Abstract
Background
Olanzapine has been reported to be an effective antiemetic in patients receiving carboplatin-based chemotherapy. However, the efficacy of a neurokinin-1 receptor antagonist (NK1RA) added to olanzapine, a 5-hydroxytryptamine-3 receptor antagonist (5-HT3RA), and dexamethasone (DEX) has not been proven. This study aimed to assess the efficacy and safety of NK1RA, in combination with three-drug antiemetic regimens containing olanzapine, in preventing nausea and vomiting induced by carboplatin-based chemotherapy.
Methods
Data were pooled for 140 patients receiving carboplatin-based chemotherapy from three multicenter, prospective, single-arm, open-label phase II studies that evaluated the efficacy and safety of olanzapine for chemotherapy-induced nausea and vomiting. The propensity score of the co-administration of NK1RA was estimated for each patient using a logistic regression model that included age, sex, and carboplatin dose. We analyzed a total of 62 patients, who were treated without NK1RA (non-NK1RA group: 31 patients) and with NK1RA (NK1RA group: 31 patients). The patients were selected using propensity score matching.
Results
The complete response rate (without emetic episodes or with no administration of rescue medication) in the overall period (0–120 h post carboplatin administration) was 93.5% in the non-NK1RA group and 96.8% in the NK1RA group, with a difference of -3.2% (95% confidence interval, -18.7% to 10.9%; P = 1.000). In terms of safety, there was no significant difference between the groups in daytime sleepiness and concentration impairment, which are the most worrisome adverse events induced by olanzapine.
Conclusions
The findings suggest that antiemetic regimens consisting of olanzapine, 5HT3RA, and DEX without NK1RA may be a treatment option for patients receiving carboplatin-based chemotherapy.
Similar content being viewed by others
Background
Carboplatin is classified as a moderate-emetic-risk chemotherapy (MEC) or high-emetic-risk chemotherapy (HEC) [1,2,3,4]. Jordan et al. conducted a systematic review and meta-analysis of randomized controlled trials that assessed the effects of adding a neurokinin-1 receptor antagonist (NK1RA) to a 5-hydroxytryptamine-3 receptor antagonist (5-HT3RA) and dexamethasone (DEX) in MEC [5]. In this study, a total of 1790 patients from seven trials were analyzed, and the results of 1538 patients for whom complete response (CR) rate could be assessed supported the NK1RA combined regimen for carboplatin-based chemotherapy with an absolute risk difference of 15% and an odds ratio of 1.96 (95% confidence interval [CI]: 1.57–2.45; p < 0.001). Currently, international antiemetic guidelines consistently recommend a three-drug antiemetic prophylaxis with NK1RA, 5-HT3RA, and DEX in patients receiving carboplatin-based chemotherapy [1,2,3,4].
Olanzapine is an antipsychotic drug that is classified as a multi-acting, receptor-targeted agent. It has been reported to be a highly effective antiemetic drug in patients receiving MEC and/or HEC [6,7,8,9,10,11,12]. Three high-quality phase II studies have reported the efficacy and safety of 5 mg olanzapine for antiemetic prophylaxis in patients receiving carboplatin-based chemotherapy [13,14,15]. Two of these studies evaluated the antiemetic effects of a four-drug combination consisting of olanzapine, NK1RA, 5-HT3RA, and DEX, and one evaluated a three-drug combination consisting of olanzapine, 5-HT3RA, and DEX. To the best of our knowledge, there are no phase III studies evaluating the efficacy and safety of olanzapine for the management of nausea and vomiting in cancer patients receiving carboplatin-based chemotherapy. Therefore, we integrated these three phase II studies and reported the efficacy and safety of olanzapine in patients receiving carboplatin-based chemotherapy and the risk factors associated with carboplatin-induced nausea and vomiting [16].
The results showed that olanzapine had an antiemetic effect with a CR rate (defined as no emetic episodes and no administration of rescue medication for nausea and vomiting) of 87.9% in the overall period (0–120 h). In the analysis of risk factors affecting carboplatin-induced nausea and vomiting, co-administration of NK1RA was not significantly associated with carboplatin-induced nausea and vomiting. This integrated analysis is the only study that analyzes the effect of NK1RA, when added to an olanzapine-containing antiemetic regimen, on carboplatin-induced nausea and vomiting. However, the efficacy of NK1RA in combination with an olanzapine-containing antiemetic regimen remains to be demonstrated. Therefore, the present study aimed to evaluate the efficacy and safety of the combination of NK1RA, olanzapine, 5-HT3RA, and DEX in preventing carboplatin-induced nausea and vomiting in a propensity score-matched analysis.
Methods
Study design
We analyzed 62 patients, treated without NK1RA (non-NK1RA group, 31 patients) and with NK1RA (NK1RA group: 31 patients), using a propensity score-matched sample from the pooled data of 140 patients receiving carboplatin-based chemotherapy. The data were from three multicenter, prospective, single-arm, open-label, phase II studies.
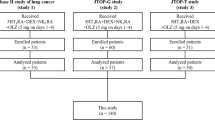
The results of these three phase II studies and the integrated analysis of the pooled data of 140 patients have been published previously [13,14,15,16]. Study 1 reported the efficacy of a four-drug combination consisting of olanzapine (orally: 5 mg on days 1–4), aprepitant (orally: 125 mg on day 1 and 80 mg on days 2 and 3), 5-HT3RA (intravenously: granisetron 1 mg, granisetron 3 mg, palonosetron 0.75 mg, or ramosetron 0.3 mg on day 1), and DEX (intravenously: 4.95 mg on day 1) in 33 patients with lung cancer [13]. Study 2 reported the efficacy of a four-drug combination consisting of olanzapine (orally: 5 mg on day 1 to 4), aprepitant (orally: 125 mg on day 1 and 80 mg on days 2 and 3), granisetron (intravenously: 1 mg on day 1), and DEX (intravenously: 9.9 mg on day 1) in 57 patients with gynecological cancer [14]. Study 3 reported the efficacy of a three-drug combination consisting of olanzapine (orally: 5 mg on day 1 to 4), granisetron (intravenously: 1 mg on day 1), and DEX (intravenously/orally: 9.9 mg/12 mg on day 1 and 6.6 mg/8 mg on days 2 and 3) in 50 patients with thoracic malignancies [15]. The patient enrollment flowchart for the present study is shown in Fig. 1.
Data collection
Data were collected from self-reported diaries. Patients reported nausea, decreased appetite, somnolence, and decreased concentration severity using a four-point scale (none, mild, moderate, and severe), as well as frequency of vomiting, and the use of rescue medication. The daily diary began from the initiation of carboplatin treatment on day 1, and entries were made over a 5-day period (Studies 1 and 3) and a 7-day period (Study 2).
Outcome
The primary endpoints for efficacy were CR rate, defined as the proportion of patients without emetic episodes or administration of rescue medication; complete control (CC) rate, defined as the proportion of patients with CR and no more than mild nausea; and total control (TC) rate, defined as the proportion of patients with CR and no nausea. The assessment periods for carboplatin-induced nausea and vomiting were 0–120 h post carboplatin administration (overall period), 0–24 h post carboplatin administration (acute period), and 24–120 h post carboplatin administration (delayed period). Additionally, the secondary endpoints for efficacy were incidences of nausea, vomiting, and decreased appetite for 5 days after the initiation of carboplatin treatment on day 1.
The endpoints for safety were incidences of somnolence and decreased concentration for 5 days after the initiation of carboplatin treatment on day 1.
Statistical analysis
Patient characteristics, rate of carboplatin-induced nausea and vomiting control, and treatment-related adverse events were summarized using descriptive statistics or reported in terms of frequencies and proportions of total patients. The propensity score of the co-administration of NK1RA was estimated for each patient using a logistic regression model that included age, sex, and carboplatin dose which most potentially affect the occurrence of chemotherapy-induced nausea and vomiting (CINV) in patients [17,18,19,20]. In the propensity score matching, 1:1 nearest neighbor matching algorithm without replacement was employed with a caliper width equal to 0.2 of the standard deviation of the logit of the propensity score [21]. The difference in the primary endpoints between the NK1RA and non-NK1RA groups was shown with a two-sided exact CI [22] and compared using Fisher’s exact test. All statistical analyses were performed using JMP 15.0.0 and SAS version 9.4 (SAS Institute, Inc., Cary, NC, USA). All P-values were two-sided, and statistical significance was set at P < 0.05.
Results
Study patients
A total of 62 patients were included in the analysis. Of these patients, 31 were in the non-NK1RA group and 31 in the NK1RA group. Baseline patient characteristics are presented in Table 1. The median ages of patients in the non-NK1RA group and those in the NK1RA group were 71 years (range, 25th and 75th percentiles, 67–76 years) and 71 years (range, 25th and 75th percentiles, 65–77 years), respectively. The proportion of males (58.1%) to females (41.9%) was similar in both groups.
Efficacy
The primary endpoints of efficacy are shown in Table 2. As shown in the table, CR rates for the overall, delayed, and acute periods in the non-NK1RA and NK1RA groups did not show any statistically significant difference. Likewise, the CC and TC rates in the non-NK1RA group, during each period, were not significantly different from those in the NK1RA group.
The secondary endpoints for efficacy are shown in Fig. 2. Patient-reported nausea, vomiting, and decreased appetite in the overall period were not significantly different between the two groups. The incidence of nausea was 12.9% in the non-NK1RA group and 16.1% in the NK1RA group (P = 1.000), that of vomiting was 6.5% in the non-NK1RA group and 3.2% in the NK1RA group (P = 1.000), and that of decreased appetite was 58.1% in the non-NK1RA group and 61.3% in the NK1RA group (P = 1.000).
Safety
Data on somnolence and decreased concentration assessed by the patients’ self-reported diaries are shown in Fig. 3. The incidence of somnolence was 83.9% in the non-NK1RA group and 80.6% in the NK1RA group. However, moderate or severe somnolence was 6.5% in the non-NK1RA group and 0% in the NK1RA group. The incidence of decreased concentration was 48.4% in the non-NK1RA group and 48.4% in the NK1RA group. However, moderate or severe decreased concentration was 3.2% in the non-NK1RA group and 0% in the NK1RA group. The peak incidence of somnolence and decreased concentration was observed on day 4 in both groups.
Discussion
To the best of our knowledge, there are no studies that have evaluated the efficacy of adding NK1RA to antiemetic therapy consisting of olanzapine, 5HT3RA, and DEX in MEC and HEC. In the present study, the prophylactic antiemetic combination regimen of olanzapine, 5-HT3RA, and DEX showed no statistical difference between groups treated with or without NK1RA for CINV control, as demonstrated by the endpoints of CR, CC, and TC rates during the overall, acute, and delayed periods. Moreover, daytime sleepiness and concentration impairment, the most worrisome adverse events associated with olanzapine administration, were unaffected by NK1RA administration. The incidences of moderate and severe daytime sleepiness and concentration impairment were rare.
In the present study, prophylactic antiemetic treatment without NK1RA had a high CR rate of 93.5%, CC rate of 93.5%, and TC rate of 87.1%. The incidence of nausea in the non-NK1RA group was also very low (12.9%). A head-to-head comparison of the antiemetic effects of olanzapine and NK1RA, each combined with palonosetron and DEX, has been reported for patients receiving HEC. [6, 7]. In these studies, the CR rates of the olanzapine and NK1RA regimens were comparable in the overall, acute, and delayed periods. Nevertheless, antiemetic prophylaxis with the olanzapine regimen resulted in a significantly higher control of nausea in the delayed and overall periods than that with the NK1RA regimen. It has been reported that when 5-HT released by anticancer drugs acts on 5-HT2b and 5-HT2c receptors, the secretion of ghrelin, an appetite-stimulating hormone, is decreased, inducing anorexia and nausea [23]. Olanzapine is an antipsychotic drug classified as a multi-acting, receptor-targeted agent that is known to antagonize 5-HT at the 5-HT2b and 5-HT2c receptors [24]. These mechanisms may account for the excellent nausea-suppressing effects of olanzapine.
The incidence of nausea, vomiting, and decreased appetite mainly peaked on day 4 in both groups, which is consistent with a recent report by Iihara et al. showing that CINV associated with carboplatin occurs on day 4 [25].
Younger age is a well-known patient-related risk factor for CINV [17,18,19,20]. In our previous study, which evaluated the efficacy of olanzapine for carboplatin-induced nausea and vomiting in younger patients, the cut-off value for age was set to 60 years, and was significantly associated with an increased risk of non-TC in the overall study period [16]. The median patient age in the present study was 71 years (range, 25th and 75th percentiles, 67–76 years) in the non-NK1RA group and 71 years (range, 25th and 75th percentiles, 65–77 years) in the NK1RA group, which had relatively older patients. Only two patients under the age of 60 years were included in both groups. Therefore, caution should be exercised when extrapolating the results of this study to younger patients, especially those aged below 60 years. We suggest that these findings should be confirmed with a randomized comparison of older and younger patients in future research.
Undesired patient sedation with 10 mg olanzapine is a problem in its antiemetic use for elderly or oversedated patients [1, 3, 11]. The J-FORCE study, which evaluated 5 mg olanzapine in patients receiving high-dose cisplatin, suggested that 5 mg olanzapine therapy does not have a significant effect on daytime somnolence and decreased concentration [12]. Our previously reported integrated analysis evaluating 5 mg of olanzapine in patients receiving carboplatin was consistent with this result [16]. This was not affected by the presence or absence of the NK1RA combination.
The present study has some limitations. First, this study had an open-label, single-arm design. Second, data was small number from three studies. But we used a propensity score-matched analysis which is a popular methodology for a retrospective study design. Third, the results of this study are not a direct comparison between patients treated with or without NK1RA. Furthermore, due to the older age of the patients included in this analysis, the results may not be applicable to younger patients. Finally, the results were obtained only in the Japanese population. In the future, a phase III trial comprising a direct comparison of the efficacy and safety of an antiemetic combination regimen of olanzapine, 5-HT3RA, and DEX, with or without NK1RA in patients receiving carboplatin-based chemotherapy is warranted.
Conclusion
These findings suggest that antiemetic combination regimens of olanzapine, 5-HT3RA, and DEX without NK1RA may be a treatment option for patients treated with carboplatin-based combination chemotherapy with an area under the curve of ≥ 5 mg/mL/min.
Availability of data and materials
The data that support the findings of this study are available from the study groups, but restrictions apply to the availability of these data, which were used under license for the current study; therefore, the data are not publicly available. However, data are available from the corresponding authors upon reasonable request and with permission from the study groups.
Abbreviations
- 5-HT3RA:
-
5-Hydroxytryptamine-3 receptor antagonist
- CC:
-
Complete control
- CI:
-
Confidence interval
- CINV:
-
Chemotherapy-induced nausea and vomiting
- CR:
-
Complete response
- DEX:
-
Dexamethasone
- ECOG PS:
-
Eastern Cooperative Oncology Group performance status
- HEC:
-
High-emetic-risk chemotherapy
- MEC:
-
Moderate-emetic-risk chemotherapy
- NK1RA:
-
Neurokinin-1 receptor antagonist
- TC:
-
Total control
References
Roila F, Molassiotis A, Herrstedt J, Aapro M, Gralla RJ, Bruera E, et al. 2016 MASCC and ESMO guideline update for the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting and of nausea and vomiting in advanced cancer patients. Ann Oncol. 2016;27:v119–33.
Hesketh PJ, Kris MG, Basch E, Bohlke K, Barbour SY, Clark-Snow RA, et al. Antiemetics: ASCO guideline update. J Clin Oncol. 2020;38:2782–97.
NCCN Clinical Practice Guidelines in Oncology: Antiemesis. version 1.2021. https://www.nccn.org/professionals/physician_gls/pdf/antiemesis.pdf. Accessed 11 Apr 2021.
Aogi K, Takeuchi H, Saeki T, Aiba K, Tamura K, Iino K, et al. Optimizing antiemetic treatment for chemotherapy-induced nausea and vomiting in Japan: update summary of the 2015 Japan society of clinical oncology clinical practice guidelines for antiemesis. Int J Clin Oncol. 2021;26:1–17.
Jordan K, Blättermann L, Hinke A, Müller-Tidow C, Jahn F. Is the addition of a neurokinin-1 receptor antagonist beneficial in moderately emetogenic chemotherapy?-a systematic review and meta-analysis. Support Care Cancer. 2018;26:21–32.
Navari RM, Gray SE, Kerr AC. Olanzapine versus aprepitant for the prevention of chemotherapy-induced nausea and vomiting: a randomized phase III trial. J Support Oncol. 2011;9:188–95.
Navari RM, Nagy CK, Le-Rademacher J, Loprinzi CL. Olanzapine versus fosaprepitant for the prevention of concurrent chemotherapy radiotherapy-induced nausea and vomiting. J Community Support Oncol. 2016;14:141–7.
Tan L, Liu J, Liu X, Chen J, Yan Z, Yang H, et al. Clinical research of olanzapine for prevention of chemotherapy-induced nausea and vomiting. J Exp Clin Cancer Res. 2009;28:131.
Babu G, Saldanha SC, Kuntegowdanahalli Chinnagiriyappa L, Jacob LA, Mallekavu SB, Dasappa L, et al. The efficacy, safety, and cost benefit of olanzapine versus aprepitant in highly emetogenic chemotherapy: a pilot study from South India. Chemother Res Pract. 2016;2016:3439707.
Wang X, Wang L, Wang H, Zhang H. Effectiveness of olanzapine combined with ondansetron in prevention of chemotherapy-induced nausea and vomiting of non-small cell lung cancer. Cell Biochem Biophys. 2015;72:471–3.
Navari RM, Qin R, Ruddy KJ, Liu H, Powell SF, Bajaj M, et al. Olanzapine for the prevention of chemotherapy-induced nausea and vomiting. N Engl J Med. 2016;375:134–42.
Hashimoto H, Abe M, Tokuyama O, Mizutani H, Uchitomi Y, Yamaguchi T, et al. Olanzapine 5 mg plus standard antiemetic therapy for the prevention of chemotherapy-induced nausea and vomiting (J-FORCE): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2020;21:242–9.
Tanaka K, Inui N, Karayama M, Yasui H, Hozumi H, Suzuki Y, et al. Olanzapine-containing antiemetic therapy for the prevention of carboplatin-induced nausea and vomiting. Cancer Chemother Pharmacol. 2019;84:147–53.
Iihara H, Shimokawa M, Hayasaki Y, Fujita Y, Abe M, Takenaka M, et al. Efficacy and safety of 5 mg olanzapine combined with aprepitant, granisetron and dexamethasone to prevent carboplatin-induced nausea and vomiting in patients with gynecologic cancer: a multi-institution phase II study. Gynecol Oncol. 2020;156:629–35.
Sakai C, Shimokawa M, Iihara H, Fujita Y, Ikemura S, Hirose C, et al. Low-dose olanzapine plus granisetron and dexamethasone for carboplatin-induced nausea and vomiting in patients with thoracic malignancies: a prospective multicenter phase II trial. Oncologist. 2021;26:e1066-72-e1072.
Yamamoto S, Iihara H, Uozumi R, Kawazoe H, Tanaka K, Fujita Y, et al. Efficacy and safety of 5 mg olanzapine for nausea and vomiting management in cancer patients receiving carboplatin: integrated study of three prospective multicenter phase II trials. BMC Cancer. 2021;21:832.
Hesketh PJ, Aapro M, Street JC, Carides AD. Evaluation of risk factors predictive of nausea and vomiting with current standard-of-care antiemetic treatment: analysis of two phase III trials of aprepitant in patients receiving cisplatin-based chemotherapy. Support Care Cancer. 2010;18:1171–7.
Warr DG, Street JC, Carides AD. Evaluation of risk factors predictive of nausea and vomiting with current standard-of-care antiemetic treatment: analysis of phase 3 trial of aprepitant in patients receiving adriamycin-cyclophosphamide-based chemotherapy. Support Care Cancer. 2011;19:807–13.
Hilarius DL, Kloeg PH, van der Wall E, van den Heuvel JJ, Gundy CM, Aaronson NK. Chemotherapy-induced nausea and vomiting in daily clinical practice: A community hospital-based study. Support Care Cancer. 2012;20:107–17.
Sekine I, Segawa Y, Kubota K, Saeki T. Risk factors of chemotherapy-induced nausea and vomiting: index for personalized antiemetic prophylaxis. Cancer Sci. 2013;104:711–7.
Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat. 2011;10:150–61.
Chan IS, Zhang Z. Test-based exact confidence intervals for the difference of two binomial proportions. Biometrics. 1999;55:1202–9.
Takeda H, Sadakane C, Hattori T, Katsurada T, Ohkawara T, Nagai K, et al. Rikkunshito, an herbal medicine, suppresses cisplatin-induced anorexia in rats via 5-HT2 receptor antagonism. Gastroenterology. 2008;134:2004–13.
Shahid M, Walker GB, Zorn SH, Wong EH. Asenapine: a novel psychopharmacologic agent with a unique human receptor signature. J Psychopharmacol. 2009;23:65–73.
Iihara H, Shimokawa M, Hayashi T, Kawazoe H, Saeki T, Aiba K, et al. A nationwide, multicenter registry study of antiemesis for carboplatin-based chemotherapy-induced nausea and vomiting in Japan. Oncologist. 2020;25:e373–80.
Acknowledgements
We are grateful to all the patients and their families for participating in this study. We would like to thank Editage (www.editage.com) for English language editing.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
S.Y., H.I., R.U., H.K., and N.I. conceived the study. S.Y., H.I., R.U., H.K., and N.I. conducted the claims data analysis. R. U. performed the statistical analyses. Y.O. and K.M. provided technical support. S.Y., H.I., R.U., H.K., K.T., Y.F., M.A., H.I., M.K., Y.H., C.H., T.S., K.N., A.S., and N.I. contributed to the interpretation of data and assisted in the preparation of the manuscript. S.Y., H.I., R.U., and H.K. drafted the manuscript. S.Y., H.I., R.U., H.K. A. S., Y.O., K.M., and N.I. critically revised the manuscript. All authors reviewed the manuscript. The author(s) read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Study 1 was approved by the Medical Review Board of the Hamamatsu University Graduate School of Medicine (16–296). Written informed consent was obtained from all patients. Study 1 was an opt-out study for the secondary use of data with approval (20–335). Studies 2 and 3 were approved by the Medical Review Board of Gifu University Graduate School of Medicine (30–002, 2018–19). Written informed consent was obtained from all patients. In studies 2 and 3, written informed consent was obtained for the secondary use of data. These studies were conducted in accordance with the Declaration of Helsinki and ethical guidelines for clinical studies.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yamamoto, S., Iihara, H., Uozumi, R. et al. Effects of adding a neurokinin-1 receptor antagonist to 5 mg olanzapine, a 5-hydroxytryptamine-3 receptor antagonist, and dexamethasone for preventing carboplatin-induced nausea and vomiting: a propensity score-matched analysis. BMC Cancer 22, 310 (2022). https://doi.org/10.1186/s12885-022-09392-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-022-09392-9