Abstract
Background
This study evaluated the distribution pattern of the Ki67-labeling index (LI) among patients at a Chinese breast cancer center, and analyzed its prognostic significance in the 2015 St Gallen consensus breast cancer classification, estrogen receptor-positive and human epidermal growth factor receptor 2-negative(ER+/HER2−)subtype.
Methods
We classified 939 women with ER+/HER2− breast cancer into three groups by Ki67-LI levels, and followed their clinicopathologic characteristics and prognoses.
Results
In the 939 eligible subjects, 342 had Ki67-LI ≤10% (Ki67Low), 281 had Ki67-LI between 10 and 30% (Ki67Medium), and 316 had Ki67-LI ≥30% (Ki67High). Although the Ki67High group had less favorable clinicopathologic factors, the Ki67Medium group’s factors varied considerably. Kaplan-Meier estimates showed that disease-free survival(DFS) for the Ki67Medium group was significantly shorter than the Ki67Low group but longer than the Ki67High group. Ki67-LI had independent prognostic significance in multivariate analysis. Other diagnostic factors, including tumor size >2 cm, positive lymph nodes, and grade III disease, were significantly associated with poorer disease-free survival only in the Ki67Medium group.
Conclusions
For patients with ER+/HER2− breast cancer, we confirmed three distinct risk patterns by Ki67-LI levels according to the 2015 St Gallen consensus. For patients with clearly low or high Ki67-LI, straightforward clinical decisions could be offered, but for patients with intermediate Ki67-LI, other factors might provide valuable information.
Similar content being viewed by others
Background
Breast cancer (BC) is a molecularly heterogeneous disease that includes at least four intrinsic subtypes with different features and prognoses [1–5]. Among estrogen receptor-positive and human epidermal growth factor receptor 2-negative (ER+/HER2−) BCs, gene expression-based assays showed that they can be divided into at least two distinct subgroups—luminal A and luminal B [4]. Although the luminal B subset has higher proliferation marker expression and worse prognosis [6], clinicopathological subtyping criteria of the two luminal groups keep changing in the literature.
The 2011 St Gallen consensus panel [7] recommended a cut-off of 14% for the Ki67-labeling index (LI) as the threshold between luminal A and B subtypes. The 2013 St Gallen consensus panel added another immunohistochemical (IHC) surrogate marker—progesterone receptor (PgR)—and increased the Ki67-LI cut-off to 20% [8]. However, at the latest 2015 St Gallen International Breast Cancer Conference, the panel recommended Ki67-LI should be interpreted upon local laboratory values, and ER+/HER2− BCs could not be classified as two distinctive groups by IHC surrogate markers, as they belong to a spectrum of disease [9].
This study evaluated Ki67-LI distribution in a Chinese BC treatment center and analyzed its prognostic significance in the 2015 St Gallen consensus of ER+/HER2− BCs.
Methods
Patients and clinical data collection
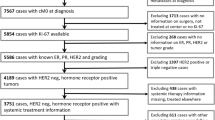
We searched breast tumor registries of the 2063 patients who had been treated for BC at the Sun Yat-Sen Memorial Hospital from March 2005 to December 2012, and for whom Ki67-LI information was available. Our hospital is equipped with one of the most comprehensive breast centers in China, and we are highly recognized by patients from all over the country. We excluded patients with non-invasive BC, ER-negative or HER2-positive disease, more than three involved lymph nodes, T4 lesions, male patients, and those with distant metastasis at first diagnosis. Finally, we included 939 patients with early invasive ER+/HER2− BCs.
Of these 939 participants, 372 (39.6%) received modified radical mastectomies and 567 (60.4%) underwent breast-conserving surgery (BCS, all with negative margins). All patients received sentinel lymph node biopsy (SLNB), of whom SLNB positive went on to axillary lymph node dissection(ALND). After surgery, 776 (82.6%) patients received adjuvant chemotherapy. The regimens mainly consisted of three types: standard cyclophosphamide, methotrexate, and 5-fluorouracil, anthracycline-based, or combined anthracycline and taxane (termed taxane-based regimen). They each received four to eight chemotherapy cycles. The main indications for radiotherapy included positive lymph nodes; primary tumor >5 cm; or BCS. In our study, chemotherapy indications were based on the National Comprehensive Cancer Network (NCCN) guidelines.
Patients were followed according to clinical protocols. The evaluated endpoint was DFS, which was defined as the interval from first diagnosis until diagnosis of local or regional BC recurrence, contralateral BC, distant metastasis, or death from BC. Patients known to be alive without recurrent disease or lost to follow-up at the time of analysis were screened at the time of their last follow-up. For those who attended no further clinical visits at our institute, essential follow-up information was collected by telephone. The cut-off date for the results presented here was February 22, 2016. This study was approved by the Ethics Committee of Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University.
Laboratory methods and group definition
IHC staining for ER, PgR, HER2 protein, and Ki67 antigen was performed on core biopsies or surgical specimens. All specimens were fixed with 10% neutral phosphate-buffered formalin and embedded in paraffin. Samples were considered ER+ if more than 10% nuclei were stained, which is the usual cut-off in larger Chinese hospitals [10]. Tumors were considered HER2+ if they received a score of three by IHC or if they were two by IHC but had amplified HER2 genes (ratio 2.0) based on fluorescence in situ hybridization (FISH). The MIB-1 clone antibody (1:100, DAKO) was used for Ki67 IHC staining; the Ki67-LI was the percentage of positively stained cancer cells (regardless of staining intensity).
Based on the 2015 St Gallen consensus standard, and the median Ki67-LI value(20%) of patients in this study, the 939 women were classified into three groups by cut-off points 10 and 30% [9]. Patients whose Ki67-LI was 30% or above were considered clearly high (Ki67High); those whose Ki67-LI was 10% or less were clearly low (Ki67Low), and those whose Ki67-LI was 10–30% were considered intermediate (Ki67Medium).
Statistical analysis
Statistical comparisons of clinicopathological characteristics within the three groups were calculated by chi-square and rank-sum tests. Kaplan-Meier survival curves were calculated; log-rank test was adopted to compare DFS within groups. Multivariate DFS analysis used the Cox proportional hazards model. SPSS 19.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analyses. All P values were two-tailed; P ≤ 0.05 was considered significant.
Results
For the 939 patients who were eligible for inclusion in this study, the median follow-up period was 64.7 months (range:4.3–120.7 months), and the mean follow-up period was 67.2 months (SD:23.4 months). The Ki67-LIs were intensively clustered at values ending with 5 or 0 (829, 88.3%). The median value of Ki67-LI was 20% for these 939 cases. Among the 939 patients, 342 cases had Ki67-LI ≤ 10%, 281 had Ki67-LI between 10 and 30%, and 316 had Ki67-LI ≥ 30%. If all 2063 cases with Ki67-LI data were included for analysis regardless of subtype, median value increased to 25%. Ki67-LIs of this cohort also intensively clustered at values ending with 5 or 0 (1886, 91.4%).
Table 1 shows the clinicopathological characteristics in each group and indicates significant differences in age distribution, menopausal status, histology, tumor size, node involvement, grade, and PgR status. No significant differences within groups were seen for median age or lymphovascular invasion (LVI) status. The proportion of early-onset BC (age <35 years) or PgR-negative/low was higher in the Ki67High group than other groups. Tumors <2 cm or without lymph node involvement were more commonly observed in the Ki67Low group. Almost all tumors in the Ki67Low group were grade I/II, whereas more than half the cases in the Ki67High group were grade III. Nevertheless, the clinical features of the Ki67Medium group were not remarkable, and for many cases, it was hard to differentiate them from the Ki67Low or Ki67High groups. The largest proportion of patients who received chemotherapy were from the Ki67High group, and the largest proportion of patients who received radiotherapy were from the Ki67Low group. The groups did not significantly differ by surgery type.
A total of 79 (8.4%) patients had BC-associated disease (Ki67Low: 10; Ki67Medium: 20; Ki67High: 49), including 12 local recurrences, seven regional nodal recurrences, three contralateral new BCs, 54 distant metastases, and three deaths from BC. The estimated 5-year DFS rate for the Ki67Medium group (93%) was significantly less than for the Ki67Low group (97%; log-rank P = 0.003), but significantly better than for the Ki67High group (85%, log-rank P = 0.014; Fig. 1).
Our univariate analysis associated age younger than 35 years, tumor >2 cm, lymph node involvement, positive LVI status, grade III disease, higher Ki67-LI group, and surgical approach (mastectomy vs. BCS) with worse prognoses (Table 2). In multivariate analysis, the Ki67-LI groups were a significant independent predictor for DFS after adjusting for clinicopathological parameters and treatments (Table 2).
To refine prognostication for the Ki67Medium group, we investigated the value of conventional clinical parameters. Interestingly, these factors, including tumor size >2 cm, lymph node status (N1 vs. N0), and grade III disease, had significant prognostic value in univariate and multivariate analyses of DFS for the Ki67Medium group (Table 3), but not the Ki67Lowor Ki67High groups (Additional file 1: Table S1 and Additional file 2: Table S2).
Discussion
In this study, we investigated clinicopathologic characteristics and prognoses of the three risk levels among patients with ER+/HER2− BCs, according to the most recent consensus from the 2015 St Gallen Conference, which focuses on local laboratory Ki67-LIs. To our knowledge, such an evaluation based on the 2015 St Gallen Breast Cancer Conference consensus has never been published before.
Ki67 is a nuclear protein expressed in all active phases of the cell cycle except resting phase G0 [11]. Therefore, it can be used as an alternative marker of proliferation by IHC assessment in many malignancies, including BC. Ki67 has been extensively evaluated in both research and clinical settings [12], and clearly offers robust prognostic and predictive information [13, 14]. The St Gallen International BC Conferences have recommended using Ki67-LI to tailor treatment in ER+/HER2− BCs from 2009 to 2015 [7–9, 15]. However, our understanding of Ki67 has expanded, including its clinical value and optimal cut-off point. The 2009 St Gallen consensus suggested Ki67-LI as a proliferation marker in choosing appropriate systemic treatment, categorizing it by three levels: low (≤15%), intermediate (16–30%), or high (>30%) [15]. After comparing Ki67-LI with PAM50 intrinsic subtyping, Cheang et al. suggested a cut-off point of 14% to distinguish luminal B from luminal A subtype in ER+/HER2− BCs [16]. The 2011 St Gallen Conference then endorsed a 14% cut-off point between luminal A and B tumors [7]. Subtyping can be used to shape decisions about the use of cytotoxic chemotherapy for luminal BCs [7, 8]. Luminal A disease generally requires only endocrine therapy, whereas chemotherapy is usually indicated for luminal B diseases. In 2013, the cut-off point was increased to 20% by the St Gallen panel [8]. Meta-analysis also showed diverse cut-off points of Ki67-LI ranging from 3.5 to 34% with various definitions [13]. A recent meta-analysis of more than 60,000 patients confirmed the prognostic value of Ki67-LI, and found 25% to be an optimal cut-off point [17].
At the most recent St Gallen Conference, in 2015, the panel recognized the controversy of using Ki67-LI by IHC assessment for clinical decisions [9]. The previous efforts of finding a universal optimal cut-off point of Ki67 might have been in vain, as Ki67 presents as a continuum. Clinicians should therefore be cautious when using a single cut-off point to dichotomize Ki67 scores [6, 18]. Particularly, reproducibility across laboratories has proven to be unacceptably poor, which is the major obstacle for its clinical use [19]. Because of this, the American Society of Clinical Oncology (ASCO) and NCCN do not currently recommend Ki67 as a routine required marker in clinical practice ([20], https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf). With the goal of harmonizing methodology, the International Ki67 in Breast Cancer Working Group proposed guidelines for Ki67 assessment [12]. However, the effect on actual practice is less than optimal [21, 22]. Studies [21] and new image analyses [23] that aim to increase concordance in Ki67 scoring are still under development.
The 2015 St Gallen consensus panel recommended interpreting Ki67-LI with local laboratory values [9], which was also suggested by the European Society for Medical Oncology (ESMO) clinical practice guidelines [24]. In our study, median Ki67-LI was 25% for all tumors and 20% for the ER+/HER2− subset. The two values reported by Cserni study were 17 and 14%, respectively [22]. Although the numerical gaps between these two centers were only 8 and 6% for two different patient sets, the numerous patients within this range should not be ignored. Of the 939 ER+/HER2− cases in our study, 214 (22.8%) had Ki67-LIs between 14 and 20%. Our Ki67-LI values also intensively clustered at those ending with 5 or 0 (91.4% for all tumors and 88.3% for ER+/HER2− subset), as did those in the Cserni study [22]. This finding reflects a common practice of pathologists. In our 939 cases of ER+/HER2− tumors, none of the Ki67-LIs were valued at 14%, and only four cases at 12% or 13%, but 103 patients—11%—had Ki67-LIs of 15%. In light of this pattern, the widely used 14% cut-off point recommended by the 2011 St Gallen Conference seems unreasonable.
When ER+/HER2− cases were stratified into three levels by median Ki67-LI value 20%, the Ki67High group (≥30%) had less-favorable clinicopathologic characteristics, including the highest percentages of patients younger than 35 years, large tumors, high histology grades, and low or negative PgR. The Ki67Low group (≤10%) had some favorable features, and features of the Ki67Medium group (10–30%) were between the other two groups. These findings confirmed the validity of these Ki67 groupings from a clinicopathologic perspective. The three groups differed significantly in DFS (P < 0.001), with the Ki67Low group having the longest DFS and the Ki67High group the shortest.
To refine prognostication within the Ki67Medium group, we examined other conventional diagnostic factors. We found that tumors larger than 2 cm, lymph node involvement, and grade III disease were all associated with poorer prognoses in the Ki67Medium group after adjusting for treatments; however, these parameters had no prognostic significance in Ki67High and Ki67Low groups. It is difficult to standardize scores for patients with Ki67Medium LIs, as they suffer from the highest variability [25, 26]. Accordingly, when making clinical decisions about the inclusion of cytotoxic chemotherapy for ER+/HER2− BCs with intermediate Ki67-LI, other conventional parameters may provide more valuable information rather than Ki67-LI, or, alternatively, multigene assays [26]. However, multigene assays are not readily available worldwide owing to high cost and technical requirements. The prognosis significance of chemotherapy was not shown in analyses for the Ki67Medium group. This result may be due to the low percentage of patients without chemotherapy. Further researches are needed to study the prognosis significance of treatments for the Ki67Medium group.
PgR status was adopted to define luminal B breast tumors by the 2013 St Gallen consensus, using the cut-off point of 20% proposed by Prat et al. [27]. However, this cut-off for PgR had no prognostic significance for DFS in our ER+/HER2− cohort. Maisonneuve et al. [28]. also found that PgR < 20% was not associated with poorer outcomes for all tumors, but only for patients with Ki67-LI of 14–20% in their study cohort. ESMO emphasized the importance of laboratory quality assurance in recommending the cut-off of 20%. The cut-off point of PgR still needs more study, just as Ki67-LI does.
This study has some limitations. First, this was a single-institution retrospective study, and its results might not be directly applicable to other institutions. Before putting these results into clinical practice, other institution should verify them with their own laboratory data. In addition, the follow-up period of this cohort was relatively short considering that in the ER+/HER2− subset, most cases of recurrence—let alone death—occur at least 5 years later [29, 30]. For this reason, we did not analyze overall survival in this study.
Conclusions
We have evaluated distribution of Ki67-LI at an Asian institution and confirmed its validity as a risk factor among ER+/HER2− BCs according to the 2015 St Gallen consensus. For patients with clearly very low or very high Ki67-LIs in early-stage BCs, the importance of Ki67 in clinical decisions is rather straightforward. However, for patients whose Ki67-LIs are in a medium range, diagnostic parameters including tumor size, lymph node involvement, and grade might provide significant clues.
Abbreviations
- ALND:
-
Axillary lymph node dissection
- ASCO:
-
American Society of Clinical Oncology
- BC:
-
Breast cancer
- BCS:
-
Breast-conserving surgery
- DFS:
-
Disease-free survival
- ER+/HER2−:
-
Estrogen receptor-positive and human epidermal growth factor receptor 2-negative
- ESMO:
-
European Society for Medical Oncology
- FISH:
-
Fluorescence in situ hybridization
- IDC:
-
Invasive ductal carcinoma
- IHC:
-
Immunohistochemical
- ILC:
-
Invasive lobular carcinoma
- IQT:
-
Interquartile range
- LI:
-
Labeling index
- LVI:
-
Lymphovascular invasion
- NCCN:
-
National Comprehensive Cancer Network
- PgR:
-
Progesterone receptor
- SLNB:
-
Sentinel lymph node biopsy
References
Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, et al. Molecular portraits of human breast tumours. Nature. 2000;406(6797):747–52.
Sotiriou C, Neo SY, McShane LM, Korn EL, Long PM, Jazaeri A, Martiat P, Fox SB, Harris AL, Liu ET. Breast cancer classification and prognosis based on gene expression profiles from a population-based study. Proc Natl Acad Sci U S A. 2003;100(18):10393–8.
Sorlie T, Tibshirani R, Parker J, Hastie T, Marron JS, Nobel A, Deng S, Johnsen H, Pesich R, Geisler S, et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci. 2003;100(14):8418–23.
Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey SS, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98(19):10869–74.
Cancer Genome Atlas N. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490(7418):61–70.
Ades F, Zardavas D, Bozovic-Spasojevic I, Pugliano L, Fumagalli D, de Azambuja E, Viale G, Sotiriou C, Piccart M. Luminal B breast cancer: molecular characterization, clinical management, and future perspectives. J Clin Oncol. 2014;32(25):2794–803.
Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thurlimann B, Senn HJ, Panel members. Strategies for subtypes—dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer. Ann Oncol. 2011;22(8):1736–47.
Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart-Gebhart M, Thurlimann B, Senn HJ, Panel members. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol. 2013;24(9):2206–23.
Coates A, Winer E, Goldhirsch A, Gelber R, Gnant M, Piccart-Gebhart M, Thürlimann B, Senn H-J, André F, Baselga J. Tailoring therapies-improving the management of early breast cancer: St GallenInternational Expert Consensus on the Primary Therapy of Early Breast Cancer 2015. Ann Oncol. 2015:26(8):1533–46. doi:10.1093/annonc/mdv221.
Su Y, Zheng Y, Zheng W, Gu K, Chen Z, Li G, Cai Q, Lu W, Shu XO. Distinct distribution and prognostic significance of molecular subtypes of breast cancer in Chinese women: a population-based cohort study. BMC Cancer. 2011;11:292.
Gerdes J, Lemke H, Baisch H, Wacker HH, Schwab U, Stein H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol. 1984;133(4):1710–5.
Dowsett M, Nielsen TO, A’Hern R, Bartlett J, Coombes RC, Cuzick J, Ellis M, Henry NL, Hugh JC, Lively T, et al. Assessment of Ki67 in breast cancer: recommendations from the International Ki67 in Breast Cancer working group. J Natl Cancer Inst. 2011;103(22):1656–64.
de Azambuja E, Cardoso F, de Castro Jr G, Colozza M, Mano MS, Durbecq V, Sotiriou C, Larsimont D, Piccart-Gebhart MJ, Paesmans M. Ki-67 as prognostic marker in early breast cancer: a meta-analysis of published studies involving 12,155 patients. Br J Cancer. 2007;96(10):1504–13.
Yerushalmi R, Woods R, Ravdin PM, Hayes MM, Gelmon KA. Ki67 in breast cancer: prognostic and predictive potential. Lancet Oncol. 2010;11(2):174–83.
Goldhirsch A, Ingle JN, Gelber RD, Coates AS, Thurlimann B, Senn HJ, Panel members. Thresholds for therapies: highlights of the St Gallen International Expert Consensus on the primary therapy of early breast cancer 2009. Ann Oncol. 2009;20(8):1319–29.
Cheang MC, Chia SK, Voduc D, Gao D, Leung S, Snider J, Watson M, Davies S, Bernard PS, Parker JS, et al. Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst. 2009;101(10):736–50.
Petrelli F, Viale G, Cabiddu M, Barni S. Prognostic value of different cut-off levels of Ki-67 in breast cancer: a systematic review and meta-analysis of 64,196 patients. Breast Cancer Res Treat. 2015;153(3):477–91.
Denkert C, von Minckwitz G. Reply to Ki67 in breast cancer: a useful prognostic marker! Ann Oncol. 2014;25(2):542–3.
Polley MY, Leung SC, McShane LM, Gao D, Hugh JC, Mastropasqua MG, Viale G, Zabaglo LA, Penault-Llorca F, Bartlett JM, et al. An international Ki67 reproducibility study. J Natl Cancer Inst. 2013;105(24):1897–906.
Harris L, Fritsche H, Mennel R, Norton L, Ravdin P, Taube S, Somerfield MR, Hayes DF, Bast Jr RC, American Society of Clinical O. American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol. 2007;25(33):5287–312.
Polley MY, Leung SC, Gao D, Mastropasqua MG, Zabaglo LA, Bartlett JM, McShane LM, Enos RA, Badve SS, Bane AL, et al. An international study to increase concordance in Ki67 scoring. Mod Pathol. 2015;28(6):778–86.
Cserni G, Voros A, Liepniece-Karele I, Bianchi S, Vezzosi V, Grabau D, Sapino A, Castellano I, Regitnig P, Foschini MP, et al. Distribution pattern of the Ki67 labelling index in breast cancer and its implications for choosing cut-off values. Breast. 2014;23(3):259–63.
Klauschen F, Wienert S, Schmitt W, Loibl S, Gerber B, Blohmer J-U, Huober J, Ruediger T, Erbstoesser E, Mehta K. Standardized Ki67 diagnostics using automated scoring-clinical validation in the GeparTrio breast cancer study. Clin Cancer Res. 2015;21(16):3651–7. doi: 10.1158/1078-0432.CCR-14-1283.
Senkus E, Kyriakides S, Ohno S, Penault-Llorca F, Poortmans P, Rutgers E, Zackrisson S, Cardoso F, Committee EG. Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26 Suppl 5:v8–v30.
Varga Z, Diebold J, Dommann-Scherrer C, Frick H, Kaup D, Noske A, Obermann E, Ohlschlegel C, Padberg B, Rakozy C, et al. How reliable is Ki-67 immunohistochemistry in grade 2 breast carcinomas? A QA study of the Swiss Working Group of Breast- and Gynecopathologists. PLoS One. 2012;7(5):e37379.
Denkert C, Budczies J, von Minckwitz G, Wienert S, Loibl S, Klauschen F. Strategies for developing Ki67 as a useful biomarker in breast cancer. Breast. 2015;24 Suppl 2:s67–72. doi:10.1016/j.breast.2015.07.017.
Prat A, Cheang MC, Martin M, Parker JS, Carrasco E, Caballero R, Tyldesley S, Gelmon K, Bernard PS, Nielsen TO, et al. Prognostic significance of progesterone receptor-positive tumor cells within immunohistochemically defined luminal A breast cancer. J Clin Oncol. 2013;31(2):203–9.
Maisonneuve P, Disalvatore D, Rotmensz N, Curigliano G, Colleoni M, Dellapasqua S, Pruneri G, Mastropasqua MG, Luini A, Bassi F, et al. Proposed new clinicopathological surrogate definitions of luminal A and luminal B (HER2-negative) intrinsic breast cancer subtypes. Breast Cancer Res. 2014;16(3):R65.
Pagani O, Price KN, Gelber RD, Castiglione-Gertsch M, Holmberg SB, Lindtner J, Thurlimann B, Collins J, Fey MF, Coates AS, et al. Patterns of recurrence of early breast cancer according to estrogen receptor status: a therapeutic target for a quarter of a century. Breast Cancer Res Treat. 2009;117(2):319–24.
Lee Y, Kang E, Lee AS, Baek H, Kim EK, Park SY, Kim JH, Kim YJ, Kim SH, Kim IA, et al. Outcomes and recurrence patterns according to breast cancer subtypes in Korean women. Breast Cancer Res Treat. 2015;151(1):183–90.
Acknowledgement
The authors thank the patients for their willingness to cooperate with our study.
This study was supported by the Grant [2013] 163 from the Key Laboratory of Malignant Tumor Molecular Mechanism and Translational Medicine of Guangzhou Bureau of Science and Information Technology.
Funding
This research was supported by the National Natural Science Foundation of China under grant No. 81372817.
Availability of data and materials
Data will not be shared for privacy reason.
Authors’ contributions
YH, RG and JHZ participated in the data collection, carried out the statistical analysis and drafted the manuscript. QL, KC, FXS and WJJ conceived of the study, and participated in its design and coordination and helped to draft the manuscript. YPY, FTL, HXJ, HLW and LJ participated in the data proofreading and manuscript revision. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
This study was approved by the Ethics Committee of Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University and no inform consent was needed for this retrospective Study.
Author information
Authors and Affiliations
Corresponding authors
Additional files
Additional file 1: Table S1.
Results of disease-free survival analysis by Cox proportional hazards model for the the Ki67Low group. (DOC 75 kb)
Additional file 2: Table S2.
Results of disease-free survival analysis by Cox proportional hazards model for the the Ki67High group. (DOC 76 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Hu, Y., Gu, R., Zhao, J. et al. Prognostic significance of Ki67 in Chinese women diagnosed with ER+/HER2− breast cancers by the 2015 St. Gallen consensus classification. BMC Cancer 17, 28 (2017). https://doi.org/10.1186/s12885-016-3021-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-016-3021-7