Abstract
Background
Cancer stem/Initiating cell (CS/IC) hypothesis argues that CS/ICs are responsible of tumour initiation, drug resistance, metastasis or disease relapse. Their detection in several cancers supports this concept. However, their origin is still misunderstood. Cell fusion is shown to take part in the formation of CS/ICs, i.e. fusion between mesenchymal stem cell and cancer cell. In a previous paper, we described that fusion leads to hybrids with metastatic capacity. This process triggered genomic rearrangements in hybrid cells together with increased metastasis development. Here, we hypothesize that cell fusion could be strong enough to provoke a cellular reprogramming and the acquisition of CS/IC properties, promoting metastasis formation.
Methods
After spontaneous cell fusion between E6E7 (IMR90 with the oncogenes E6 and E7) and RST (IMR90 fully transformed) cell lines, hybrid cells were selected by dual antibiotic selection. Cancer stem cells capacities were evaluated regarding capacity to form spheres, expression of stem cell markers and the presence of ALDHhigh cells.
Results
Our data show that after cell fusion, all hybrids contain a percentage of cells with CS/ICs properties, regarding. Importantly, we lastly showed that NANOG inhibition in H1 hybrid decreases this migration capacity while having no effect on the corresponding parental cells.
Conclusions
Altogether these results indicate that the combination of CS/ICs properties and genomic rearrangement in hybrids is likely to be key to tumour progression.
Similar content being viewed by others
Background
A century ago, Aichel formulated the hypothesis that cell fusion (macrophage/cancer cell) could be at the origin of metastatic cells. From that date, several papers have demonstrated that diverted cell fusion could be a process leading to the development of tumours and metastasis. Cell fusion occurs at different stages of tumour development from initiation to the development of metastasis [1, 2]. Cell fusion events were detected in animal model [3, 4] and in human tumours [5,6,7]. Furthermore, Gast et al. recently identified hybrids in the blood of pancreatic cancer patients, and their presence is associated with a poor prognosis [3]. These data highlight the importance of cell fusion and hybrid cells in the mechanism of dissemination. However, the regulation of cell fusion in tumour and the consequences for the hybrid are still misunderstood.
Tumours present heterogeneous cancer cell population with genetic and phenotypic differences. To explain this diversity, many models were established. The model of clonal evolution presents that the heterogeneity arises through stochastic genetic and epigenetic changed that confers heritable phenotypic differences upon cancer cells. Then, the fittest cells will be selected in the tumour following a Darwinian evolution [8]. A second model, described a hierarchical organisation of tumours, where cancer stem/initiating cells (CS/ICs) are at the top of the pyramid [9, 10]. CS/ICs have an unlimited capacity for self-renewal and can also generate non CS/ICs progeny (differentiated progeny) [11]. Their origin is still an ongoing debate but two main hypotheses hold sway. First, CS/ICs could arise from normal stem cells that accumulate mutations leading to transformation. Second, differentiated cells acquire mutations that lead to reprogramming [12]. Fusion events may also be involved in the generation of CS/ICs. In fact, fusion between normal stem cells such as mesenchymal stem cells and bone marrow-derived stem cells, on the one hand, and cancer cells on the other, leads to the formation of hybrids with tumorigenic and stemness properties, i.e. the hallmarks of CS/ICs [13,14,15]. Furthermore, CS/ICs exhibit stem cell markers (OCT4 and NANOG) and have an increased metastatic capacity [16].
Metastasis is a complex and still only partially understood process. Several studies have described the acquisition of metastatic capacity at the early stage of tumour development, after a burst event leading to invasive cancer and metastasis [17,18,19]. In addition, several papers demonstrate that CS/ICs are composed of different clones, of which some known as metastasis-initiating cells (MIC) could have the capacity to metastasise [20, 21]. However, how CS/IC become MIC is still not understood.
In a previous study, we demonstrated that spontaneous fusion between IMR90 E6-E7 (E6E7) fibroblasts with IMR90 E6-E7- HRASG12V -SmallT-hTERT (RST) fibroblasts is a burst event leading to cellular heterogeneity and the acquisition of metastatic capacity by hybrids [22]. The goal of this paper was to test, in that model, the hypothesis that cell fusion triggers the acquisition of CS/IC properties, leading to the development of metastatic capacity.
Results
Fused cells exhibit ALDHhigh activity immediately after cell fusion
As previously described [22], hybrid cell lines (H1 to H4) were established after spontaneous cell fusion of E6E7 (IMR90 immortalized with E6 and E7) and RST (IMR90 transformed with E6, E7, RAS, Smallt and hTERT) (fusion event represents less than 1% of the population). RST and hybrid cell lines developed Undifferentiated Pleomorphic Sarcomas after subcutaneous injection in mice. However, hybrid cell lines possess an increased migration capacity in vitro and a higher metastatic capacity (absent in parental cell lines) [22].
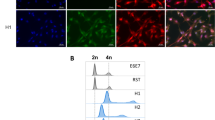
Elevated aldehyde dehydrogenase (ALDH) activity is considered a suitable marker for the identification of normal and cancer stem cells, as defined by ALDHhigh cells [23, 24]. Cell populations with high ALDH activity has been detected in many sarcoma histotypes and enabled the identification of CS/ICs [25,26,27]. To validate this marker in our model, parental and hybrid cell lines were cells sorted according to their ALDH activity (ALDHlow versus ALDHhigh cells) (Fig. S1 A). They were sorted and plated in conditions to grow as spheres. The frequency of CS/ICs in these two populations was determined by Extreme Limiting Dilution Analysis (ELDA) method [28] (Fig. S1 B). The frequency of CS/IC ranges from 0 to 1/873 in ALDHlow cells and from 1/3203 to 1/82 in ALDHhigh cells. For all the cell lines except E6E7, the frequency of CS/ICs is significantly enriched in ALDHhigh populations. This confirms that ALDH activity is a consistent marker of CS/ICs in this cell line model. Hence, we used this measured ALDH activity to quantify CS/ICs in parental cell lines and hybrids. ALDHhigh cell percentage was evaluated after 72 h, the time to get spontaneous hybrids, of coculture of RST and E6E7 (Fig. 1, Fig. S2) for RST cells (DsRed+ cells), E6E7 cells (CFP+ cells) and hybrids cells (DsRed+/CFP+ cells).
The experiment was repeated three times and each time in one co-culture, hybrids cells had a percentage of ALDHhigh cells two-fold higher than in the other samples (co-culture 5 in the experiment represented). Since parental and fused cells both presented the CS/IC marker, we wondered whether it was due to the transmission or acquisition of CS/IC properties following cell fusion.
Identification of a population with stem cell properties in hybrid cell lines
To assess the presence of CS/ICs in parental and hybrid cell lines, several CS/IC markers were tested in the hybrid clones and parental cell lines. First, we sought to detect ALDHhigh cells. We found that they were present in all parental and hybrid cell lines (Fig. 2 a), and that this phenotype persisted in all passages. Their percentage was not significantly different between RST, H2, H3 and H4.
a. Percentage of ALDHhigh cells in parental and hybrid cell lines evaluated by flow cytometry. * p value < 0,05; n = 4. b. Number of spheres after 10 days of culture in non-adherent condition. n = 6 c. Evaluation of stem cell frequency by ELDA method in spheres formed to evaluate self-renew capacity. d. Expression of NANOG and OCT4 by western blotting analysis in the monolayer culture of each cell lines. β-actin was used as loading control. Full-length blots are presented in Supplementary Fig. 4
However, their population was higher in H1 than in the parental cell lines E6E7 and RST. In non-adherent condition and SVF-free medium at day 10, all cell lines had developed spheres (Fig. 2 b, Fig. S3 B). To further investigate their self-renewal capacities, these spheres were dissociated at day 12 when they were completely formed, and were re-plated in a 96-well plate ultra-low attachment. Except for E6E7 in which the number of spheres was too low to complete the experiment, all cell lines demonstrated the formation of secondary spheres at a frequency ranging from 1/37.5 (H1) to 1/1281 (H4) (Fig. 2c).
To go further, the CS/IC population was also evaluated by the detection of two stem cell markers: NANOG and OCT4 [28]. NANOG was expressed in parental and hybrid cell lines, with a stronger expression in all hybrid cell lines (Fig. 2d, Fig. S4). OCT4 expression was weak in RST, H2 and H3, higher in E6E7 and H1, and not detected in H4. Hybrids H1, H2 and H3 thus have a strong NANOG expression with a moderate (H1) or weak (H2 and H3) OCT4 expression. The parental cells have a moderate NANOG expression with a moderate (E6E7) or weak (RST) OCT4 expression.
To sum up, RST and hybrid cell lines have a population of cells with the capacity to form spheres and to self-renew, to express stem cells markers, and possessing a high ALDH activity. Therefore, RST and hybrid cell lines contain CS/IC population. Their proportion seems to be identical between RST and H2, H3 and H4. Only H1 has a higher percentage of CS/ICs.
Generation of ALDHhigh cells after cell fusion
Because a population of cells in RST and all hybrid cell lines were found to possess CS/ICs properties, we investigated whether these properties are generated upon cell fusion or is transmitted from RST to the new hybrid. E6E7low were thus cocultured for 72 h with RSTlow or RSThigh and E6E7high with RSTlow or RSThigh. After 72 h of co-culture, cell fusion events were counted by cytometry (Fig. 3a, Fig. S5).
A higher frequency of fusion events was observed with the combination E6E7low/RSThigh and E6E7high/RSThigh. After co-culture, dual antibiotic selection was added to select hybrids. Four clones were established in the E6E7low/RSTlow condition, one clone each with E6E7low/RSThigh and E6E7high/RSTlow, and none with E6E7high/RSThigh. The percentage of ALDHhigh cells was determined in all these hybrid clones. Interestingly, ALDHhigh cells (Fig. 3b) were detected in all clones, ranging from 5.9% (HL1) to 49% (LL4). Hence, the fusion of negative ALDH cells may generate ALDHhigh cells.
Inhibition of NANOG decreases migration capacity in hybrid
As previously described [22], H1 migrated significantly more than RST. To determine whether CS/ICs are involved in the increasing migration capacity of the hybrid, NANOG, which was strongly expressed by all hybrids, was inhibited by siRNA (Fig. 4a) and migration capacity was evaluated (Fig. 4b, c).
a. At the top, expression of NANOG and β-actin evaluated by western blotting in wild type cell lines and after 72 h of incubation with siRNA negative control or siRNA NANOG. At the bottom, quantification of NANOG expression normalized by β-actin expression (loading control). Full-length blots/gels are presented in Supplementary Fig. 6. b. Images of scratch test migration assay at 0 h and 10 h after inhibition of NANOG by siRNA compared to negative control siRNA. c. Evaluation of migration with scratch test for RST and H1 in wild type cell lines and after incubation with non-targeted siRNA or siRNA NANOG. *** p value < 0.001 (Mann-Whitney test) (n = 16)
RST with siRNA against NANOG did not modify migration compared to RST WT and RST with non-targeted siRNA. Contrasting, inhibition of NANOG in H1 significantly decreased its migration capacity compared to the negative control (Fig. 4c). RST and H1 both contain CS/ICs. However, the inhibition of the principal marker of this population does not have the same impact in the parental and in H1 hybrid. Unlike RST CS/ICs, CS/ICs from H1 can migrate in vitro and this capacity is NANOG-dependent.
Discussion
In the present study, we tested the hypothesis that the tumour aggressiveness and metastatic capacity acquired by hybrids [22] could be associated with the stemness phenotype developed by hybrids upon fusion. The results show that all hybrid cell lines had a population of cells with stemness properties. The percentage of CS/ICs remained stable during the passages. However, these CS/IC populations did not demonstrate the same capacity to grow as spheres, the same expression of OCT4 and NANOG or the same percentage of ALDHhigh cells. For example, H1 highly expressed NANOG and OCT4 whereas the other hybrids expressed NANOG similarly but weakly expressed OCT4. H1 also had a higher percentage of ALDHhigh cells and a greater capacity to develop spheres. Fusion between E6E7 and RST led to the formation of hybrids with heterogeneous properties, as metabolism and in vitro migration and invasion capacity [22] but were all able to develop metastases in mice. Since cell fusion is a driver of cell diversity and heterogeneity, we hypothesise that the number of hybrid cells with stemness properties is different in each hybrid, and that their own properties are also different.
Hybrid cells with CS/IC properties have been shown to form after fusion between stem cells (normal or tumoral) and differentiated cells [13, 29,30,31,32]. In the present study, the fusion of E6E7 ALDHlow and RST ALDHlow led to the formation of a population of cells with ALDHhigh activity. ALDH activity is known to be a valuable marker of CS/IC populations in soft tissue sarcomas [26,27,28]. While in vivo experiments remain to be done because limiting dilution in mice is the golden standard to demonstrate the presence of CSCs, these data together with previous publications show that cell fusion can produce a hybrid with stemness capacity, even if neither parent possesses it. We and others have already shown that cell fusion involves massive genomic reorganization [1, 33,34,35]. Such alterations disturb the expression of several genes and pathways. Over-expression of genes involved in stem cell maintenance, self-renewal and pluripotency, as well as the down-expression of genes associated with differentiation, could lead to reprogramming towards cells with stemness properties. Cell fusion might also modify epigenetic markers that unlock the expression of stemness markers like NANOG and OCT4, which are directly involved in cell reprogramming [36].
The inhibition of NANOG leads to the decrease of H1 migration. Even though our study lacks an in vivo experiment with stable knockdown, our results correlate with data already reported in bladder, ovarian and breast cancer [37,38,39]. NANOG is known to be a marker of poor prognosis in several cancers [40,41,42]. This transcription factor is not only a cancer stem cell marker; it also promotes important characteristics of CS/IC such as drug resistance, cancer cell motility and tumour metastasis [43]. NANOG positively regulates MMP-2 and MMP-9, which are factors involved in migration and metastasis [37, 44]. Interestingly, in the present fusion model, the inhibition of NANOG had an impact only on the hybrid cell line and not on the parental RST. Because the inhibition of NANOG impacts cells with stemness properties [45], we hypothesise that CS/IC populations are different in parental and hybrid cell lines. In fact, only cells surviving fusion and having extra chromosomes, genomic rearrangements, epigenetic modifications and stemness properties can become “super” cells able to disseminate and metastasize.
Methods
Cell lines and hybrid generation
Cell lines, hybrid cell selection and culture conditions were already described [22]. Parental cell lines were as follows: 1) E6E7 was IMR90 (human foetal lung fibroblast) partially transformed, harbouring two human papilloma viral ONC E6 and E7, targeting p53 and pRB respectively; 2) RST was IMR90 fully transformed harbouring the ONC HRAS G12V, SV40 small T, and hTERT in addition to E6 and E7. These cell lines were established according to the model described by Hahn et al. [46, 47] and were kindly provided by Martin Teichmann. After 72 h of coculture of E6E7 and RST, hybrids were selected by double antibiotic selection (puromycin and blasticidin; Life Technologies, Thermo Fisher Scientific). Fluorescence and genomic analysis were performed to validate the selection of cells from fusion.
Sphere formation assay
Cells were cultured in suspension in Ultralow Attachment (ULA) plate in DMEM/F12 supplemented with N2-supplement (ThermoFisher). Every three days 10 ng/ml of EGF (R&D systems) and bFGF (ThermoFisher) were added. In a 6-well plates 10,000 cells were plated and after 10 days the number of spheres was manually counted.
To assess stem cell frequency in formed spheres, spheres were dissociated using trypsin and mechanical dissociation. Then, cells were counted and 500–250–100-10 cells / 100 μl were seeded in a 96-well plate with ultra-low attachment. The presence of spheres were determined manually after 10 days and results were analysed with the ELDA method [48].
Protein extraction and Western blotting
This section was performed as already described [34]. Cells were rinsed in PBS 1X and lysed for 20 min at 4 °C in RIPA buffer (R0278, Sigma Aldrich) supplemented by phosphatase/protease inhibitor cocktail (1X, Sigma Aldrich). Proteins were collected in supernatant after 15 min of centrifugation at 13000 g and quantified by DC protein assay kit, Biorad. On Mini Proteane TGX stain free 4–15% (Biorad), 40 μg of total proteins were loaded. Transfers to PVDF membrane were performed using a dry transfer system (iBlot2, ThermoFisher Scientific) and membranes were blocked in 5% non-fat dry milk in 0.1% PBS-Tween solution. Membranes were incubated overnight with the primary antibody: mouse anti-NANOG (GTX627421, Genetex, 1/200), rabbit anti-OCT4 (653,702, Biolegend, 1/200) and mouse anti-β-actin (A5316, Sigma Aldrich, 1/1000) at 4 °C overnight. After washing, membranes were incubated for 1 h at room temperature with appropriate secondary antibodies: anti-rabbit HRP (7074S, Ozyme, 1/1000) and anti-mouse HRP (7076S, Ozyme, 1/1000). After incubation with chemiluminescent substrate (ECL Immobilon Western, WBKLS0100, Merck), signals were detected using PXi (Syngene).
Aldehyde dehydrogenase activity
The ALDFELUOR kit (Stem Cell Technologies, Vancouver, Canada) was used to detect ALDH activity according to the manufacturer’s instructions. Briefly, 500,000 cells in 1 ml Aldefluor assay buffer were stained with 5 μl of Aldefluor reagent and 500 μl were used as a negative control with 10 μl of DEAB. Cells were incubated for 45 min at 37 °C. Flow cytometry was performed with BD LSRFortessa (BD Biosciences, Franklin Lakes, NJ, USA) and analysed with FlowJo software.
For cell sorting, dead cells were excluded based on light scatter characteristics and only DsRed-positive cells and CFP-positive cells were selected for RST and E6E7 respectively. Gates were selected in order to choose the brightest (ALDHhigh) and the dimmest (ALDHhigh) cells compared to DEAB-negative control. Cell sorting was done with FACS Melody (BD Biosciences, Franklin Lakes, NJ, USA).
For the quantification of ALDHhigh cells in hybrids right after cell fusion (Fig. 1), parental cell lines E6E7 and RST were cocultured for 72 h in a 6-well plates. Then, cells were harvested and ALDEFLUOR assay was performed as described above. This experiment was repeated three times, with 5 cocolture for each experiment.
siRNA NANOG
Cells were harvested, counted and diluted in order to obtain 125,000 cells per well in a 6-well plate. After 24 h, lipofectamine RNAiMAX (13,778,030, ThermoFisher Scientific) was diluted in OPTI-MEM (31,985,062, ThermoFisher Scientific). A solution of 10 nM of siRNA NANOG (s36649, ThermoFisher Scientific, Waltham, MA, USA) or 10 nM of siRNA negative control (4,390,843, ThermoFisher) was prepared with OPTI-MEM. Lipofectamine and siRNA were slowly mixed and incubated for 20 min at room temperature. Then, these complexes were added to the cells and incubated in culture conditions. After 6 h, medium was changed and replaced by culture medium without antibiotics. Optimal inhibition of NANOG, without changes in negative control compared to wild type cells, was detected at 72 h by western blotting.
Scratch test migration assay
This section was performed as already described [22]. For the wound healing assay, 4 × 105 cells were plated onto a 6-well plate. Twenty-four hours later, a strip of cells was removed from the monolayer of cells using a pipette tip. Phase contrast images were acquired with a 10× objective at the time of the scratch and 10 h later using a Nikon Eclipse TS100 microscope. Quantification was done using Image J. Data at 10 h were normalized to the size of the wound at 0 h and results were plotted using GraphPad (La Jolla, CA, USA) software.
Statistical analysis
Statistical analysis were performed with GraphPad (La Jolla, CA, USA) software.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Berndt B, Zänker KS, Dittmar T. Cell fusion is a potent inducer of aneuploidy and drug resistance in tumor cell/ normal cell hybrids. Crit Rev Oncog. 2013;18:97–113.
Duelli D, Lazebnik Y. Cell fusion: A hidden enemy? Cancer Cell. 2003;3:445–8.
Gast CE, Silk AD, Zarour L, Riegler L, Burkhart JG, Gustafson KT, et al. Cell fusion potentiates tumor heterogeneity and reveals circulating hybrid cells that correlate with stage and survival. Sci Adv. 2018;4. https://doi.org/10.1126/sciadv.aat7828.
Powell AE, Anderson EC, Davies PS, Silk AD, Pelz C, Impey S, et al. Fusion between intestinal epithelial cells and macrophages in a Cancer context results in nuclear reprogramming. Cancer Res. 2011;71:1497–505.
Chakraborty A, Lazova R, Davies S, Bäckvall H, Ponten F, Brash D, et al. Donor DNA in a renal cell carcinoma metastasis from a bone marrow transplant recipient. Bone Marrow Transplant. 2004;34:183–6.
Yilmaz Y, Lazova R, Qumsiyeh M, Cooper D, Pawelek J. Donor Y chromosome in renal carcinoma cells of a female BMT recipient: visualization of putative BMT-tumor hybrids by FISH. Bone Marrow Transplant. 2005;35:1021–4.
Clawson GA, Matters GL, Xin P, McGovern C, Wafula E, de Pamphilis C, et al. “Stealth dissemination” of macrophage-tumor cell fusions cultured from blood of patients with pancreatic ductal adenocarcinoma. PLoS ONE. 2017;12. https://doi.org/10.1371/journal.pone.0184451.
Nowell PC. The clonal evolution of tumor cell populations. Science. 1976;194:23–8.
Dalerba P, Cho RW, Clarke MF. Cancer stem cells: models and concepts. Annu Rev Med. 2007;58:267–84.
Vermeulen L, Sprick MR, Kemper K, Stassi G, Medema JP. Cancer stem cells--old concepts, new insights. Cell Death Differ. 2008;15:947–58.
Baccelli I, Trumpp A. The evolving concept of cancer and metastasis stem cells. J Cell Biol. 2012;198:281–93.
Bjerkvig R, Tysnes BB, Aboody KS, Najbauer J, Terzis AJA. The origin of the cancer stem cell: current controversies and new insights. Nat Rev Cancer. 2005;5:899–904.
Fahlbusch SS, Keil S, Epplen JT, Zänker KS, Dittmar T. Comparison of hybrid clones derived from human breast epithelial cells and three different cancer cell lines regarding in vitro cancer stem/ initiating cell properties. BMC Cancer. 2020;20:446.
Rappa G, Mercapide J, Lorico A. Spontaneous formation of tumorigenic hybrids between breast Cancer and multipotent stromal cells is a source of tumor heterogeneity. Am J Pathol. 2012;180:2504–15.
Zhang S, Mercado-Uribe I, Xing Z, Sun B, Kuang J, Liu J. Generation of cancer stem-like cells through the formation of polyploid giant cancer cells. Oncogene. 2014;33:116–28.
Zhang L-N, Kong C-F, Zhao D, Cong X-L, Wang S-S, Ma L, et al. Fusion with mesenchymal stem cells differentially affects tumorigenic and metastatic abilities of lung cancer cells. J Cell Physiol. 2019;234:3570–82.
Gao R, Davis A, McDonald TO, Sei E, Shi X, Wang Y, et al. Punctuated copy number evolution and clonal stasis in triple-negative breast Cancer. Nat Genet. 2016;48:1119–30.
Notta F, Chan-Seng-Yue M, Lemire M, Li Y, Wilson GW, Connor AA, et al. A renewed model of pancreatic cancer evolution based on genomic rearrangement patterns. Nature. 2016;538:378–82.
Turajlic S, Xu H, Litchfield K, Rowan A, Chambers T, Lopez JI, et al. Tracking Cancer Evolution Reveals Constrained Routes to Metastases: TRACERx Renal. Cell. 2018;173:581–94 e12.
Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M, et al. Distinct populations of Cancer stem cells determine tumor growth and metastatic activity in human pancreatic Cancer. Cell Stem Cell. 2007;1:313–23.
Pang R, Law WL, Chu ACY, Poon JT, Lam CSC, Chow AKM, et al. A subpopulation of CD26+ cancer stem cells with metastatic capacity in human colorectal cancer. Cell Stem Cell. 2010;6:603–15.
Lartigue L, Merle C, Lagarde P, Delespaul L, Lesluyes T, Le Guellec S, et al. Genome remodeling upon mesenchymal tumor cell fusion contributes to tumor progression and metastatic spread. Oncogene. 2020;39:4198–211.
Charafe-Jauffret E, Ginestier C, Iovino F, Wicinski J, Cervera N, Finetti P, et al. Breast Cancer cell lines contain functional Cancer stem cells with metastatic capacity and a distinct molecular signature. Cancer Res. 2009;69:1302–13.
Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1:555–67.
Honoki K, Fujii H, Kubo A, Kido A, Mori T, Tanaka Y, et al. Possible involvement of stem-like populations with elevated ALDH1 in sarcomas for chemotherapeutic drug resistance. Oncol Rep. 2010;24:501–5.
Lohberger B, Rinner B, Stuendl N, Absenger M, Liegl-Atzwanger B, Walzer SM, et al. Aldehyde dehydrogenase 1, a potential marker for Cancer stem cells in human sarcoma. PLoS One. 2012;7. https://doi.org/10.1371/journal.pone.0043664.
Nakahata K, Uehara S, Nishikawa S, Kawatsu M, Zenitani M, Oue T, et al. Aldehyde dehydrogenase 1 (ALDH1) is a potential marker for Cancer stem cells in Embryonal Rhabdomyosarcoma. PLoS One. 2015;10:e0125454.
Trucco M, Loeb D. Sarcoma stem cells: do we know what we are looking for? Sarcoma. 2012;2012:291705.
Melzer C, von der Ohe J, Lehnert H, Ungefroren H, Hass R. Cancer stem cell niche models and contribution by mesenchymal stroma/stem cells. Mol Cancer. 2017;16:28.
Wei H-J, Nickoloff JA, Chen W-H, Liu H-Y, Lo W-C, Chang Y-T, et al. FOXF1 mediates mesenchymal stem cell fusion-induced reprogramming of lung cancer cells. Oncotarget. 2014;5:9514–29.
Xu M-H, Gao X, Luo D, Zhou X-D, Xiong W, Liu G-X. EMT and Acquisition of Stem Cell-Like Properties Are Involved in Spontaneous Formation of Tumorigenic Hybrids between Lung Cancer and Bone Marrow-Derived Mesenchymal Stem Cells. PLOS ONE. 2014;9:e87893.
Xue J, Zhu Y, Sun Z, Ji R, Zhang X, Xu W, et al. Tumorigenic hybrids between mesenchymal stem cells and gastric cancer cells enhanced cancer proliferation, migration and stemness. BMC Cancer. 2015;15:793.
Delespaul L, Merle C, Lesluyes T, Lagarde P, Le Guellec S, Pérot G, et al. Fusion-mediated chromosomal instability promotes aneuploidy patterns that resemble human tumors. Oncogene. 2019;38:6083–94.
Merle C, Thébault N, LeGuellec S, Baud J, Pérot G, Lesluyes T, et al. Tetraploidization of immortalized myoblasts induced by cell fusion drives myogenic sarcoma development with DMD deletion. Cancers. 2020;12:1281.
Zhou X, Merchak K, Lee W, Grande JP, Cascalho M, Platt JL. Cell fusion connects Oncogenesis with tumor evolution. Am J Pathol. 2015;185:2049–60.
Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–72.
Gawlik-Rzemieniewska N, Galilejczyk A, Krawczyk M, Bednarek I. Silencing expression of the NANOG gene and changes in migration and metastasis of urinary bladder cancer cells. Arch Med Sci AMS. 2016;12:889–97.
Siu MKY, Wong ESY, Kong DSH, Chan HY, Jiang L, Wong OGW, et al. Stem cell transcription factor NANOG controls cell migration and invasion via dysregulation of E-cadherin and FoxJ1 and contributes to adverse clinical outcome in ovarian cancers. Oncogene. 2013;32:3500–9.
Wang D, Lu P, Zhang H, Luo M, Zhang X, Wei X, et al. Oct-4 and Nanog promote the epithelial-mesenchymal transition of breast cancer stem cells and are associated with poor prognosis in breast cancer patients. Oncotarget. 2014;5:10803–15.
Nagata T, Shimada Y, Sekine S, Hori R, Matsui K, Okumura T, et al. Prognostic significance of NANOG and KLF4 for breast cancer. Breast Cancer Tokyo Jpn. 2014;21:96–101.
Rasti A, Mehrazma M, Madjd Z, Abolhasani M, Saeednejad Zanjani L, Asgari M. Co-expression of Cancer stem cell markers OCT4 and NANOG predicts poor prognosis in renal cell carcinomas. Sci Rep. 2018;8:11739.
Watanabe M, Ohnishi Y, Inoue H, Wato M, Tanaka A, Kakudo K, et al. NANOG expression correlates with differentiation, metastasis and resistance to preoperative adjuvant therapy in oral squamous cell carcinoma. Oncol Lett. 2014;7:35–40.
Wang M-L, Chiou S-H, Wu C-W. Targeting cancer stem cells: emerging role of Nanog transcription factor. OncoTargets Ther. 2013;6:1207–20.
Siu MKY, Wong ESY, Chan HY, Ngan HYS, Chan KYK, Cheung ANY. Overexpression of NANOG in gestational trophoblastic diseases: effect on apoptosis, cell invasion, and clinical outcome. Am J Pathol. 2008;173:1165–72.
Jeter CR, Liu B, Liu X, Chen X, Liu C, Calhoun-Davis T, et al. NANOG promotes cancer stem cell characteristics and prostate cancer resistance to androgen deprivation. Oncogene. 2011;30:3833–45.
Durrieu-Gaillard S, Dumay-Odelot H, Boldina G, Tourasse NJ, Allard D, André F, et al. Regulation of RNA polymerase III transcription during transformation of human IMR90 fibroblasts with defined genetic elements. Cell Cycle. 2018;17:605–15.
Hahn WC, Counter CM, Lundberg AS, Beijersbergen RL, Brooks MW, Weinberg RA. Creation of human tumour cells with defined genetic elements. Nature. 1999;400:464–8.
Hu Y, Smyth GK. ELDA: extreme limiting dilution analysis for comparing depleted and enriched populations in stem cell and other assays. J Immunol Methods. 2009;347:70–8.
Acknowledgements
Not applicable.
Funding
The Fondation ARC pour la Recherche Contre le Cancer, Fondation Recherche Médicale, Association Pour Corentin and Phil’Anthrope supported this work.
Author information
Authors and Affiliations
Contributions
C.M. and F.C. wrote the main manuscript text, P. L and C.M. performed the experiments, L.L., C.M. and F.C. designed the project. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure S1.
A. Cytometry dot plot. ALDHlow cells in blue and ALDHhigh cells in green. Top: cell line incubated with DEAB as negative control. Bottom: cell line without DEAB. B. Evaluation of stem cell frequency by ELDA method in ALDHlow and ALDHhigh cells after cell sorting. “-“: absence of CS/ICs. Figure S2. Percentage of ALDHhigh cells evaluated in parental and hybrid cell population after 72 h of coculture between E6E7 and RST (n = 17). Percentages were not statistically different. Figure S3. A. Cytometry dot plot representing cell populations selected for cell sorting. B. Images of spheres developed in non-adherent cell culture conditions; scale bar = 50 μm. Figure S4. Full length blots. Figure S5. Cytometry dot plot with gates used to determine percentage of fused cells after 72 h of co-culture. x-axis represents CFP fluorescence intensity and y-axis represents DsRed fluorescence intensity. Figure S6. Full length blots.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Merle, C., Lagarde, P., Lartigue, L. et al. Acquisition of cancer stem cell capacities after spontaneous cell fusion. BMC Cancer 21, 241 (2021). https://doi.org/10.1186/s12885-021-07979-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-021-07979-2