Abstract
Background
Hepatocellular carcinoma (HCC) is one of the leading causes of cancer-related deaths worldwide. Sorafenib is the first-line treatment for advanced HCC, but the anti-cancer effects remain to be improved as indicated by its low response rates and failure to prolong the progression-free survival (PFS). Thus, it is urgent to explore approaches to improve the clinical outcome.
Materials and methods
The effect of Sorafenib in HCC was analyzed by SRB (sulforhodamine B) assay in normoxia and hypoxia, respectively. The different dose combination effect of CT707 and sorafenib was analyzed by SRB assay in hypoxia. Flow cytometry assay was used to detect the cell apoptosis rate with CT707 and sorafenib treatment in hypoxia. Western blotting was used to detect the expression levels of apoptosis -related proteins and the mechanism of CT707 overcome the resistance of sorafenib in hypoxia.
Results
Our study showed that the characteristic intratumor hypoxia of advanced HCC is one of the major factors which mediated the drug resistance towards sorafenib in HCC. And CT-707, a novel multi-kinase inhibitor, could sensitize the hypoxic HCC cells towards sorafenib. Further studies showed that CT-707 abolished the nuclear translocation of Yes Associate-Protein (YAP), which has been demonstrated as one of mechanism of hypoxia-mediated sorafenib-resistance in HCC.
Conclusions
Overall, this study not only favors the development of this novel multi-kinase inhibitor CT-707 as a therapeutic agent against HCC, but also provides a potential strategy to overcome the hypoxia-mediated resistance to sorafenib in HCC patients.
Similar content being viewed by others
Introduction
Hepatocellular carcinoma (HCC) is the leading cause of cancer-related deaths malignant solid tumor worldwide [1, 2]. Most HCC patients are diagnosed at advanced stage, which are generally suffered from the low efficiency to hepatic resection and liver transplantation therapy [3], therefore largely dependent on the systematic therapy including multi-kinase inhibitors or immunotherapy. Sorafenib, a multikinase inhibitor mainly targeting vascular endothelial growth factor receptors (VEGFR) 1–3, is the firstly-approved first-line kinase inhibitor for advance HCC treatment [4]. Nevertheless, a large number of HCC patients have low response towards sorafenib, or develop resistance to sorafenib very soon, and the progression free survival (PFS) almost remained unchanged with sorafenib treatment [5]. Several lines of evidence have implicated that intratumor hypoxia plays critical roles to the low efficacy of sorafenib in HCC patients [6,7,8]. Particularly, our previous study reveals that hypoxia-mediates resistance of sorafenib in HCC is conferred by the nuclear translocation of Yes-associated protein (YAP) which promoted cell survival and escaped apoptosis [9].
YAP is a transcriptional co-activator negatively-regulated by the upstream components of Hippo pathway, nuclear translocation of YAP would transactivate a variety of target genes to promote cell proliferation and inhibit apoptosis [10]. The aberrant over-activation of YAP was extensively observed in HCC tumor samples; particularly, in those hypoxic regions [11]. Therefore, the dysregulation of YAP not only leads to tumorigenesis under normoxia, but also plays indispensable roles in hypoxia-mediated malignant progression, including hypoxia-mediated sorafenib resistance [12]. In our recent study, we utilized a cell-based YAP-TEADs luciferase reporter system and identified a multi-kinase inhibitor CT-707, a novel anticancer drug candidate approved by National Medical Products Administration (NMPA) for phase I clinical trial (NCT02695550), as an novel YAP inhibitor possessing enhanced anti-cancer activity against hypoxic HCC [13], but whether CT-707 could abrogated the hypoxia-mediated resistance towards anti-cancer drugs remains unknown.
In this study, we demonstrated that CT-707 greatly improved the anticancer effects of sorafenib under hypoxia, reinforced the apoptotic population in sorafenib groups. These effects were accompanied with the inhibitory effect against YAP activity by CT-707. Given that CT-707 is multi-kinase inhibitor undergoing clinical trial, our findings demonstrate that this agent could overcome the hypoxia-mediated sorafenib resistance, provide a promising therapeutic strategy for the treatment of those HCC patients suffering from sorafenib resistance.
Materials and Methods
Materials
Sorafenib was obtained from Aladdin, CT-707 was provided by Centaurus Biopharma. The primary antibodies against cleaved Poly (ADP-ribose) polymerase (PARP), anti-YAP, phospho-YAP (Ser127) were purchased from Cell Signaling Technology. The primary antibody against β-actin and horseradish peroxidase (HRP)-labeled secondary anti-rabbit, anti-goat, and anti-mouse antibodies were purchased from Santa Cruz Biotechnology.
Cell lines and culture
The human HCC cell lines HepG2, SMMC-7721 and Bel-7402 were purchased from Shanghai Institutes for Biological Sciences (Chinese Academy of Sciences, Shanghai, China). Bel-7402 and SMMC-7721 were normally cultured in RPMI1640 medium (Gibco, Grand Island, NY, USA), all supplement with 10% FBS (Gibco, Grand Island, NY, USA) in a 5% CO2 humidified incubator at 37 degrees. And in order to get hypoxic cells. Cells were exposed to hypoxic conditions (1% O2) in a hypoxia incubator filled with a mixture of 1% O2, 5% CO2and 94% N2.
Cell proliferation assay
SMMC-7721 and Bel-7402 cells were treated with various concentrations of CT-707 in the first 24 h and then with various doses of sorafenib for 48 h in normoxic or hypoxic condition, and cell proliferation were measured by microscopy (Leica DM4000 B) and sulforhodamine B (SRB) protein assay (Sigma, S1402) [14]. For SRB, Cells (7000/well) were incubated with 10% trichloroacetic acid (TCA) for 1 h (4 °C) and washed it at least 3 times by water, then stained with sulforhodamine B for 30 min. The sulforhodamine B was washed away with 1% cold acetic acid, and 100 μl of 1% Tris-base was added to each well. The optical density (OD) was determined at 515 nm by a Multiskan Spectrum plate reader (Thermo Electron Corporation, Marietta, OH, USA). For clonogenic assay, Cells were observed and photographed by 10X objective, the results were analyzed by Leica Microscope Imaging Software.
Flow cytometry
Propidium iodide (PI) staining and annexin V-PI (AV-PI) staining were used to detect the apoptosis of cells by flow cytometry on a FACS Calibur cytometer (Becton Dickinson) as described previously [15].
Western blot
Cells were lysed with the loading buffer. Proteins were fractionated on 10% SDS-PAGE and transferred to polyvinylidene fluoride membrane (Millipore Corporation). The following antibodies were used: anti-YAP (Cell Signaling Technology, 4912 s), phospho-YAP (Ser127) (Cell Signaling Technology, #4911), β-actin (Santa Cruz, sc-1615), cleaved-PARP (Santa Cruz, sc-7150).
Statistical analysis
The results are expressed as the mean ± SD of at least 3 independent experiments. Differences between two means were analyzed by student's t-test and were considered statistically significant when P < 0.05.
Results
Hypoxia mediated sorafenib resistance of HCC cells
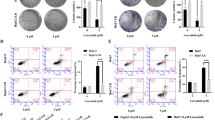
First, we confirmed the antitumor effect of the sorafenib in human HCC cell lines, SMMC-7721 and Bel-7402 in normoxia and hypoxia. Cells were treated with serial concentrations of sorafenib for 72 h, and then SRB staining assay was used to detect the survival fractions. The survival curve of sorafenib is shown in Fig. 1A. When cells were exposed to serial concentrations of sorafenib in normoxia or hypoxia, the survival rate of sorafenib in normoxia or hypoxia were determined respectively, and the results suggested that hypoxia significantly reduced the anti-cancer effect of sorafenib. To specifically evaluate the differential antitumor effect in normoxia and hypoxia, we calculated the half maximal inhibitory concentration (IC50) [16, 17]. The IC50 values of sorafenib in normoxia and hypoxia were 10.01 μM and 47.99 μM respectively (Fig. 1B). Similar results were obtained on Bel-7402 (Supplemental Fig. 1). These data demonstrate that hypoxic microenvironment conferred the resistance of HCC cells towards sorafenib.
Hypoxia mediated sorafenib resistance in hepatocellular carcinoma. A SMMC-7721 cell was treated with serial concentrations of sorafenib in normoxia and hypoxia and cell survival were detected using SRB assay. B The IC50 values of sorafenib in normoxia and hypoxia. Data are representative of 3 independent experiments and are expressed as the mean ± SD. The symbols refer to ** P < 0.01 and *** P < 0.001, respectively
Cytotoxicity of the combination of sorafenib and CT-707 in human HCC cell lines
Our previous study has uncovered the enhanced anti-cancer capacity of CT-707 (Fig. 2A) against the hypoxic HCC cells [13]; therefore, we were encouraged to ask whether the combination of sorafenib and CT-707 would exert enhanced effects under hypoxia. Normoxic or hypoxic SMMC-7721 cells were pre-treated with serial concentrations of CT-707 for the first 24 h, and then subjected to serial concentrations of sorafenib for the next 48 h. SRB assay was used to monitor the survival fractions of each group. As shown in Fig. 2B, the combination of sorafenib and CT-707 achieved synergistic anti-cancer effects under hypoxia, which was greater than that under normoxia. Specifically, when cells were exposed to sorafenib (20 μM), CT-707 (3 μM), or their combination in normoxia, the inhibition rates were 83.94%, 32.07%, and 92.94%, respectively. In the contrast, those under hypoxia were 29.76%, 40.32%, and 91.29%, respectively (Fig. 2C). These data revealed that the hypoxia-mediated resistance was remarkably attenuated by CT-707, and the combination of these two agents elicited robustly enhanced anti-cancer activities against hypoxic HCC cells than that under normoxia. Similar results were also achieved when cells were co-exposed to CT-707 at 4 μM (Fig. 2C).
Hypoxia-induced sorafenib resistance can be overcome by CT-707 in vitro. A Chemical structures of CT-707. B, C SMMC-7721 cell was treated with sorafenib at serial concentrations and CT-707 at different concentrations (2 μM, 3 μM, 4 μM), and the inhibition ratio was then determined by SRB assays. Data are representative of 3 independent experiments and are expressed as the mean ± SD. The symbols refer to ** P < 0.01 and *** P < 0.001, respectively. D SMMC-7721 cell was treated with sorafenib (10 μM), CT-707(3 μM) or both, and the cell density was observed by optical microscope
To further demonstrate that CT-707 could overcome the hypoxia-mediated resistance of sorafenib, we treated SMMC-7721 cells with sorafenib (10 μM), CT-707 (3 μM) or both under normoxia and hypoxia, respectively; and observed the cell morphology using optical microscope. Representative pictures of SMMC-7721 and Bel-7402 after 72 h in cell culture dishes were displayed in Fig. 2D and Supplemental Fig. 2. Combination treatment resulted in significant inhibition to the proliferation of SMMC-7721 and Bel-7402 under hypoxia, while the mono-treatment induced moderate inhibition. Taken together, these data suggested that the hypoxic resistance of sorafenib in HCC cells could be greatly abolished by CT-707.
CT-707 treatment strengthens the apoptosis-induction by sorafenib in hypoxic HCC cells
To further confirm that CT-707 could overcome sorafenib resistance under hypoxia, we assessed the apoptosis of SMMC-7721 and Bel-7402 cells after 72 h treatment by sorafenib (15 μM), CT-707(4 μM) or both. The results detected by PI staining following FACS analysis were shown in Fig. 3A and Supplemental Fig. 3, the apoptosis ratio (early + late apoptosis) of control, sorafenib, CT-707 and combination groups in SMMC-7721 were 7.49%, 15.90%, 21.60% and 71.03% respectively; and those in Bel-7402 were 0.08%, 34.40%, 27.71% and 61.76% respectively. The results indicated that the combination of these two agents enhanced the apoptosis in HCC compared with mono-treatment.
The combination of Sorafenib and CT-707 induced enhanced apoptosis of hepatocellular carcinoma cells. A SMMC-7721 cell was treated with sorafenib (15 μM), CT-707(4 μM) or both, and the cell apoptosis was detected by AV/PI staining (the units of the y-axis and x-axis are fluorescence intensity. y-axis: PI staining; x-axis: Annexin V). B SMMC-7721 was treated with sorafenib (20 μM), CT-707(3 μM) or both, and the protein expression levels of cleaved PARP (c-PARP) and β-actin in the cell lines were determined using western blot analysis. Full-length blots/gels are presented in Supplementary materials Figure 1A
Because most apoptotic cell death undergoes the caspase-dependent pathway [18], we further examine the activation of the caspase cascade of SMMC-7721 cell line after 72-h treatment by sorafenib (20 μM), CT-707(3 μM) or the combination using Western blotting. As demonstrated by Fig. 3A, the combined treatment of CT-707 an sorafenib significantly triggered caspase activation as indicated by the robust cleavage of PARP, the substrate of caspases cascade, which denoting more apoptosis in the combination groups. These findings collectively verified that the combination of CT-707 with sorafenib could significantly enhance the hypoxic anti-cancer activities in HCC.
CT-707 inhibits YAP nuclear translocation in HCC cell lines
Above-mentioned data illustrated the capability of CT-707 that increased the hypoxic HCC cell susceptibility towards sorafenib. Mounting evidence has implicated the critical roles of YAP signaling in hypoxia-mediated drug resistance [19, 20]. Particularly, our previous studies demonstrated that hypoxia-activated YAP pathway contributed to the decreased drug response of HCC cells towards sorafenib or SN-38 [9, 12]. Based on these findings, we performed a functional screening and identified CT-707 as a novel YAP inhibitor, and this agent possessed superior activity under hypoxia by suppressing hypoxia-induced YAP translocation. Therefore, we next investigated whether CT-707 overcome the resistance to sorafenib through its YAP-inhibitory effect.
The core components of the Hippo pathway include the mammalian sterile 20-like kinases (MSTs) and large tumor suppressor kinases (LATSs), impose negative regulation on YAP by phosphorylation on residue Ser127, leading to the cytoplasmic retention of YAP protein. On the contrary, the unphosphorylated YAP would translocate into the nucleus and exert its transactivation function. We assessed the phosphorylated and total levels of YAP in SMMC-7721 cells after 24-h exposure to sorafenib (10 μM), CT-707 (3 μM) or both using Western blot analysis. The results showed that hypoxia caused decreased levels of p-YAP (Ser127), denoting the nuclear translocation of YAP under hypoxic microenvironment (Fig. 4A). While CT-707 exposure under hypoxia significantly induced the phosphorylated YAP, which indicated that in these cells, YAP protein was detained in the cytoplasm (Fig. 4A). These results implied that CT-707 overcome the resistance of sorafenib in hypoxia by preventing YAP nuclear translocation.
CT-707 increase sorafenib-induced apoptosis under hypoxia by suppressing YAP target genes. A The protein levels of YAP, P-YAP and β-actin in SMMC-7721 cell were detected by western blot analysis in the same conditions as Fig. 2 (D). B A proposed model of crosstalk among Hippo signaling, sorafenib and CT-707 response in hypoxia. On the left, hypoxia caused decreased levels of p-YAP (Ser127), denoting the nuclear translocation of YAP under hypoxic microenvironment, which induce sorafenib resistance. On the right, CT-707 overcome the resistance of sorafenib in hypoxia by preventing YAP nuclear translocation. Full-length blots/gels are presented in Supplementary materials Figure 1B-D
Discussion
HCC is one of the most common cancer in the world. And the incidence of HCC has increased rapidly worldwide in the last decade [21]. The molecular pathogenesis of HCC varies according to the differential genotoxic insults and aetiologias including hepatitis B, C and other confounding factors such as tobacco use, obesity and alcohol abuse [22,23,24]. Sorafenib is the firstly-approved multikinase inhibitor to treat advanced HCC [25]. Clinical studies have shown some survival benefits on the time to progression (TTP) and overall survival (OS), but the benefits of sorafenib generally could not be sustained for long time treatment due to the acquisition of resistance [26, 27]. Therefore, it is urgent to explore potential strategies to alleviate sorafenib resistance in HCC.
HCC is a type of hypervascular tumor and angiogenesis plays an important role in the development of the disease [28]. Given the sophistication and intricacy of angiogenetic process, it would be extremely difficult for sorafenib to completely suppress blood vessel formation, particularly those microvessels inside HCC tumors [29]. Recent studies showed that Axitinib, a multiple tyrosine kinase inhibitor targeting VEGFR1, VEGFR2, VEGFR3, PDGFR and c-Kit, could improve the anticancer effects of sorafenib in advanced HCC patients [30]. Similar observation was obtained on apatinib, which is also a VEGFR2 inhibitor and showing synergistic effects with sorafenib [31, 32]. Notably, both combination regimens have been evaluated in clinical trials (NCT00678392, NCT02329860).
On the other hand, the clinical outcome of anti-angiogenesis strategies remains debated, recent findings reveal that anti-angiogenic treatment has limited efficacy due to therapy-induced blood vessel alterations, often followed by severe intratumor hypoxia, tumor adaptation, progression and metastasis [33]. Particularly, mounting evidence has established the causal link between hypoxia and reduced susceptibility towards sorafenib. Liang and colleagues found that HIF1α which was induced by hypoxia may contribute to the hypoxic resistance of sorafenib, and EF24, a curcumin analogue, could synergistically strengthen the antitumor effects of sorafenib and overcome sorafenib resistance by inhibiting HIF-1α and promoting proteasomal degradation by up-regulating tumor suppressor Von Hippel-Lindau [8]. Another study conducted by Lin et al. uncovers a critical function for METTL3-mediated m6A modification in the hypoxic tumor microenvironment and identifies FOXO3 as an important target of m6A modification in the resistance of HCC to sorafenib therapy [34]. Our previous study demonstrated that hypoxia induced the nuclear translocation of YAP, subsequently transactivated YAP target genes which promoted cell survival and escaped apoptosis, thereby leading to sorafenib resistance [9, 12].
The important roles of YAP in those HCC tumors presenting hypoxic region raise the feasibility of targeting YAP to interfere with hypoxia-related malignancy including sorafenib resistance. According to our previous study, Statins, the inhibitors of hydroxymethylglutaryl-CoA reductase (HMGCR), could ameliorate hypoxia-provoked nuclear YAP and improve the anti-cancer activity of sorafenib both in vitro and in vivo [9]. However, the mono-treatment of Statins failed to exert robust anticancer activities, which may hamper the clinical application of HMGCR inhibitors in HCC treatment [35]. Therefore, the possibility to combine more potent YAP inhibitor with sorafenib to combat with the hypoxia-caused resistance deserve to be further explored.
CT-707 is a novel multikinase inhibitor that was recently approved by the NMPA for clinical trial in NSCLC. Preclinical study found that CT-707 shows anti-cancer activities against different cancer models, including inhibition of both tumor growth and metastasis. Our previous study showed that CT-707 displayed an ability which inhibits the nuclear translocation of YAP on HCC models [36], so there is a possibility that CT-707 can overcome sorafenib-resistance in hypoxia by inhibiting the dephosphorylation and nuclear translocation of YAP, and the present study demonstrated the hypoxia-mediated sorafenib resistance indeed abated by the co-exposure to CT-707, accompanied with the apoptosis induction [13].
Conclusions
In summary, our findings demonstrated that the combination of sorafenib and CT-707 exerted synergistic in vitro activity in hypoxic HCC cells. Further studies showed that CT-707 inhibited the activation of YAP through interfering with the dephosphorylation and nuclear translocation of YAP under hypoxia, which would reverse the sorafenib-resistance in HCC under hypoxia (Fig. 4B). And our study provides a promising therapeutic strategy for HCC and expands the horizon for the clinical applications of improving the effect of sorafenib in advanced HCC patients.
Availability of data and materials
The data used to support the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
- HCC:
-
Hepatocellular Carcinoma
- PFS:
-
Progression-Free Survival
- SRB:
-
The sulforhodamine B (SRB) assay
- YAP:
-
Yes Associate-Protein
- VEGFR:
-
Vascular Endothelial Growth Factor Receptors
- NMPA:
-
National Medical Products Administration
- PARP:
-
Poly (ADP-ribose) polymerase
- TCA:
-
Trichloroacetic Acid
- PI:
-
Propidium Iodide
- AV-PI:
-
Annexin V-PI
- IC50 :
-
Inhibitory Concentration
- TTP:
-
The Time to Progression
- OS:
-
Overall Survival
- HIF-1α:
-
Hypoxia-inducible factor 1-alpha
- FOXO3:
-
Forkhead Box O3
- HMGCR:
-
Hydroxymethylglutaryl-CoA Reductase
- NSCLC:
-
Non-Small Cell Lung Cancer
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. https://doi.org/10.3322/caac.21590.
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. https://doi.org/10.3322/caac.21262.
Llovet JM, Villanueva A, Lachenmayer A, Finn RS. Advances in targeted therapies for hepatocellular carcinoma in the genomic era. Nat Rev Clin Oncol. 2015;12(8):436. https://doi.org/10.1038/nrclinonc.2015.103.
Gores GJ. Decade in review-hepatocellular carcinoma. HCC-subtypes, stratification and sorafenib. Nat Rev Gastroenterol Hepatol. 2014;11(11):645–7. https://doi.org/10.1038/nrgastro.2014.157.
Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–90. https://doi.org/10.1056/nejmoa0708857.
Mendez-Blanco C, Fondevila F, Garcia-Palomo A, Gonzalez-Gallego J, Mauriz JL. Sorafenib resistance in hepatocarcinoma: role of hypoxia-inducible factors. Exp Mol Med. 2018;50(10):1–9. https://doi.org/10.1038/s12276-018-0159-1.
Zhu YJ, Zheng B, Wang HY, Chen L. New knowledge of the mechanisms of sorafenib resistance in liver cancer. Acta Pharmacol Sin. 2017;38(5):614–22. https://doi.org/10.1038/aps.2017.5.
Liang Y, Zheng T, Song R, Wang J, Yin D, Wang L, Liu H, Tian L, Fang X, Meng X, et al. Hypoxia-mediated sorafenib resistance can be overcome by EF24 through Von Hippel-Lindau tumor suppressor-dependent HIF-1alpha inhibition in hepatocellular carcinoma. Hepatology. 2013;57(5):1847–57. https://doi.org/10.1002/hep.26224.
Zhou TY, Zhuang LH, Hu Y, Zhou YL, Lin WK, Wang DD, Wan ZQ, Chang LL, Chen Y, Ying MD, et al. Inactivation of hypoxia-induced YAP by statins overcomes hypoxic resistance tosorafenib in hepatocellular carcinoma cells. Sci Rep. 2016;6:30483. https://doi.org/10.1038/srep30483.
Liu H, Du S, Lei T, Wang H, He X, Tong R, Wang Y. Multifaceted regulation and functions of YAP/TAZ in tumors (Review). Oncol Rep. 2018;40(1):16–28. https://doi.org/10.3892/or.2018.6423.
Liu Y, Wang X, Yang Y. Hepatic Hippo signaling inhibits development of hepatocellular carcinoma. Clin Mol Hepatol. 2020;26(4):742–50. https://doi.org/10.3350/cmh.2020.0178.
Dai XY, Zhuang LH, Wang DD, Zhou TY, Chang LL, Gai RH, Zhu DF, Yang B, Zhu H, He QJ. Nuclear translocation and activation of YAP by hypoxia contributes to the chemoresistance of SN38 in hepatocellular carcinoma cells. Oncotarget. 2016;7(6):6933–47. https://doi.org/10.18632/oncotarget.6903.
Zhu H, Wang DD, Yuan T, Yan FJ, Zeng CM, Dai XY, Chen ZB, Chen Y, Zhou T, Fan GH, et al. Multi-kinase inhibitor CT-707 targets liver cancer by interrupting the hypoxia-activated IGF-1R-YAP axis. Cancer Res. 2018. https://doi.org/10.1158/0008-5472.can-17-1548.
Zhu H, Huang M, Yang F, Chen Y, Miao ZH, Qian XH, Xu YF, Qin YX, Luo HB, Shen X, et al. R16, a novel amonafide analogue, induces apoptosis and G2-M arrest via poisoning topoisomerase II. Mol Cancer Ther. 2007;6(2):484–95. https://doi.org/10.1158/1535-7163.mct-06-0584.
Zheng L, Fu Y, Zhuang L, Gai R, Ma J, Lou J, Zhu H, He Q, Yang B. Simultaneous NF-kappaB inhibition and E-cadherin upregulation mediate mutually synergistic anticancer activity of celastrol and SAHA in vitro and in vivo. Int J Cancer. 2014;135(7):1721–32. https://doi.org/10.1002/ijc.28810.
Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. https://doi.org/10.1016/0065-2571(84)90007-4.
Chou TC, Talalay P. Generalized equations for the analysis of inhibitions of Michaelis-Menten and higher-order kinetic systems with two or more mutually exclusive and nonexclusive inhibitors. Eur J Biochem. 1981;115(1):207–16. https://doi.org/10.1111/j.1432-1033.1981.tb06218.x.
Oliver FJ, de la Rubia G, Rolli V, Ruiz-Ruiz MC, de Murcia G, Murcia JM. Importance of poly(ADP-ribose) polymerase and its cleavage in apoptosis. Lesson from an uncleavable mutant. J Biol Chem. 1998;273(50):33533–9. https://doi.org/10.1074/jbc.273.50.33533.
Zhou Y, Wang Y, Zhou W, Chen T, Wu Q, Chutturghoon VK, Lin B, Geng L, Yang Z, Zhou L, et al. YAP promotes multi-drug resistance and inhibits autophagy-related cell death in hepatocellular carcinoma via the RAC1-ROS-mTOR pathway. Cancer Cell Int. 2019;19:179. https://doi.org/10.1186/s12935-019-0898-7.
Gujral TS, Kirschner MW. Hippo pathway mediates resistance to cytotoxic drugs. Proc Natl Acad Sci U S A. 2017;114(18):E3729–38. https://doi.org/10.1073/pnas.1703096114.
Kim E, Viatour P. Hepatocellular carcinoma: old friends and new tricks. Exp Mol Med. 2020;52(12):1898–907. https://doi.org/10.1038/s12276-020-00527-1.
Tanaka Y, Kurbanov F, Mano S, Orito E, Vargas V, Esteban JI, Yuen MF, Lai CL, Kramvis A, Kew MC, et al. Molecular tracing of the global hepatitis C virus epidemic predicts regional patterns of hepatocellular carcinoma mortality. Gastroenterology. 2006;130(3):703–14. https://doi.org/10.1053/j.gastro.2006.01.032.
El-Serag HB, Tran T, Everhart JE. Diabetes increases the risk of chronic liver disease and hepatocellular carcinoma. Gastroenterology. 2004;126(2):460–8. https://doi.org/10.1053/j.gastro.2003.10.065.
Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348(17):1625–38. https://doi.org/10.1056/nejmoa021423.
Raza A, Sood GK. Hepatocellular carcinoma review: current treatment, and evidence-based medicine. World J Gastroenterol. 2014;20(15):4115–27. https://doi.org/10.3748/wjg.v20.i47.18059.
Gao JJ, Shi ZY, Xia JF, Inagaki Y, Tang W. Sorafenib-based combined molecule targeting in treatment of hepatocellular carcinoma. World J Gastroenterol. 2015;21(42):12059–70. https://doi.org/10.3748/wjg.v21.i42.12059.
Woo HY, Heo J. Sorafenib in liver cancer. Expert Opin Pharmacother. 2012;13(7):1059–67. https://doi.org/10.1517/14656566.2012.679930.
Pecchi A, Besutti G, De Santis M, Del Giovane C, Nosseir S, Tarantino G, Di Benedetto F, Torricelli P. Post-transplantation hepatocellular carcinoma recurrence: Patterns and relation between vascularity and differentiation degree. World J Hepatol. 2015;7(2):276–84. https://doi.org/10.4254/wjh.v7.i2.276.
Sun H, Zhu MS, Wu WR, Shi XD, Xu LB. Role of anti-angiogenesis therapy in the management of hepatocellular carcinoma. The jury is still out. World J Hepatol. 2014;6(12):830–5. https://doi.org/10.4254/wjh.v6.i12.830.
Lin ZZ, Chen BB, Hung YP, Huang PH, Shen YC, Shao YY, Hsu CH, Cheng AL, Lee RC, Chao Y, et al. A Multicenter Phase II Study of Second-Line Axitinib for Patients with Advanced Hepatocellular Carcinoma Failing First-Line Sorafenib Monotherapy. Oncologist. 2020;25(9):e1280–5. https://doi.org/10.1634/theoncologist.2020-0143.
He W, Liao L, Hu D, Li B, Wang C, Qiu J, Liao Y, Liu W, Yang Z, Zhang Y, et al. Apatinib versus sorafenib in patients with advanced hepatocellular carcinoma: a preliminary study. Ann Transl Med. 2020;8(16):1000. https://doi.org/10.21037/atm-20-5298.
Scott AJ, Messersmith WA, Jimeno A. Apatinib: a promising oral antiangiogenic agent in the treatment of multiple solid tumors. Drugs Today (Barc). 2015;51(4):223–9. https://doi.org/10.1358/dot.2015.51.4.2320599.
Vasudev NS, Reynolds AR. Anti-angiogenic therapy for cancer: current progress, unresolved questions and future directions. Angiogenesis. 2014;17(3):471–94. https://doi.org/10.1007/s10456-014-9420-y.
Lin Z, Niu Y, Wan A, Chen D, Liang H, Chen X, Sun L, Zhan S, Chen L, Cheng C, et al. RNA m(6) A methylation regulates sorafenib resistance in liver cancer through FOXO3-mediated autophagy. EMBO J. 2020;39(12):e103181. https://doi.org/10.15252/embj.2019103181.
Mansourian PG, Yoneda M, Krishna Rao M, Martinez FJ, Thomas E, Schiff ER. Effects of statins on the risk of hepatocellular carcinoma. Gastroenterol Hepatol (N Y). 2014;10(7):417–26.
Wang DD, Chen Y, Chen ZB, Yan FJ, Dai XY, Ying MD, Cao J, Ma J, Luo PH, Han YX, et al. CT-707, a Novel FAK Inhibitor, Synergizes with Cabozantinib to Suppress Hepatocellular Carcinoma by Blocking Cabozantinib-Induced FAK Activation. Mol Cancer Ther. 2016;15(12):2916–25. https://doi.org/10.1158/1535-7163.mct-16-0282.
Acknowledgements
Not applicable.
Funding
This work was supported by the Natural Science Foundation of China (No. 81773753, No. 81973349).
Author information
Authors and Affiliations
Contributions
All authors participated in the drafting of the article or its critical revision for important intellectual content and approved the publication of the final version of the manuscript. ZC, TY, BY and HZ had full access to all the study data and take responsibility for data integrity and the accuracy of data analysis results. ZC, QH, BY and HZ conceived and designed the study. ZC, TY, FY, QX and NL performed the cell biology and molecular biology experiments. ZC, TY, SY and BZ collected and analyzed the data. CZ, TY organized figures and wrote the manuscript. All authors have read and approved the manuscript and ensure that this is the case.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors have agreed to be published.
Competing interests
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Supplemental figure 1. Bel-7402 cell wastreated with serial concentrations of sorafenib in normoxia and hypoxia andcell survival were detected using SRB assay. Data are representative of 3independent experiments and are expressed as the mean ± SD. The symbols *** P < 0.001. Supplemental figure 2. Bel-7402 cell was treated with sorafenib (10 μM), CT-707(3 μM) or both, and thecell density was observed by optical microscope. Supplemental figure 3. Bel-7402 cell was treated with sorafenib (15μM), CT-707(4 μM) or both, and the cell apoptosis was detected by AV/PIstaining (the units of the y-axis and x-axis are fluorescence intensity.y-axis: PI staining; x-axis: Annexin V).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chen, Z., Yuan, T., Yan, F. et al. CT-707 overcomes hypoxia-mediated sorafenib resistance in Hepatocellular carcinoma by inhibiting YAP signaling. BMC Cancer 22, 425 (2022). https://doi.org/10.1186/s12885-022-09520-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-022-09520-5