China Guidelines for Pharmacoeconomic Evaluations and Manual, both the costs and the utility values were discounted at an annual rate of 3% [22].
Results
Patient characteristics
Clinical data pertaining to a total of 1753 consecutive NPC patients treated with IMRT were reviewed. Finally, 394 patients were eligible for propensity score-matching (Fig. 1). Out of the entire cohort of 394 LA-NPC patients, 50 patients treated with nimotuzumab were categorized as nimotuzumab group and 344 patients who did not receive nimotuzumab were categorized as non-nimotuzumab group. The basic characteristics of all patients are summarized in Table 1. To generate a comparable non-nimotuzumab group in a ratio of 1:2, 100 patients were selected by PSM from 344 patients. Gender, age, T stage, and N stage were used as matching factors and patient characteristics were well balanced between the two propensity-matched groups. Ultimately, nimotuzumab group included 50 patients who had received 2–3 cycles of IC followed by CCRT with nimotuzumab, and non-nimotuzumab group included 100 patients who had received 2–3 cycles of IC followed by CCRT without nimotuzumab. The baseline characteristics of PSM cohorts are summarized in Table 2.
In the entire cohort of 394 patients, the median age of patients was 47 (range 17–70) years, the ratio of male (n = 274) to female (n = 120) was 2.28:1, and the median duration of follow-up was 45 months (range 5–73).
Survival outcomes and effectiveness
In the original unmatched cohort of 394 patients, the 3-year OS, PFS, LRRFS and DMFS rates for nimotuzumab group vs. non-nimotuzumab group were 98.00% versus 88.66% (P = 0.013), 88.00% versus 79.94% (P = 0.099), 96.00% versus 95.06% (P = 0.525), and 92.00% versus 89.24% (P = 0.469), respectively. Significant difference in OS was observed between the two groups. The survival curves for OS and PFS are shown in Fig. 2.
In the propensity-matched cohort of 150 patients, the 3-year OS, PFS, LRRFS and DMFS rates for nimotuzumab group versus non-nimotuzumab group were 98.00% versus 91.00% (P = 0.032), 88.00% versus 83.00% (P = 0.306), 96.00% versus 93.00% (P = 0.444) and 92.00% versus 93.00% (P = 0.991.), respectively. Significant difference in OS was observed between the two groups. The survival curves for OS and PFS are shown in Fig. 3.
Multivariate analysis
The OS and PFS of the 394 eligible patients were analyzed by Cox regression models (Table 3). Based on the results of previous studies and the univariate analysis, multivariate analysis was performed to evaluate the following potential prognostic factors: age, gender, T stage, N stage, clinical stage, and nimotuzumab. The results indicated that nimotuzumab treatment [hazard ratio (HR) = 0.124, 95% confidence interval (CI): 0.017–0.902, P = 0.039] had prognostic significance for OS. Higher N stage was an independent predictor of poorer OS and PFS.
Cost-effectiveness analysis and sensitivity analysis
After propensity score-matching, the total costs of nimotuzumab group (n = 50) and non-nimotuzumab group (n = 100) were $ 34,135.80 and $ 19,771.17, respectively. Addition of nimotuzumab increased the treatment costs by $14,364.63. Thus, the cost-effectiveness ratio (C/E%) of 3-year OS and 3-year PFS in nimotuzumab group vs. non-nimotuzumab group were $ 348.32 versus $ 217.27 and $ 387.91 versus $ 238.21, respectively. The difference of effectiveness (3-year OS) was 7%. Therefore, the ICER was calculated as $ 2052.09, which means that every one percent increase in overall survival rate by using nimotuzumab costed an additional $ 2052.09.
We varied overall survival and progression-free survival by ± 10% in the sensitivity analyses; thus, the C/E% of 3-year OS and 3-year PFS in nimotuzumab group versus non-nimotuzumab group were $ 387.91 versus $ 244.09 and $ 437.64 versus $ 270.84, respectively.
Adverse reactions
Table 4 showed the incidence of acute toxicity in 394 patients. Hematological toxicity was the most frequently observed adverse reaction in the Nimotuzumab group. Nevertheless, there were no significant differences between the two groups with respect to hematologic parameters (P > 0.05). There was no significant between-group difference with respect to hepatoxicity, nephrotoxicity, gastrointestinal reactions, or acute radiation dermatitis and mucositis (P > 0.05 for all). Overall, treatment toxicity was well-tolerated, and no treatment-related deaths occurred in either group.
Discussion
Radio-chemotherapy is the standard treatment modality for stage III-IV NPC. Even with the best available treatment according to guidelines, approximately 5–15% of patients develop local failure, and 15–30% develop distant failure [23]. To further improve the therapeutic outcomes, many clinical trials have explored the effects of radiotherapy and chemotherapy administered in combination with novel therapies. With in-depth characterization of the molecular mechanisms of carcinogenesis and cancer progression, molecular targeted therapy for NPC patients has become a research hotspot [10]. The high expression of EGFR in NPC has been evaluated as a potential therapeutic target. Activation of EGFR pathway was shown to promote tumor cell growth, invasion and angiogenesis, prevent apoptosis, and induce chemoresistance and radioresistance [24].
Although there is no clear consensus, most studies suggest that anti-EGFR monoclonal antibodies, especially nimotuzumab and cetuximab, confer significant benefits in patients with LA-NPC. According to a meta-analysis, addition of anti-EGFR monoclonal antibodies to standard therapy for NPC significantly improved OS (HR, 0.51; 95% CI, 0.39–0.66) compared to standard therapy alone [25]. In a case–control study based on intelligence platform, concurrent administration of nimotuzumab/cetuximab with IC was found to be more effective, with a significant improvement in 3-year disease-free survival rate (84.3% vs. 74.3% P = 0.027) [9].
As the most commonly used anti-EGFR monoclonal antibody, cetuximab has shown good curative effect in the treatment of NPC; however, its use is associated with severe adverse reactions, such as oral mucositis and itchy rash [26]. To minimize the toxicity, a drug with a lower affinity constant, nimotuzumab, was developed; nimotuzumab shows a high uptake by tumor and low uptake by normal tissues [27]. Nimotuzumab selectively binds to tumors with moderate to high EGFR expression and rarely causes severe adverse reactions of skin and mucosa. Besides, it displays a longer half-life and elevated area under the curve than cetuximab at equivalent doses [28]. Many clinical trials have demonstrated that concomitant administration of nimotuzumab with concurrent radiotherapy may facilitate radiosensitivity and thus increase treatment efficacy [12, 29, 30]. A phase II clinical study of IC and sequential nimotuzumab combined with CCRT for NPC in stage N3 yielded a satisfactory survival benefit and tolerable toxicity, with 3-year OS, DMFS, and PFS rates of 85.6, 81.9, and 79.5%, respectively [29]. A retrospective paired analysis found that, compared to CCRT alone, CCRT plus nimotuzumab significantly improved the 5-year OS (96.8% vs. 82.3%; P = 0.001), DMFS (90.3% vs. 80.6%, P = 0.012), and PFS (83.9% vs. 71.0%, P = 0.006) rates [30]. These findings indicate a synergistic effect of nimotuzumab and radiotherapy in NPC.
The current study retrospectively analyzed the therapeutic efficacy in 394 patients with stage III-IV EGFR-positive NPC who received standard treatment with or without nimotuzumab. Consistent with previous studies, addition of nimotuzumab to standard treatment was shown to confer significant survival benefit and tolerable adverse reactions for LA-NPC. In the propensity-matched nimotuzumab group, the 3-year OS was 98.00%. The 3-year OS rate in the nimotuzumab group was significantly greater than that in the non-nimotuzumab group (98.00% vs. 91.00%, P = 0.032). On multivariate analysis, nimotuzumab was a significant prognostic factor for OS.
We also assessed the cost-effectiveness of the survival benefits conferred by nimotuzumab in the matched cohort. The average treatment cost in the nimotuzumab group was higher than that in the non-nimotuzumab group by $14,364.63. The C/E% of 3-year OS in nimotuzumab group and non-nimotuzumab group were $ 348.32 and $ 217.27, respectively. The ICER was calculated as $ 2052.09. The results of sensitivity analysis of 3-year OS and 3-year PFS were consistent with this finding. This implies that, although nimotuzumab can confer significant survival benefit, its addition to the current standard treatment for LA-NPC patients is unlikely to be considered as cost effective given the commonly accepted thresholds for cancer drugs in China. Based on the cost-effectiveness analysis, we recommend screening of high-risk patients to receive targeted therapy of nimotuzumab. Nimotuzumab may help improve outcomes of high-risk NPC patients. The high-risk factors of NPC include N2-3 stage, large primary tumor volume, unsatisfactory tumor response after IC, and high Epstein Barr virus DNA level after IC [31].
To the best of our knowledge, this is the first study that evaluated the cost-effectiveness of adding nimotuzumab to standard treatment for stage III-IV EGFR-positive NPC. A key strength of this study was the use of propensity score-matching method to minimize the influence of confounding factors; this allowed for a more robust cost-effectiveness analysis. Moreover, by refining costs and benefits, this analysis provides a comprehensive cost and benefit assessment for clinicians developing treatment plans. However, we acknowledge some limitations in this research. First, as a single-center study, due caution should be exercised while interpreting and extrapolating the results to other populations. Second, due to the small number of cases included in this study, larger studies are required to provide more definitive evidence.
Conclusion
Addition of nimotuzumab to the current standard treatment for LA-NPC was found to confer significant survival benefits; however, it was not found to be cost effective. Thus, targeted therapy with nimotuzumab should be recommended only for high-risk patients. Clinical trials with sufficiently large patient cohorts are required to confirm the efficacy, feasibility, and cost-effectiveness of nimotuzumab.
Availability of data and materials
The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- LA-NPC:
-
Locoregionally advanced nasopharyngeal carcinoma
- CCRT:
-
Concurrent chemoradiotherapy
- IC:
-
Induction chemotherapy
- IMRT:
-
Intensity-modulated radiotherapy
- EGFR:
-
Epidermal growth factor receptor
- NTZ:
-
Nimotuzumab
- PSM:
-
Propensity score-matching
- AJCC:
-
American Joint Committee on Cancer
- KPS:
-
Karnofsky performance score
- VMAT:
-
Volumetric Modulated Arc Therapy
- GTV-P:
-
Primary gross tumor volume
- GTV-N:
-
Gross tumor volume of the cervical metastatic lymph nodes
- MRI:
-
Magnetic resonance imaging
- OAR:
-
Organ at risk
- CT:
-
Chest computed tomography
- PET-CT:
-
18F-Fluorodeoxyglucose positron emission tomography and CT
- OS:
-
Overall survival
- PFS:
-
Progression-free survival
- LRRFS:
-
Locoregional relapse-free survival
- DMFS:
-
Distant metastasis-free survival
- RTOG:
-
Radiation therapy oncology group
- USD:
-
U.S. Dollars
- CNY:
-
China Yuan
- C/E%:
-
Cost-effectiveness ratio
- ICER:
-
Incremental cost-effectiveness ratio
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424.
Chua MLK, Wee JTS, Hui EP, Chan ATC. Nasopharyngeal carcinoma. Lancet. 2016;387:1012–24.
Yang Q, Cao SM, Guo L, et al. Induction chemotherapy followed by concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: long-term results of a phase III multicentre randomised controlled trial. Eur J Cancer. 2019;119:87–96.
Lee AWM, Tung SY, Ng WT, et al. A multicenter, phase 3, randomized trial of concurrent chemoradiotherapy plus adjuvant chemotherapy versus radiotherapy alone in patients with regionally advanced nasopharyngeal carcinoma: 10-year outcomes for efficacy and toxicity. Cancer. 2017;123:4147–57.
Mazzola R, Fiorentino A, Ricchetti F, et al. An update on radiation therapy in head and neck cancers. Expert Rev Anticancer Ther. 2018;18(4):359–64.
Blanchard P, Lee A, Marguet S, et al. Chemotherapy and radiotherapy in nasopharyngeal carcinoma: an update of the MAC-NPC meta-analysis. Lancet Oncol. 2015;16:645–55.
Ribassin-Majed L, Marguet S, Lee AWM, et al. What is the best treatment of locally advanced nasopharyngeal carcinoma? An individual patient data network meta-analysis. J Clin Oncol. 2017;35:498–505.
Liang ZG, Lin GX, Ye JX, et al. Cetuximab or nimotuzumab versus cisplatin concurrent with radiotherapy for local-regionally advanced nasopharyngeal carcinoma: a meta-analysis. Asian Pac J Cancer Prev. 2018;19:1397–404.
Peng H, Tang LL, Liu X, et al. Anti-EGFR targeted therapy delivered before versus during radiotherapy in locoregionally advanced nasopharyngeal carcinoma: a big-data, intelligence platform-based analysis. BMC Cancer. 2018;18:323.
Wang F, Jiang C, Ye Z, et al. Treatment outcomes of 257 patients with locoregionally advanced nasopharyngeal carcinoma treated with nimotuzumab plus intensity-modulated radiotherapy with or without chemotherapy: a single-institution experience. Transl Oncol. 2018a;11:65–73.
Wang F, Jiang F, Ye Z, et al. Efficacy and safety of nimotuzumab with neoadjuvant chemotherapy followed by concurrent chemoradiotherapy for locoregionally advanced nasopharyngeal carcinoma. Oncotarget. 2017;8:75544–56.
Lu Y, Chen D, Liang J, et al. Administration of nimotuzumab combined with cisplatin plus 5-fluorouracil as induction therapy improves treatment response and tolerance in patients with locally advanced nasopharyngeal carcinoma receiving concurrent radiochemotherapy: a multicenter randomized controlled study. BMC Cancer. 2019;19:1262.
Fei Z, Chen C, Huang Y, et al. Metabolic tumor volume and conformal radiotherapy based on prognostic PET/CT for treatment of nasopharyngeal carcinoma. Medicine (Baltimore). 2019;98:e16327.
Chen C, Fei Z, Pan J, Bai P, Chen L. Significance of primary tumor volume and T-stage on prognosis in nasopharyngeal carcinoma treated with intensity-modulated radiation therapy. Jpn J Clin Oncol. 2011;41:537–42.
Mazzola R, Alongi P, Ricchetti F, et al. 18F-Fluorodeoxyglucose-PET/CT in locally advanced head and neck cancer can influence the stage migration and nodal radiation treatment volumes. Radiol Med. 2017;122(12):952–9.
Shafrin J, Skornicki M, Brauer M, et al. An exploratory case study of the impact of expanding cost-effectiveness analysis for second-line nivolumab for patients with squamous non-small cell lung cancer in Canada: does it make a difference? Health Policy. 2018;122:607–13.
Kovic B, Xie F. Economic evaluation of bevacizumab for the first-line treatment of newly diagnosed glioblastoma multiforme. J Clin Oncol. 2015;33:2296–302.
Stintzing S, van Oostrum I, Pescott CP, Ronga P, Heeg B, Heinemann V. Cost-effectiveness of FOLFIRI + cetuximab vs FOLFIRI + bevacizumab in the first-line treatment of RAS wild-type metastatic colorectal cancer in Germany: data from the FIRE-3 (AIO KRK-0306) study. J Med Econ. 2020;23:1–8.
Zhang P, Wen F, Fu P, Yang Y, Li Q. Addition of docetaxel and/or zoledronic acid to standard of care for hormone-naive prostate cancer: a cost-effectiveness analysis. Tumori. 2017;103:380–6.
Jin C, Zheng H, Zhan M, Wen F, Xu T. Cost-effectiveness analysis of gemcitabine plus cisplatin versus fluorouracil plus cisplatin in the first-line setting for Chinese patients with metastatic nasopharyngeal carcinoma. Eur Arch Otorhinolaryngol. 2020;277:577–84.
Zhang H, Wang Y, Jiang ZM, et al. Impact of nutrition support on clinical outcome and cost-effectiveness analysis in patients at nutritional risk: a prospective cohort study with propensity score matching. Nutrition. 2017;37:53–9.
Chen Z, Zhan M, Tian F, Xu T. Cost-effectiveness analysis of the addition of bevacizumab to temozolomide therapy for the treatment of unresected glioblastoma. Oncol Lett. 2020;19:424–30.
Peng L, Liu JQ, Chen YP, Ma J. The next decade of clinical trials in locoregionally advanced nasopharyngeal carcinoma. Br J Radiol. 2019;92:1–11.
Sigismund S, Avanzato D, Lanzetti L. Emerging functions of the EGFR in cancer. Mol Oncol. 2018;12:3–20.
Peng L, Liu ZL, Xu C, et al. The efficacy and safety of anti-epidermal growth factor receptor monoclonal antibodies in nasopharyngeal carcinoma: literature-based meta-analyses. J Cancer. 2018;9:4510–20.
Feng HX, Guo SP, Li GR, et al. Toxicity of concurrent chemoradiotherapy with cetuximab for locoregionally advanced nasopharyngeal carcinoma. Med Oncol. 2014;31:170.
Ramakrishnan MS, Eswaraiah A, Crombet T, et al. Nimotuzumab, a promising therapeutic monoclonal for treatment of tumors of epithelial origin. MAbs. 2009;1(1):41–8.
Wang F, Jiang C, Ye Z, et al. Efficacy and safety of nimotuzumab plus radiotherapy with or without cisplatin-based chemotherapy in an elderly patient subgroup (aged 60 and older) with nasopharyngeal carcinoma. Transl Oncol. 2018b;11:338–45.
Zhang S, Huang X, Zhou L, et al. An open-label, single-arm phase II clinical study of induction chemotherapy and sequential Nimotuzumab combined with concurrent chemoradiotherapy in N3M0 stage nasopharyngeal carcinoma. JBUON. 2018;23(6):1656–61.
Yao JJ, Zhang LL, Gao TS, et al. Comparing treatment outcomes of concurrent chemoradiotherapy with or without nimotuzumab in patients with locoregionally advanced nasopharyngeal carcinoma. Cancer Biol Ther. 2018;19:1102–7.
Liu LT, Tang LQ, Chen QY, et al. The prognostic value of plasma epstein-barr viral DNA and tumor response to neoadjuvant chemotherapy in advanced-stage nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2015;93:862–9.
Acknowledgements
Not applicable.
Funding
“Startup Fund for scientific research, Fujian Medical University (2017XQ1210)” funded this study. The funding source has no role in study design, data collection, analysis, interpretation, the writing of the manuscript, or the decision to submit the study.
Author information
Authors and Affiliations
Contributions
Study concept and design: CC, ZF, XQ. Acquisition, analysis, or interpretation of data: All authors. Drafting of the manuscript: ZF, TX. Critical revision of the manuscript for important intellectual content: All authors. Statistical analysis: ZF, TX. Study supervision: CC. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Ethics Committee of Fujian cancer hospital approved the study protocol (No. YKT2020-O11-01), and this study was conducted in accordance with the Declaration of Helsinki. Because informed consent was deemed unnecessary by the ethical committee, the requirement to obtain written informed consent was waived.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Fei, Z., Xu, T., Li, M. et al. Effectiveness and cost-effectiveness analysis of nimotuzumab for the radiotherapy of locoregionally advanced nasopharyngeal carcinoma. Radiat Oncol 15, 230 (2020). https://doi.org/10.1186/s13014-020-01674-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13014-020-01674-5