Abstract
Background
Cancer surgery can promote tumour metastases and worsen prognosis, however, the effect of perioperative complications on metastatic disease remains unclear. In this study we sought to evaluate the effect of common perioperative complications including perioperative blood loss, hypothermia, and sepsis on tumour metastases in a murine model.
Methods
Prior to surgery, pulmonary metastases were established by intravenous challenge of CT26LacZ colon cancer cells in BALB/c mice. Surgical stress was generated through partial hepatectomy (PH) or left nephrectomy (LN). Sepsis was induced by puncturing the cecum to express stool into the abdomen. Hemorrhagic shock was induced by removal of 30% of total blood volume (i.e. stage 3 hemorrhage) via the saphenous vein. Hypothermia was induced by removing the heating apparatus during surgery and lowering core body temperatures to 30 °C. Lung tumour burden was quantified 3 days following surgery.
Results
Surgically stressed mice subjected to stage 3 hemorrhage or hypothermia did not show an additional increase in lung tumour burden. In contrast, surgically stressed mice subjected to intraoperative sepsis demonstrated an additional 2-fold increase in the number of tumour metastases. Furthermore, natural killer (NK) cell function, as assessed by YAC-1 tumour cell lysis, was significantly attenuated in surgically stressed mice subjected to intraoperative sepsis. Both NK cell-mediated cytotoxic function and lung tumour burden were improved with perioperative administration of polyI:C, which is a toll-like receptor (TLR)-3 ligand.
Conclusions
Perioperative sepsis alone, but not hemorrhage or hypothermia, enhances the prometastatic effect of surgery in murine models of cancer. Understanding the cellular mechanisms underlying perioperative immune suppression will facilitate the development of immunomodulation strategies that can attenuate metastatic disease.
Similar content being viewed by others
Background
Severe trauma causes compensatory changes in immune, neural, endocrine, and metabolic function [1]. Likewise, surgical stress can lead to the onset of prothrombotic and immunosuppressive changes during the postoperative period [2, 3]. Correlative studies have confirmed an association between postoperative complications, immune suppression, and worsened cancer prognosis [4,5,6,7]. Moreover, our group and others have proposed surgery-induced cellular immune suppression as a primary factor in the progression of cancer, including local recurrence and metastatic disease [8,9,10,11,12,13,14,15,16,17,18,19,20]. In humans, suppression of the cellular immune response following major surgery appears to peak at 3 days [21], but can also persist for weeks [17, 22, 23]. These immunosuppressive changes are characterized by an imbalance in plasma cytokine levels (i.e. a decrease in the levels of interleukin (IL)-2 [24] and IL-12 [25] and an increase in the levels of IL-6 [24, 26, 27] and IL-10 [28]) and a decrease in the number and function of circulating CD8+ T cells [29], dendritic cells [30], and natural killer (NK) cells [8, 12, 31]. Specifically, our group reported on the association between coagulation and NK cell function in the development of metastases following cancer surgery [8]; while, more recently, we employed validated murine models of surgical stress and spontaneous metastases [11] to provide in vivo evidence of global NK cell dysfunction in postoperative metastatic disease.
Modern surgical techniques minimize the adverse consequences of perioperative events, such as intraoperative blood loss, sepsis, and hypothermia. Despite this, however, severe intraoperative blood loss occurs in approximately 6–10% of patients with advanced cancer [32], while surgery accounts for 30% of all sepsis diagnoses in the US annually [33]. Furthermore, 8.5% of all cancer-related deaths are due to the concurrent onset of severe sepsis [34], and hypothermia, which is defined as a core body temperature of < 36 °C, occurs in 70% of postoperative patients [35].
Clinical studies in cancer patients have confirmed an association between perioperative factors such as hypothermia [36], blood loss [37, 38], and postoperative infections [39, 40], and increased cancer recurrence and reduced cancer-specific survival following cancer surgery.
Despite the epidemiological evidence linking perioperative complications with increased surgical stress and worsened cancer outcomes, the role of intraoperative blood loss, sepsis, and hypothermia in immunosuppression and metastatic disease remains poorly understood. Our study incorporates three surgical murine models of colorectal cancer (CRC) to investigate the effect of blood loss, sepsis, and hypothermia on NK cell function and metastatic disease. Taking measures to reduce perioperative complications and/or employing preoperative neoadjuvant immunotherapy will help to improve survival outcomes and reduce cancer recurrence.
Methods
Cell lines
CT26LacZ mouse colon carcinoma and YAC-1 mouse lymphoma cells were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). CT26LacZ cells were cultured in HyQ high glucose Dulbecco’s modified Eagles medium (GE healthcare, Mississauga, ON, CA) supplemented with 10% fetal bovine serum (CanSera, Etobicoke, ON, CA). YAC-1 cells were cultured in HyClone™ Roswell Park Memorial Institute medium (RPMI)-1640 medium (GE healthcare, Mississauga, ON, CA) supplemented with 10% fetal bovine serum (CanSera, Etobicoke, ON, CA) and 1× of Penicillin/Streptomycin (Invitrogen, Carlsbad, CA, USA).
Animals
Female age-matched (6–8 weeks old at study initiation) BALB/c mice (Charles River Laboratories, Wilmington, MA, USA) were housed in specific pathogen-free conditions. The number of mice employed per experiment is indicated in the figure legends. Animal studies complied with the Canadian Council on Animal Care guidelines and were approved by the University of Ottawa Animal Research Ethics Board.
Induction of experimental metastasis and surgical stress
Mice were subjected to 2.5% isofluorane (Baxter Corporations, Mississauga, ON, CA) for the induction and maintenance of anesthesia. Routine perioperative care for mice, including the subcutaneous administration of buprenorphine (0.05 mg/kg) for pain control the day of surgery and every 8 h for 2 days following surgery, was conducted in concordance with University of Ottawa protocols. Surgical stress was induced via an abdominal laparotomy (i.e. 3-cm midline incision), which was preceded by an intravenous challenge with 3e5 CT26LacZ cells to establish pulmonary metastases. Abdominal laparotomy was commenced 10 min following tumor inoculation, as previously described [11]. Animals were euthanized at 18 h or 3 days following tumor inoculation and their lungs were stained with X-gal (Bioshop Canada Inc., Burlington, ON, CA), as described previously [41]. The total number of surface metastases on the largest lung lobe (left lobe) were quantified using a stereomicroscope (Leica Microsystems, Richmond Hill, ON, CA).
Hypovolemic stress model
Hypovolemia was induced by preoperatively bleeding mice prior to tumour inoculation. Mice were bled either 20% (300 uL) or 30% (450 uL) of their total blood volume by puncturing the saphenous vein just above the foot. Systolic arterial pressure (SAP) in conscious mice before and after saphenous vein bleeding was measured using a tail-cuff sphygmomanometer. Mice were kept in a warmed black box and an inflatable cuff was applied to the base of the tail. The tail of each mouse was then placed on a piezoelectric sensor for analysis of the pressure waveforms.
Hypothermia stress model
Intraoperative hypothermic shock was induced by placing mice directly on the metal surgical surface without a heating pad immediately following tumour inoculation. Mice were kept under hypothermic conditions and anesthesia for approximately 2 h and were subsequently housed under normothermic conditions. Rectal temperatures were recorded every 15 min throughout the procedure to verify that hypothermia was maintained.
Sepsis stress model
Intraoperative polymicrobial sepsis was induced in mice by cecal puncture at the time of abdominal laparotomy (i.e. 3-cm midline incision). Polymicrobial sepsis was confirmed by Gram stain of peritoneal lavage fluid, which was isolated 18 h following surgery. Bacterial counts were determined by serial dilution of peritoneal lavage fluid and overnight culture on tryptic soy broth agar plates at 37 °C. We also investigated whether antibiotic treatment with Imipenem, which was administered intravenously at 0.5 mg, or treatment with poly(I:C), a toll-like receptor (TLR)-3 ligand, at 150 μg/200 μL PBS had an impact on lung tumour burden.
Ex-vivo NK cell cytotoxicity assay
Chromium-release assays were conducted as previously described [42]. Briefly, splenocytes were isolated from surgically stressed and control mice 18 h after surgery (n = 3 for each treatment group and each E:T ratio). Pooled and sorted NK cells were resuspended at a concentration of 2.5 × 106 cells/mL. These cells were then mixed with chromium-labeled YAC-1 target cells, which were resuspended at a concentration of 3 × 104 cells/mL at various effector-to-target (E:T) ratios (i.e. 50:1, 25:1, 12:1, and 6:1).
Statistical analysis
Statistical tests were performed using GraphPad Prism (GraphPad, San Diego, CA, USA). One-way ANOVAs, factorial ANOVAs with Tukey correction for multiple comparisons, and student’s t-tests with equal variances were conducted. Data were reported as the mean ± standard error of the mean (SEM). An alpha value of < 0.05 was considered to be statistically significant.
Results
Severe hypovolemia increases pulmonary metastases, but is not additive when combined with surgical stress
To discern the effect of hypovolemic shock on perioperative metastases, mice were first exsanguinated through the saphenous vein while systolic blood pressure was measured using a tail cuff sphygmomanometer (Fig. 1a). A significant reduction in tail cuff blood pressure was observed following the loss of 450 uL of blood, which is representative of 30% of the total blood volume of a 25-g mouse (71.67 ± 2.186 mmHg vs. 107.7 ± 1.453 mmHg, p = 0.0002); moreover, the reduction in tail cuff blood pressure was exacerbated when 450 uL of blood loss was combined with surgical stress (61.67 ± 1.667 mmHg vs. 107.7 ± 1.453 mmHg, p < 0.0001) (Fig. 1b). In the absence of surgical stress, hypovolemic changes due to 30% blood loss significantly increased the number of pulmonary metastases when compared to mice that did not undergo any blood loss (438.3 ± 56.89 vs. 205.0 ± 24.88, p = 0.0212) (Fig. 1c). In addition to hypovolemia, surgical stress alone significantly increased pulmonary metastases compared to mice that did no undergo surgery (287.5 ± 39.01 vs. 95.40 ± 32.35, p = 0.0065). However, a combination of 30% blood loss and surgical stress did not further increase the number of pulmonary metastases above surgical stress alone (285.6 ± 35.94 vs. 287.5 ± 39.01, p = 0.9725) (Fig. 1d).
Hemorrhagic shock does not increase metastatic disease. a Experimental overview. BALB/c mice were bled through the saphenous vein (indicated by the white arrow) and subsequently injected intravenously (IV) through the tail vein with 3 × 105 CT26LacZ cells. Approximately 1 h later, surgical stress (sx) was generated by laparotomy (Lap) (5 cm incision). Mice were sacrificed at 72 h to quantify lung metastases. b Blood pressure is reduced following surgical stress and blood loss. Blood pressure (mmHg) was measured following a 5-day training period (Day 1–5), prior to bleeding (Pre), immediately following bleeding (Post-BL), and immediately following surgical stress (Post-Sx and BL, n = 3). c Blood loss increases metastatic burden. Lung metastases were measured on Day 3 following no blood loss (no BL, n = 3) or 20% (20% BL, n = 3) or 30% blood loss (30% BL, n = 4). d Blood loss does not increase metastatic disease in conjunction with surgical stress. Lung metastases were measure on Day 3 in mice that did not undergo surgical stress (No Sx, n = 5) and animals undergoing a laparotomy (Lap, n = 4) alone or in combination with 30% blood loss (Lap + 30% BL, n = 5). Error bars represent ± SEM
Severe hypothermia does not increase pulmonary metastases
Next, we sought to assess the effect of perioperative hypothermic shock on metastases in a surgical murine model. Anaesthetized animals were maintained at either normothermic (35.22 °C–37.95 °C) or hypothermic (26.35 °C–29.03 °C) temperatures for a 3-h period following injection of the tumour cells and surgical stress (Fig. 2a and b). We observed that hypothermia alone did not significantly increase pulmonary metastases when compared to normothermic mice (27.75 ± 7.667 vs. 21.38 ± 7.720, p = 0.5673) (Fig. 2c). Surgical stress in normothermic conditions did significantly increase the number of pulmonary metastases compared to mice that did not undergo surgery (391.5 ± 38.18 vs. 136.4 ± 21.47, p = 0.0024) (Fig. 2d). When combined with surgical stress, however, hypothermia did not further increase the number of pulmonary metastases when compared to mice that underwent surgical stress under normothermic conditions (391.5 ± 38.18 vs. 341.8 ± 80.90, p = 0.6265).
Hypothermia does not increase metastatic disease. a Experimental overview. BALB/c mice were injected intravenously (IV) through the tail vein with 3 × 105 CT26lacZ cells. Mice undergoing hypothermia were placed directly on the metal block (shown) while control mice were kept under normothermic conditions with heating pads. One hour into anesthesia, surgical stress (Sx) was generated by laparatomy (Lap). Mice were removed from anesthesia after 2 h total and kept in normal housing conditions. Temperature was measured during anesthesia using a rectal probe (as shown). Mice were sacrificed at 72 h to quantify lung metastases. b Maintenance of body temperature. Mice kept under normothermic conditions alone ranged from 31.88 °C to 38.16 °C in comparison to hypothermic conditions which ranged from 23.98 °C to 32.75 °C for 100 min. c Hypothermia alone does not increase lung metastases. The impact of body temperature upon lung metastases was assessed in mice maintained at normothermic (n = 8) and hypothermic (n = 8) conditions. d Prometastic effects of surgical stress are not impacted by hypothermic conditions. Lung metastases were measured in mice that did not undergo surgery (No Sx, n = 5) and mice undergoing a laparotomy under normothermic (Lap, n = 4) and hypothermic conditions (Hypothermic + Lap, n = 5). Error bars represent ± SEM
Perioperative polymicrobial sepsis increases pulmonary metastases compared to surgical stress alone
Next, we assessed the effects of sepsis on pulmonary metastases by contaminating the peritoneal cavity with stool expressed through caecal puncture (Fig. 3a). Peritoneal lavage contained both Gram-negative and Gram-positive bacteria, as well as coccus and bacillus-shaped bacteria, confirming that the caecal puncture lead to polymicrobial sepsis (Fig. 3b). Surgical stress alone resulted in a significant increase in pulmonary metastases when compared to control mice (374.3 ± 20.68 vs. 266.4 ± 16.15, p = 0.0006); furthermore, we observed that a combination of polymicrobial sepsis and surgical stress resulted in a significant increase in pulmonary metastases when compared to mice that underwent surgical stress in the absence of sepsis (480.3 ± 18.98 vs. 374.3 ± 20.68, p = 0.0010) (Fig. 3c). Previous studies have shown that suppression of NK cell cytotoxic activity is responsible for the increase in cancer burden following surgical stress. In this study, we demonstrate that sepsis, in conjunction with surgical stress, significantly attenuated NK cell cytotoxic activity below that of surgical stress alone (Fig. 3d).
Sepsis increases metastatic disease burden. a Experimental overview. BALB/c mice were injected intravenously (IV) through the tail vein with 3 × 105 CT26lacZ cells. One hour later, surgical stress (Sx) was generated by laparatomy (Lap) or laparatomy with caecal puncture (Lap + CP). The caecum was externalized and punctured using an 18G needle (as shown). Mice were sacrificed at 72 h to quantify lung metastases. b Detection of bacteria in the peritoneal cavity. A representative Gram stain of peritoneal lavage fluid from a mouse that underwent Lap + CP indicates polymicrobial sepsis due to the presence of both cocci and bacilli. c Sepsis increases lung metastases. Lung metastases were quantified in mice that did not undergo surgical stress (No Sx, n = 13), laparotomy alone (Lap, n = 10), or laparotomy combined with sepsis (Lap + Sepsis, n = 19). d Intraoperative sepsis impairs NK cell-mediated cytotoxicity. Chromium release killing assay of YAC-1 tumour target cells by DX5+ NK cells isolated from animals that did not undergo surgical stress (No Sx), laparotomy alone (Lap) in the presence or absence of sepsis (Lap + Sepsis). Effector (DX5+ NK cells) to target (Yac1) (E:T) ratios of 6:1, 25:1, and 50:1 are shown (n = 3 for each treatment group and each E:T ratio). Error bars represent ± SEM
Perioperative NK cell stimulation reduces metastases and restores NK cell function in the presence of sepsis
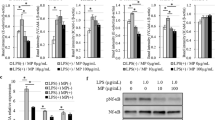
We have shown that postoperative immune dysfunction can be ameliorated through the use of NK cell-stimulating agents such as poly(I:C), a double-stranded RNA mimetic. In this study, we examined whether sepsis-induced postoperative pulmonary metastases could be suppressed by perioperative immune stimulation with polyI:C (Fig. 4a). We demonstrated that treatment with polyI:C alone significantly decreased the number of pulmonary metastases compared to mice undergoing surgical stress and sepsis with no perioperative therapy (18.25 ± 8.390 vs. 175.0 ± 13.48, p = 0.0002); moreover, antibiotics alone did not affect the number of pulmonary metastases when compared to surgically stressed mice with sepsis that were not administered perioperative therapy (185.3 ± 19.38 vs. 175.0 ± 13.48, p = 0.6794) (Fig. 4a). Furthermore, antibiotic therapy did not further decrease the number of pulmonary metastases in mice undergoing surgical stress and sepsis in the presence of polyI:C (25.80 ± 4.306 vs. 18.25 ± 8.390, p = 0.4213). In support of a role for NK cells in mediating this effect we also demonstrated that the addition of polyI:C enhanced NK cell cytotoxic activity by 10-fold, a magnitude similar to the reduction in metastases seen with perioperative use (Fig. 4b).
Prometastatic effects of sepsis are reversed with perioperative NK cell activation. a Lung metastases are reduced in polyI:C treated animals. Lung metastases were quantified in untreated animals (No Sx, n = 4) and following laparotomy (Lap + Sepsis, n = 4) without or with perioperative antibiotic treatment administered subcutaneously (Abx, Imipenem 0.5 mg, n = 4), preoperative polyI:C treatment (PolyI:C,150 μg/200 μL PBS, n = 4), and a combination of antibiotic and polyI:C treatment (Lap + Sepsis + Abx + PolyI:C, n = 5). b Preoperative PolyI:C restores NK cell cytotoxicity. Chromium release killing assay of YAC-1 tumour target cells by DX5+ NK cells isolated from animals undergoing treatments as above. Effector (DX5+ NK cells) to target (Yac1) (E:T) ratios of 6:1, 12:1, 25:1, and 50:1 are shown (n = 3 for each treatment group and each E:T ratio). Error bars represent ± SEM
Discussion
Perioperative complications, specifically infection, decrease long-term survival [5, 43] and promote recurrence in patients with CRC [44]. Although hypovolemia in the absence of surgical stress did lead to an increase in pulmonary metastases, our findings demonstrate that neither severe intraoperative hypovolemia nor hypothermia impact the prometastatic effects of surgical stress. Correlative clinical studies confirm that postoperative infections following surgery can accelerate the time to cancer recurrence [45,46,47]. Here, using murine models we demonstrate that polymicrobial sepsis in conjunction with surgical stress facilitates the development of perioperative lung metastases. Our results suggest that the combined immunosuppressive effects of surgical trauma and sepsis dampen anti-tumour immune responses, ultimately leading to an increase in metastases. In addition to the immunosuppressive effects of surgical stress, severe sepsis can induce lymphocyte exhaustion [48], apoptosis of immune cells [49, 50], and a predominance of immunoregulatory cells, including regulatory T cells [51, 52] and myeloid-derived suppressor cells [53]. This highly suppressive environment likely worsens the already immunosuppressive environment present in most cancer patients in need of surgical intervention [54, 55]. Thus, the immunosuppressive effects of surgery, sepsis, and cancer may interact to severely dampen immune activation and increase the likelihood of cancer recurrence and metastatic disease.
Our findings also suggest that sepsis induces its prometastatic effect by inhibiting NK cell cytotoxic function. In the cancer microenvironment, the anti-tumour function of NK cells is suppressed [56], while a decrease in NK cell number and function in patients undergoing surgery for CRC is associated with heightened mortality and cancer recurrence suggesting that the suppressive effects of sepsis likely exacerbate the already impaired NK cell function [57, 58]. In agreement with our findings, previous studies have demonstrated that sepsis in a non-surgical context can impair NK cell cytotoxicity [59], a finding that has been attributed to a heightened activation of regulatory cell subsets [60]. In particular, murine sepsis models have shown that an increase in regulatory T cells contributes to post-sepsis immunosuppression and potentiates tumour growth [61]. NK cells are a critical component of anti-tumour immunity and so, based on our findings, we suggest that the inhibition of NK cell function is a key player in perioperative cancer recurrence following surgical stress and septic insult.
Tumour-infiltrating NK cells and lymphocytes are associated with improved prognosis in several malignancies [62,63,64,65,66]. The enhancement of preoperative NK cell activation with PolyI:C, a TLR3 ligand, to counteract the immunosuppressive effects of surgery and sepsis and attenuate perioperative metastases formation is largely in agreement with the inhibitory effects of poly(I:C) upon tumour outgrowth in non-surgical models of lung metastases [67]. While polyI:C is ineffective in primates because of inactivation by natural enzymes, other NK stimulators, such as poly-ICLC [68] (stabilized with poly-lysine) or a virus-derived TLR agonist, like the influenza vaccine, could be safely and effectively employed in the perioperative period. Taken together, boosting NK cell activation may counteract the immunosuppressive effects of sepsis and protect against the development of metastatic disease and has potential as a perioperative cancer immunotherapeutic strategy.
Conclusions
In conclusion, our study is the first to utilize a murine model to investigate the effects of surgical complications on cancer recurrence in the perioperative period. Our findings demonstrate that intraoperative sepsis, but not intraoperative blood loss or hypothermia, contributes to the development of greater metastatic disease. We also demonstrate that perioperative sepsis-induced metastases are mediated by a suppression of NK cell cytotoxicity and can be reversed by TLR-mediated stimulation of NK cells. Further studies are required to determine whether enhancing NK cell function can prevent the development of perioperative metastatic disease in patients undergoing cancer surgery.
Change history
17 April 2018
It has been highlighted that the original manuscript [1] contains a typesetting error in Fig. 1 and the Fig. 1c panel gas been inadvertently duplicated in panel Fig. 1d. This does not affect the results and conclusions of the article. The correct version of Fig. 1 is included with this Correction. The original article has been updated.
Abbreviations
- BL:
-
Blood loss
- CP:
-
Caecal puncture
- CRC:
-
Colorectal cancer
- E:T ratio:
-
Effector-to-target ratio
- IL:
-
Interleukin
- IV:
-
Intravenous
- LN:
-
Left nephrectomy
- NK cell:
-
Natural killer cell
- PH:
-
Partial hepatectomy (PH)
- SAP:
-
Systolic arterial pressure
- SEM:
-
Standard error of the mean
- TLR:
-
Toll-like receptor
References
Desborough JP. The stress response to trauma and surgery. Br J Anaesth. 2000;85:109–17.
Caine GJ, Stonelake PS, Lip GY, Kehoe ST. The hypercoagulable state of malignancy: pathogenesis and current debate. Neoplasia. 2002;4:465–73. https://doi.org/10.1038/sj.neo.7900263.
Kimura F, Shimizu H, Yoshidome H, Ohtsuka M, Miyazaki M. Immunosuppression following surgical and traumatic injury. Surg Today. 2010;40:793–808. https://doi.org/10.1007/s00595-010-4323-z.
Young J, Han CH, Kim YK. Postoperative complications influence prognosis and recurrence patterns in Periampullary cancer. World J Surg. 2013;37:2234–41.
Artinyan A, Chen GJ, Berger DH. Infectious postoperative complications decrease long-term survival in patients undergoing curative surgery for colorectal cancer. Ann Surg. 2015;261:497–505.
Khuri SF, Henderson WG, Depalma RG. Determinants of long-term survival after major surgery and the adverse effect of postoperative complications. Ann Surg. 2005;242:326–43.
Aahlin EK, Olsen F, Uleberg B, Jacobsen BK, Lassen K. Major postoperative complications are associated with impaired long-term survival after gastro-esophageal and pancreatic cancer surgery: a complete national cohort study. BMC Surg. 2016:1–8. https://doi.org/10.1186/s12893-016-0149-y.
Seth R, Tai L-H, Falls T, de Souza CT, Bell JC, Carrier M, et al. Surgical stress promotes the development of cancer metastases by a coagulation-dependent mechanism involving natural killer cells in a murine model. Ann Surg. 2013;258:158–68. https://doi.org/10.1097/SLA.0b013e31826fcbdb.
Ananth AA, Tai LH, Lansdell C, Alkayyal AA, Baxter KE, Angka L, et al. Surgical stress abrogates pre-existing protective T cell mediated anti-tumor immunity leading to postoperative cancer recurrence. PLoS One. 2016;11:e0155947. https://doi.org/10.1371/journal.pone.0155947.
Tai L-H, Zhang J, Scott KJ, de Souza CT, Alkayyal AA, Ananth AA, et al. Perioperative influenza vaccination reduces postoperative metastatic disease by reversing surgery-induced dysfunction in natural killer cells. Clin Cancer Res. 2013;19:5104–15. https://doi.org/10.1158/1078-0432.CCR-13-0246.
Tai L-H, Tanese de Souza C, Sahi S, Zhang J, Alkayyal AA, Ananth AA, et al. A mouse tumor model of surgical stress to explore the mechanisms of postoperative immunosuppression and evaluate novel perioperative immunotherapies. J Vis Exp. 2014; https://doi.org/10.3791/51253.
Tai LH, De Souza CT, Bélanger S, Ly L, Alkayyal AA, Zhang J, et al. Preventing postoperative metastatic disease by inhibiting surgery-induced dysfunction in natural killer cells. Cancer Res. 2013;73:97–107.
Goldfarb Y, Sorski L, Benish M, Levi B, Melamed R, Ben-Eliyahu S. Improving postoperative immune status and resistance to cancer metastasis: a combined perioperative approach of immunostimulation and prevention of excessive surgical stress responses. Ann Surg. 2011;253:798–810. https://doi.org/10.1097/SLA.0b013e318211d7b5.
Benish M, Bartal I, Goldfarb Y, Levi B, Avraham R, Raz A, et al. Perioperative use of beta-blockers and COX-2 inhibitors may improve immune competence and reduce the risk of tumor metastasis. Ann Surg Oncol. 2008;15:2042–52. https://doi.org/10.1245/s10434-008-9890-5.
Glasner A, Avraham R, Rosenne E, Benish M, Zmora O, Shemer S, et al. Improving survival rates in two models of spontaneous postoperative metastasis in mice by combined administration of a beta-adrenergic antagonist and a cyclooxygenase-2 inhibitor. J Immunol. 2010;184:2449–57. https://doi.org/10.4049/jimmunol.0903301.
Colacchio TA, Yeager MP, Hildebrandt LW. Perioperative immunomodulation in cancer surgery. Am J Surg. 1994;167:174–9. http://www.ncbi.nlm.nih.gov/pubmed/8311130
Da Costa ML, Redmond P, Bouchier-Hayes DJ. The effect of laparotomy and laparoscopy on the establishment of spontaneous tumor metastases. Surgery. 1998;124:516–25. http://www.ncbi.nlm.nih.gov/pubmed/9736904
Shiromizu A, Suematsu T, Yamaguchi K, Shiraishi N, Adachi Y, Kitano S. Effect of laparotomy and laparoscopy on the establishment of lung metastasis in a murine model. Surgery. 2000;128:799–805. https://doi.org/10.1067/msy.2000.108047.
Ben-Eliyahu S, Page GG, Yirmiya R, Shakhar G. Evidence that stress and surgical interventions promote tumor development by suppressing natural killer cell activity. Int J Cancer. 1999;80:880–8.
Tsuchiya Y, Sawada S, Yoshioka I, Ohashi Y, Matsuo M, Harimaya Y, et al. Increased surgical stress promotes tumor metastasis. Surgery. 2003;133:547–55. https://doi.org/10.1067/msy.2003.141.
Coffey JC, Wang JH, Smith MJF, Bouchier-Hayes D, Cotter TG, Redmond HP. Excisional surgery for cancer cure: therapy at a cost. Lancet Oncol. 2003;4:760–8. http://www.ncbi.nlm.nih.gov/pubmed/14662433
Da Costa ML, Redmond HP, Finnegan N, Flynn M, Bouchier-Hayes D. Laparotomy and laparoscopy differentially accelerate experimental flank tumour growth. Br J Surg. 1998;85:1439–42. https://doi.org/10.1046/j.1365-2168.1998.00853.x.
Espi A, Arenas J, Garcia-Granero E, Marti E, Lledo S. Relationship of curative surgery on natural killer cell activity in colorectal cancer. Dis Colon Rectum. 1996;39:429–34. http://www.ncbi.nlm.nih.gov/pubmed/8878504
Baxevanis CN, Papilas K, Dedoussis GV, Pavlis T, Papamichail M. Abnormal cytokine serum levels correlate with impaired cellular immune responses after surgery. Clin Immunol Immunopathol. 1994;71:82–8. http://www.ncbi.nlm.nih.gov/pubmed/8137562
Ahlers O, Nachtigall I, Lenze J, Goldmann A, Schulte E, Hohne C, et al. Intraoperative thoracic epidural anaesthesia attenuates stress-induced immunosuppression in patients undergoing major abdominal surgery. Br J Anaesth. 2008;101:781–7. https://doi.org/10.1093/bja/aen287.
Nakazaki H. Preoperative and postoperative cytokines in patients with cancer. Cancer. 1992;70:709–13. http://www.ncbi.nlm.nih.gov/pubmed/1320454
Ogawa K, Hirai M, Katsube T, Murayama M, Hamaguchi K, Shimakawa T, et al. Suppression of cellular immunity by surgical stress. Surgery. 2000;127:329–36. https://doi.org/10.1067/msy.2000.103498.
Whitson BA, D’Cunha J, Maddaus MA. Minimally invasive cancer surgery improves patient survival rates through less perioperative immunosuppression. Med Hypotheses. 2007;68:1328–32. https://doi.org/10.1016/j.mehy.2006.09.063.
Bartal I, Melamed R, Greenfeld K, Atzil S, Glasner A, Domankevich V, et al. Immune perturbations in patients along the perioperative period: alterations in cell surface markers and leukocyte subtypes before and after surgery. Brain Behav Immun. 2010;24:376–86. https://doi.org/10.1016/j.bbi.2009.02.010.
Ho CS, Lopez JA, Vuckovic S, Pyke CM, Hockey RL, Hart DN. Surgical and physical stress increases circulating blood dendritic cell counts independently of monocyte counts. Blood. 2001;98:140–5. http://www.ncbi.nlm.nih.gov/pubmed/11418473
Greenfeld K, Avraham R, Benish M, Goldfarb Y, Rosenne E, Shapira Y, et al. Immune suppression while awaiting surgery and following it: dissociations between plasma cytokine levels, their induced production, and NK cell cytotoxicity. Brain Behav Immun. 2007;21:503–13. https://doi.org/10.1016/j.bbi.2006.12.006.
Pereira J, Phan T. Management of bleeding in patients with advanced cancer. Oncologist. 2004;9:561–70. https://doi.org/10.1634/theoncologist.9-5-561.
Vogel TR, Dombrovskiy VY, Lowry SF. Trends in postoperative sepsis: are we improving outcomes? Surg Infect. 2009;10:71–8. https://doi.org/10.1089/sur.2008.046.
Williams MD, Braun LA, Cooper LM, Johnston J, Weiss RV, Qualy RL, et al. Hospitalized cancer patients with severe sepsis: analysis of incidence, mortality, and associated costs of care. Crit Care. 2004;8:R291–8. https://doi.org/10.1186/cc2893.
Forstot RM. The etiology and management of inadvertent perioperative hypothermia. J Clin Anesth. 1995;7:657–74. http://www.ncbi.nlm.nih.gov/pubmed/8747566
Moslemi-kebria M, El-nashar SA. Cytoreductive surgery for ovarian cancer and perioperative morbidity. Obstet Gynecol. 2012;119:590–6.
Jiang W, Fang YJ, Wu XJ, Wang FL, Lu ZH, Zhang RX, et al. Intraoperative blood loss independently predicts survival and recurrence after resection of colorectal cancer liver metastasis. PLoS One. 2013;8:e76125. https://doi.org/10.1371/journal.pone.0076125.
Katz SC, Shia J, Jarnagin WR, Fong Y, Blumgart LH, Dematteo RP. Operative blood loss independently predicts recurrence. Ann Surg. 2009;249:617–23.
Nespoli A, Gianotti L, Totis M, Bovo G, Nespoli L, Chiodini P, et al. Correlation between postoperative infections and long-term survival after colorectal resection for cancer. Tumori. 2004;90:485–90. http://www.ncbi.nlm.nih.gov/pubmed/15656334
Yamashita K, Makino T, Miyata H, Miyazaki Y, Takahashi T. Postoperative infectious complications are associated with adverse oncologic outcomes in esophageal cancer patients undergoing preoperative chemotherapy. Ann Surg Oncol. 2016;23:2106–14.
Kirstein JM, Graham KC, Mackenzie LT, Johnston DE, Martin LJ, Tuck AB, et al. Effect of anti-fibrinolytic therapy on experimental melanoma metastasis. Clin Exp Metastasis. 2009;26:121–31. https://doi.org/10.1007/s10585-008-9221-z.
Patel R, Belanger S, Tai LH, Troke AD, Makrigiannis AP. Effect of Ly49 haplotype variance on NK cell function and education. J Immunol. 2010;185:4783–92. https://doi.org/10.4049/jimmunol.1001287.
Odermatt M, Miskovic D, Flashman K, Khan J, Senapati A, O’Leary D, et al. Major postoperative complications following elective resection for colorectal cancer decrease long-term survival but not the time to recurrence. Color Dis. 2015;17:141–9.
Alonso S, Pascual M, Salvans S, Mayol X, Mojal S, Gil MJ, et al. Postoperative intra-abdominal infection and colorectal cancer recurrence: a prospective matched cohort study of inflammatory and angiogenic responses as mechanisms involved in this association. Eur J Surg Oncol. 2015;41:208–14. https://doi.org/10.1016/j.ejso.2014.10.052.
Tsujimoto H, Ueno H, Hashiguchi Y, Ono S, Ichikura T, Hase K. Postoperative infections are associated with adverse outcome after resection with curative intent for colorectal cancer. Oncol Lett. 2010;1:119–25. https://doi.org/10.3892/ol_00000022.
Schietroma M, Pessia B, Carlei F, Cecilia EM, Amicucci G. Intestinal permeability, systemic endotoxemia, and bacterial translocation after open or laparoscopic resection for colon cancer: a prospective randomized study. Int J Color Dis. 2013;28:1651–60. https://doi.org/10.1007/s00384-013-1751-4.
Varty PP, Linehan IP, Boulos PB. Intra-abdominal sepsis and survival after surgery for colorectal cancer. Br J Surg. 1994;81:915–8. http://www.ncbi.nlm.nih.gov/pubmed/8044621
Boomer JS, Shuherk-shaffer J, Hotchkiss RS, Green JM. A prospective analysis of lymphocyte phenotype and function over the course of acute sepsis. Crit Care. 2012;16:R112.
Hotchkiss RS, Tinsley KW, Swanson PE, Schmieg RE Jr, Hui JJ, Chang KC, et al. Sepsis-induced apoptosis causes progressive profound depletion of B and CD4+ T lymphocytes in humans. J Immunol. 2001;166:6952–63. http://www.ncbi.nlm.nih.gov/pubmed/11359857
Hotchkiss RS, Swanson PE, Freeman BD, Tinsley KW, Cobb JP, Matuschak GM, et al. Apoptotic cell death in patients with sepsis, shock, and multiple organ dysfunction. Crit Care Med. 1999;27:1230–51. http://www.ncbi.nlm.nih.gov/pubmed/10446814
Venet F, Chung CS, Kherouf H, Geeraert A, Malcus C, Poitevin F, et al. Increased circulating regulatory T cells (CD4(+)CD25 (+)CD127 (−)) contribute to lymphocyte anergy in septic shock patients. Intensive Care Med. 2009;35:678–86. https://doi.org/10.1007/s00134-008-1337-8.
Wisnoski N, Chung CS, Chen Y, Huang X, Ayala A. The contribution of CD4+ CD25+ T-regulatory-cells to immune suppression in sepsis. Shock. 2007;27:251–7. https://doi.org/10.1097/01.shk.0000239780.33398.e4.
Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–74. https://doi.org/10.1038/nri2506.
Lindau D, Gielen P, Kroesen M, Wesseling P, Adema GJ. The immunosuppressive tumour network: myeloid-derived suppressor cells, regulatory T cells and natural killer T cells. Immunology. 2013;138:105–15. https://doi.org/10.1111/imm.12036.
Keskinov AA, Shurin MR. Myeloid regulatory cells in tumor spreading and metastasis. Immunobiology. 2015;220:236–42. https://doi.org/10.1016/j.imbio.2014.07.017.
Pahl J, Cerwenka A. Immunobiology tricking the balance : NK cells in anti-cancer immunity. Immunobiology. 2017;222:11–20. https://doi.org/10.1016/j.imbio.2015.07.012.
Tartter PI, Steinberg B, Barron DM, Martinelli G. The prognostic significance of natural killer cytotoxicity in patients with colorectal cancer. Arch Surg. 1987;122:1264–8. http://www.ncbi.nlm.nih.gov/pubmed/3675190
Peng YP, Zhu Y, Zhang JJ, Xu ZK, Qian ZY, Dai CC, et al. Comprehensive analysis of the percentage of surface receptors and cytotoxic granules positive natural killer cells in patients with pancreatic cancer, gastric cancer, and colorectal cancer. J Transl Med. 2013;11:262. https://doi.org/10.1186/1479-5876-11-262.
Maturana P, Puente J, Miranda D, Sepulveda C, Wolf ME, Mosnaim AD. Natural killer cell activity in patients with septic shock. J Crit Care. 1991;6:42–5.
Kessel A, Bamberger E, Masalha M, Toubi E. The role of T regulatory cells in human sepsis. J Autoimmun. 2009;32:211–5. https://doi.org/10.1016/j.jaut.2009.02.014.
Cavassani KA, Carson WF 4th, Moreira AP, Wen H, Schaller MA, Ishii M, et al. The post sepsis-induced expansion and enhanced function of regulatory T cells create an environment to potentiate tumor growth. Blood. 2010;115:4403–11. https://doi.org/10.1182/blood-2009-09-241083.
Villegas FR, Coca S, Villarrubia VG, Jiménez R, Chillón MJ, Jareño J, et al. Prognostic significance of tumor infiltrating natural killer cells subset CD57 in patients with squamous cell lung cancer. Lung Cancer. 2002;35:23–8.
Ishigami S, Natsugoe S, Tokuda K, Nakajo A, Che X, Iwashige H, et al. Prognostic value of intratumoral natural killer cells in gastric carcinoma. Cancer. 2000;88:577–83.
Coca S, Perez-Piqueras J, Martinez D, Colmenarejo A, Saez MA, Vallejo C, et al. The prognostic significance of intratumoral natural killer cells in patients with colorectal carcinoma. Cancer. 1997;79:2320–8. http://www.ncbi.nlm.nih.gov/pubmed/9191519
Xu B, Chen L, Li J, Zheng X, Shi L, Jiang J. Prognostic value of tumor infiltrating NK cells and macrophages in stage II+III esophageal cancer patients. Oncotarget. 2016;7:74904–16.
Deschoolmeester V, Baay M, Van Marck E, Weyler J, Vermeulen P, Lardon F, et al. Tumor infiltrating lymphocytes: an intriguing player in the survival of colorectal cancer patients. BMC Immunol. 2010;11:19. https://doi.org/10.1186/1471-2172-11-19.
Forte G, Rega A, Morello S, Luciano A, Arra C, Pinto A, et al. Polyinosinic-polycytidylic acid limits tumor outgrowth in a mouse model of metastatic lung cancer. J Immunol. 2012;188:5357–64. https://doi.org/10.4049/jimmunol.1103811.
Hartman LLR, Crawford JR, Makale MT, Milburn M, Joshi S, Salazar AM, et al. Pediatric phase II trials of poly-ICLC in the management of newly diagnosed and recurrent brain tumors. J Pediatr Hematol Oncol. 2014;36:451–7. https://doi.org/10.1097/MPH.0000000000000047.
Acknowledgements
Not applicable
Funding
This work was supported by funding from the Cancer Research Society (20491) and Canadian Cancer Society Research Institute Innovation Award (703424). P.S. is a recipient of the American Society for Hematology (ASH) HONORS award. The funding bodies had no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Contributions
All authors read and approved the final manuscript. Experimental conception and design - L-HT, AAA, RS, AA, JZ, CTS and RCA. Data Acquisition: L-HT, AAA, RS, AA, JZ, and CTS. Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): L-HT, AAA, RS, AA, JZ, PS, MAK and RCA. Writing, review, and revision of manuscript: L-HT, AAA, RS, PS, MAK, and RCA. Study Supervision: L-HT and RCA.
Corresponding author
Ethics declarations
Ethics approval
Animal studies complied with the Canadian Council on Animal Care guidelines and were approved by the University of Ottawa Animal Research Ethics Board (ME-1664).
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional information
The original version of this article was corrected: “the original manuscript contained a typesetting error in Fig. 1 and the Fig. 1c panel had been inadvertently duplicated in panel Fig. 1d.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Tai, LH., Ananth, A.A., Seth, R. et al. Sepsis increases perioperative metastases in a murine model. BMC Cancer 18, 277 (2018). https://doi.org/10.1186/s12885-018-4173-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-018-4173-4