Abstract
Background
Sequential biopsy of breast cancer is used to assess biomarker effects and drug efficacy. The preoperative “window of opportunity” setting is advantageous to test biomarker changes in response to therapeutic agents in previously untreated primary cancers. This study tested the consistency over time of paired, sequential biomarker measurements on primary, operable breast cancer in the absence of drug therapy.
Methods
Immunohistochemistry was performed for ER, PR and Ki67 on paired preoperative/operative tumor samples taken from untreated patients within 2 weeks of each other. Microarray analysis on mRNA extracted from formalin fixed paraffin embedded cores was performed using Affymetrix based arrays on paired core biopsies analysed using Ingenuity Pathway Analysis (IPA) and Gene Set Analysis (GSA).
Results
In 41 core/resection pairs, the recognised trend to lower ER, PR and Ki67 score on resected material was confirmed. Concordance for ER, PR and Ki67 without changing biomarker status (e.g. ER+ to ER-) was 90, 74 and 80 % respectively. However, in 23 paired core samples (diagnostic core v on table core), Ki67 using a cut off of 13.25 % was concordant in 22/23 (96 %) and differences in ER and PR immunohistochemistry by Allred or Quickscore between the pairs did not impact hormone receptor status. IPA and GSA demonstrated substantial gene expression changes between paired cores at the mRNA level, including reduced expression of ER pathway analysis on the second core, despite the absence of drug intervention.
Conclusions
Sequential core biopsies of primary breast cancer (but not core versus resection) was consistent and is appropriate to assess the effects of drug therapy in vivo on ER, PR and Ki67 using immunohistochemistry. Conversely, studies utilising mRNA expression may require non-treatment controls to distinguish therapeutic from biopsy differences.
Similar content being viewed by others
Background
Biomarker studies based on the use of core biopsy and/or resection specimens for translational research in breast cancer are useful to evaluate effects of therapeutic intervention in neoadjuvant, pre-surgical and metastatic studies. Previous studies have sought differences in ER, PR and HER2 between core biopsies and resected surgical specimens in primary breast cancer and noted discordance (usually a reduction in expression) ranging from 1.2 to 35 % [1–4]. Concerns remain that core biopsy and surgical specimens may be a source of bias in clinical trials [5]. The reporting of diagnostic specimens [6] and recommendations for tumor marker prognostic studies [7] are well established with recommendations in breast cancer as to the appropriate use of tumor markers [8]. Recently, Ki67 has come to prominence as a biomarker in breast cancer of prognostic and predictive potential [9, 10].
In the clinical setting, sequential tumor core biopsy has become accepted in neoadjuvant and window of opportunity studies to seek early evidence of therapeutic efficacy [11–13]. This has included neoadjuvant endocrine trials [14, 15] and novel agents [13] or repurposing drugs [12, 16] in window of opportunity studies. The relative simplicity, accessibility and specificity of immunohistochemistry on formalin fixed, paraffin embedded (FFPE) remains attractive. Trials have identified Ki67 at 2 weeks as a predictor of relapse free survival [14] or efficacy respectively [17] and as a prognostic marker for adjuvant chemotherapy [18, 19]. Other studies have demonstrated changes in gene expression associated with response to neoadjuvant therapy [20] although signatures of response to chemotherapy have to date been rare [21].
Based on the suggestion that Ki67 may have prognostic and predictive value, the neoadjuvant Alliance ALTERNATE trial (NCT01953588) utilises changes in Ki67 after 1 month of endocrine therapy as a decision tool for subsequent continuation of endocrine therapy or switch to chemotherapy in postmenopausal women with ER positive primary breast cancer. The POETIC (Peri-operative Endocrine Treatment for Individualising Care) Trial (CR-UK/07/015) will evaluate the importance of Ki67 (and other biomarkers) after 2 weeks of treatment with a non-steroidal aromatase inhibitor in predicting long-term outcome. These, and other, clinical trials are predicated on breast cancer biopsy material reflecting therapeutic effect. However, the consistency of markers examined by immunohistochemistry [22] and (for premenopausal women) the effect of differences in the endocrine environment [23] could modify immunohistochemical and gene expression data (in the absence of therapeutic intervention) and hence may influence interpretation of drug efficacy in such settings.
Core biopsy is now considered the tumor sample of choice for ER, PR and HER2 assessment, given the excellent fixation possible [24]. The effects of tissue handling on RNA yield and integrity [25] or comparison between proteins expressed at the centre or periphery of breast cancer [26] are established. However, comparative studies for ER, PR, Ki67 or mRNA expression on paired core biopsies in the absence of therapeutic intervention are needed to test for the consistency between sequential core biopsies and to consider the potential for a wounding effect which might interfere with therapeutic assessment. This study examined paired primary breast cancer biopsies with a 2 week interval between sampling, using immunohistochemistry for ER, PR and Ki67 and mRNA gene expression.
Methods
Immunohistochemistry comparison between core biopsy and resection specimens
To re-evaluate the consistency of staining between core biopsy and breast cancer resection specimens, 41 Caucasian women with histologically proven stage I or II primary breast cancer gave written, informed consent to participation under the auspices of the Tayside Local Research Ethics Committee (Fig. 1). Patients taking hormone replacement therapy (HRT) or oral contraception were excluded; 26 women were postmenopausal and 15 women premenopausal. FFPE paired biopsies at the time of diagnosis (core biopsy) and 2 weeks later at resection (from the surgical resected specimen taken at pathology cut up) were examined. The resected tumor was delivered fresh to the pathology laboratory (in under 30 min), the margins inked, the specimen sliced at 5–10 mm intervals and fixed overnight in neutral buffered formalin prior to final dissection and block selection. Core biopsies taken at the time of diagnosis were compared with tissue microarrays (TMA) made from the resected specimen. For the TMA, 6 × 0.6 mm cores of invasive disease were selected to avoid prior biopsy sites by a specialist breast pathologist. No therapeutic intervention occurred between the two sampling time points.
Immunohistochemistry was performed on 4 μm sections of FFPE tissues using standard methodologies [27] using primary antibodies for estrogen receptor alpha (ER) antibody 6 F11 (1:200; Novocastra Laboratories Ltd), progesterone receptor (PR) antibody clone 16 (1:800; Novocastra Laboratories Ltd) and NCL-L-Ki67-MM1 (Anti-Ki67, monoclonal antibody, Leica Microsystems). Negative controls (lacking primary antibody) were performed for all staining runs.
Samples were scored independently to agreement by two authors (PGR and LBJ) for an average of the cores scored- usually all six on the TMA- using the Quickscore method assessing intensity and proportion (hence for example 6 × 2 reflects % cells staining x intensity) for ER, PR [28] and using a cut off of 20 % for Ki67 [9].
Immunohistochemistry comparison between paired core biopsies
To eliminate potential tissue handling, fixation and processing differences, core biopsies were taken 2 weeks apart (n = 24) from consenting patients under a separate Tayside Local Research Ethics Committee permission as control tissues from a pre-surgical metformin trial [12]. All tissues were placed immediately in neutral buffered formalin and following overnight fixation processed to paraffin blocks at a single laboratory.
For the paired cores, immunohistochemistry for ER and PR was performed as described above and scored using the Quickscore method [28] and independently by the Allred method [29]. Immunohistochemistry was conducted blinded to the clinical data and scored by a single specialist breast pathologist (LBJ). Following light microscopy review, slides were scanned into a virtual microscopy format using an Aperio ScanScope XT TM (Aperio Technologies, Vista, Ca., USA) at the x40 objective utilizing standard compression methodology.
The Ki67 index (percentage of nuclear positive cells) per invasive tumor was calculated using manual annotation of the virtual microscopy slide by means of a Wacom Bamboo Pen & Touch tablet device (Wacom Corporation, Saitama Japan) within the WebScope environment (version 10.2.0.2319) of the Aperio Spectrum Plus system version 10.2.2.2317. The annotations were assessed by the Aperio IHC nuclear Algorithm version 10. Only invasive tumor cells were assessed; great care was taken to exclude normal epithelial, in situ epithelial, stromal and inflammatory elements. A mean 5600 nuclei (range 601–39,788) per invasive tumor was assessed to obtain the Ki67 index. A minimum of 1000 invasive tumor cells was examined except for one pre-treatment and one post-treatment core (601 and 825 cells respectively).
RNA Microarray
For RNA microarray analysis, FFPE core biopsy samples from 12 otherwise unselected patients from the control arm of a preoperative clinical trial [12] were examined. These represent 12 pairs of the 24 paired samples from the immunohistochemistry comparison between paired core biopsies where there was sufficient tumour material in the core for RNA extraction and analysisconfirmed on a Haematoxylin and Eosin slide was confirmed by a specialist breast pathologist (LBJ). RNA extraction and Breast Cancer Disease-Specific Array (DSA) gene expression profiling was performed as previously described [12].
Data were corrected for background noise, summarized and normalized using RMA in Partek® Genomics Suite™ software, 6.5 beta © 2009 (Partek Inc., St. Louis, MO, USA). Principle component analysis (PCA) revealed that the main variance associated with the first principle component was array quality. An additional transformation based in singular value decomposition was performed to remove this technical variation. The data was subsequently log2 transformed.
Differential gene selection
Reliably detected genes were selected by removing the probe sets with a variance below the mean global variance. The genes were then filtered based on fold change (>1.3 for less stringent and 1.5 for stringent selection) to select the differentially expressed probe sets between the second biopsy and the baseline biopsy. A student’s t-test without multiple testing corrections was performed and significant genes (p-value < 0.05 for less stringent and p-value < 0.005 for stringent selection) selected for further analysis.
Ingenuity Pathway Analysis (IPA)
Ingenuity Pathway Analysis (IPA) analysis mapped genes differentially expressed between baseline and follow-up biopsies to biological pathways using the standard commercial software (IPA, http://www.ingenuity.com)
Gene Set Analysis (GSA)
Gene Set Analysis (GSA) examined whether members of a particular biological pathway occur toward the top or the bottom of a rank-ordered gene list including all gene expression measurements ranked by differential expression between baseline and second core biopsy. This analysis takes into account information from members of a pathway that would not make it to the top most differentially expressed gene list (used for the IPA analysis above). GSA was performed using the BRB Array Tools software package (http://linus.nci.nih.gov/BRB-ArrayTools.html, US NCI Biometrics Branch) for 2987 gene sets collectively representing most known biological and metabolic pathways in Gene Ontology (GO, http://www.geneontology.org). To be included, a GO gene set required a minimum of 10 and a maximum of 200 genes. Significance was estimated with a permutation test (n = 1000). The null hypothesis was that the average degree of differential expression of members of a given gene set between the baseline and second biopsy was the same as expected from a random permutation of biopsy labels. IPA software was used to generate pathway figures for the significant gene sets.
Results
Comparison between core biopsy and resection specimens
In tumor samples from 41 women (Table 1) there was a clinically significant change (loss) of ER between the diagnostic core and the resection specimen in cancers from 4/41 (10 %) women across the threshold for adjuvant endocrine therapy of a Quickscore of 4/18, although the ER score changed in a further 18 women, but would not change the clinical impact (Fig. 2 and Table 2). Loss of ER was identified in 3/15 (20 %) premenopausal women and PR changes occurred in both premenopausal and postmenopausal women. For Ki67 (Fig. 3), there was also a loss of staining in assessable samples to below 20 % in 1/15 (7 %) premenopausal and 4/25 (16 %) postmenopausal women and a rise above 20 % in 2/15 (14 %) premenopausal and 1/25 (4 %) postmenopausal women; Ki67 was not assessable on one core.
Immunohistochemistry comparison between paired core biopsies
In paired core biopsies from 17 women, using the Quickscore method, in 2/17 (12 %) there was reduced expression of ER in the second core biopsy and in 3/17 (18 %) increased expression of ER in the second core (Fig. 2). In none of these five patients would the change in ER have led to a therapeutically important switch whether the Quickscore or Allred score was applied.
For PR in 6/17 (35 %) women there was reduced expression of PR in the second core biopsy and in 3/17 (18 %) increased expression of PR in the second core. In none of these nine patients would the change in PR have led to a therapeutically important switch whether the Quickscore or Allred score was used.
Ki67 was available on 23 paired core biopsies (including the 17 for ER and PR pairs). Using 20 % as a cut off [9], 5/23 (22 %) tumor samples would have crossed the 20 % threshold between the paired samples: 2/23 (9 %) patients would have crossed from above to below 20 % and tumor samples from a further 3/23 (13 %) patients from below to above 20 %. However, using 13.25 % as the cut off [10], only 1/23 (4 %) tumors would have crossed the 13.25 % boundary comparing the two cores (Fig. 3).
RNA microarray
Microarray analysis was successfully completed on all 12 paired samples. By paired t-test differences in gene expression profile were identified between the diagnostic and surgical core biopsy.
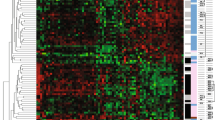
By GSA (Fig. 4), the differences between the two biopsies suggested changes in pathways involving myc, apoptosis and p53 amongst others in the second biopsy compared with the first. Several elements of cellular metabolism and immunological pathways were identified as overexpressed (Fig. 5a) in the second biopsy as compared with the first whereas, the Rho, integrin and potentially significantly the ER pathways were relatively underexpressed (Fig. 5b) in the second core biopsy.
IPA set in context a number of gene expression changes among which pathways involving PI3K, MEKK and IGF-1 may be of particular relevance in the setting of breast cancer.
Discussion
Minimising bias in clinical molecular marker studies in preoperative trials using paired samples is critical to assess the efficacy and target effects of endocrine agents (for example the ALTERNATE and POETIC trials), novel therapy [13] or new indications for established drugs [12] and to change clinical management, at least in the trial setting (ALTERNATE).
Immunohistochemistry comparison between core biopsy and resection specimens
To date there have been multiple comparisons of core biopsies and surgical resections for ER, PR, Ki67 for tumor grade and HER2 (Table 3) demonstrating a mean concordance of 92.4 % for ER (Fig. 6a), 84 % for PR (Fig. 6b) and 67.4 % for Ki67 (Fig. 6c), comparable to the data presented here. Reporting comparisons between ER, Ki67 and other biomarkers in this setting may be potentially misleading for well-rehearsed reasons [1, 5, 30] minimised by the use of (paired) core biopsies and consistent tissue handling. We revisited whether the changes in ER might be secondary to changes in circulating estradiol, confirming plausible evidence for premenopausal women [23], but likely due to tissue handling and processing at least in postmenopausal women [1, 5, 25].
a Funnel plot for 24 studies on ER concordance between diagnostic cores and surgical specimen. Mean concordance is 92.38 %. Excluding the seven studies that fall outside the 99 % Confidence Interval, changed the mean to 95.63 %. b Funnel plot for 19 studies on PgR concordance between diagnostic cores and surgical specimen. Mean concordance is 84 %. Excluding the two studies that fall outside the 99 % Confidence Interval has not changed the mean. c Funnel plot for five studies on Ki67 concordance between diagnostic cores and surgical specimen. Mean concordance is 67.4 %. Excluding the study that fall outside the 99 % Confidence Interval, changed the mean to 69.75 %
Immunohistochemistry comparison between paired core biopsies
Paired core biopsies of primary breast cancer before/after drug therapy has become popular [12, 13, 16], although quality standards for Ki67 have been of concern [9, 10]. In a trial setting [12], variations in specimen processing, specimen handling, laboratory processing and immunohistochemical staining and scoring were minimised, although patient selection (ER positive T1c and T2 cancers) occurred.
Slight variation of immunohistochemical scoring of ER and PR between paired cores, potentially attributable to geographic targeting differences over time, rarely crossed the boundary for clinical decision making. For Ki67, the cut point was key: at 20 % [9], 5/23 (22 %) paired tumor samples would have crossed the threshold, compared with only 1/23 (4 %) tumors using 13.25 %, in concordance with expert opinion [10] confirming a Ki67 boundary of 13.25 % is appropriate when seeking evidence of a drug effect.
While intra-tumoral heterogeneity has been considered elsewhere [26], the single cores at each time point may reflect clinical reality in small cancers for window of opportunity, pre-operative or neoadjuvant trials. Given the consensus, for a number of tumor types, that needle biopsy specimens result in reliable immunohistochemistry [1, 31], this study provides reassurance that immunohistochemical measurement of ER, PR and Ki67 from core biopsy pairs is consistent over 2 weeks.
RNA microarray
By GSA, the changes expression of genes integral to cell cycle and apoptosis (Fig. 4), overexpression of cellular metabolism and immunological pathways (Fig. 5a) and underexpression of cell motility and cell adhesion (Fig. 5b) suggest that in the time frames of the biopsy, perturbation of such pathways remains several days after the initial wounding effect of the first core biopsy. The reduction in mRNA expression of the ER pathway (Fig. 5b) following the first biopsy holds potential concern and is in contrast to the only other published study of eight patients where no change was noted [32]. However, mRNA changes do not exactly reflect semiquantiative immunohistochemistry and ER mRNA imperfectly correlates with the level of ER protein expression [33]. The immunohistochemical studies on the same series of samples reported here provide comfort that for the technology most widely used in clinical practice (immunohistochemistry), ER on a second core biopsy may not be compromised.
IPA set in context a number of gene expression changes among which pathways involving PI3K, MEKK and IGF-1 [34, 35] may be of particular relevance in the setting of breast cancer.
These microarray data, within the limits of the experimental design, sample numbers and analytical techniques employed, suggest that core biopsy of primary breast cancer may generate a “wounding” effect evident on subsequent mRNA analysis. The time course, duration and variations in gene expression as a consequence of tumor and patient variability were not assessed within this study and are clinically challenging to obtain [25]. However, core biopsy may influence the mRNA expression profile of sequential clinical samples used in clinical trials and requires careful evaluation.
Conclusions
This study provides reassurance that sequential core biopsy (but not core versus resection) should be an appropriate way to assess the effects of drugs on primary tumor ER, PR and Ki67 (with a cut off of 13.25 %) within the context of window of opportunity and neoadjuvant trials. By contrast, mRNA analyses may demonstrate multiple changes between paired samples reflecting the wounding effect of core biopsy, which for ER at least is not reflected at the level of immunohistochemistry. Sequential core biopsy may be used with confidence when seeking evidence of ER, PR and Ki67 changes in the preoperative setting for primary breast cancer.
Abbreviations
- DSA:
-
Disease Specific Array
- ECLIA:
-
Electrochemoluminescence immunoassay
- ER:
-
Estrogen receptor
- FFPE:
-
Formalin fixed paraffin embedded tissue
- GSA:
-
Gene Set Analysis
- HER2:
-
Human epidermal growth factor type 2
- HRt:
-
Hormone replacement therapy
- IGF-1:
-
Insulin like growth factor-1
- IPA:
-
Ingenuity Pathway Analysis
- MEKK:
-
Mitogen activated protein kinase kinase
- mRNA:
-
Messenger RNA
- PCA:
-
Principle component analysis
- PI3K:
-
Phosphoinositol-3-kinase
- PR:
-
Progesterone receptor
- TMA:
-
Tissue MicroArray
References
Mann GB, Fahey VD, Feleppa F, Buchanan MR. Reliance on hormone receptor assays of surgical specimens may compromise outcome in patients with breast cancer. J Clin Oncol. 2005;23(22):5148–54.
Douglas-Jones AG, Collett N, Morgan JM, Jasani B. Comparison of core oestrogen receptor (ER) assay with excised tumour: intratumoral distribution of ER in breast carcinoma. J Clin Pathol. 2001;54(12):951–5.
Arnedos M, Nerurkar A, Osin P, A'Hern R, Smith IE, Dowsett M. Discordance between core needle biopsy (CNB) and excisional biopsy (EB) for estrogen receptor (ER), progesterone receptor (PgR) and HER2 status in early breast cancer (EBC). Ann Oncol. 2009;20(12):1948–52.
Li S, Yang X, Zhang Y, Fan L, Zhang F, Chen L, Zhou Y, Chen X, Jiang J. Assessment accuracy of core needle biopsy for hormone receptors in breast cancer: a meta-analysis. Breast Cancer Res Treat. 2012;135(2):325–34.
Ransohoff DF, Gourlay ML. Sources of bias in specimens for research about molecular markers for cancer. J Clin Oncol. 2010;28(4):698–704.
Bossuyt PMM. Better standards for better reporting of RCTs - a revised CONSORT statement should further improve standards of reporting. Br Med J. 2001;322(7298):1317–8.
McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM. Re: Reporting recommendations for tumor marker prognostic studies (REMARK) - Reply. J Natl Cancer Inst. 2005;97(24):1855–6.
Harris L, Fritsche H, Mennel R, Norton L, Ravdin P, Taube S, Somerfield MR, Hayes DF, Bast RC. American society of clinical oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol. 2007;25(33):5287–312.
Yerushalmi R, Woods R, Ravdin PM, Hayes MM, Gelmon KA. Ki67 in breast cancer: prognostic and predictive potential. Lancet Oncol. 2010;11(2):174–83.
Dowsett M, Nielsen TO, A'Hern R, Bartlett J, Coombes RC, Cuzick J, Ellis M, Henry NL, Hugh JC, Lively T, et al. Assessment of Ki67 in breast cancer: recommendations from the International Ki67 in Breast Cancer Working Group. J Natl Cancer Inst. 2011;103(22):1656–64.
Dowsett M, Dunbier A, Anderson H, Salter J, Detre S, Jones R, Skene A, Dixon M, Smith IE. Biomarkers and predictive factors of response to neoadjuvant treatment. Breast Cancer Res. 2009;11:S5.
Hadad S, Iwamoto T, Jordan L, Purdie C, Bray S, Baker L, Jellema G, Deharo S, Hardie DG, Pusztai L, et al. Evidence for biological effects of metformin in operable breast cancer: a pre-operative, window-of-opportunity, randomized trial. Breast Cancer Res Treat. 2011;128(3):783–94.
Macaskill EJ, Bartlett JM, Sabine VS, Faratian D, Renshaw L, White S, Campbell FM, Young O, Williams L, Thomas JS, et al. The mammalian target of rapamycin inhibitor everolimus (RAD001) in early breast cancer: results of a pre-operative study. Breast Cancer Res Treat. 2011;128(3):725–34.
Dowsett M, Smith IE. Re: Prognostic value of Ki67 expression after short-term presurgical endocrine therapy for primary breast cancer - Response. J Natl Cancer Inst. 2007;99(13):1053–4.
Ellis MJ, Coop A, Singh B, Tao Y, Llombart-Cussac A, Janicke F, Mauriac L, Quebe-Fehling E, Chaudri-Ross HA, Evans DB, et al. Letrozole inhibits tumor proliferation more effectively than tamoxifen independent of HER1/2 expression status. Cancer Res. 2003;63(19):6523–31.
Hadad SM, Coates P, Jordan LB, Dowling RJ, Chang MC, Done SJ, Purdie CA, Goodwin PJ, Stambolic V, Moulder-Thompson S, et al. Evidence for biological effects of metformin in operable breast cancer: biomarker analysis in a pre-operative window of opportunity randomized trial. Breast Cancer Res Treat. 2015.
Baselga J, Semiglazov V, van Dam P, Manikhas A, Bellet M, Mayordomo J, Campone M, Kubista E, Greil R, Bianchi G, et al. Phase II randomized study of neoadjuvant everolimus plus letrozole compared with placebo plus letrozole in patients with estrogen receptor-positive breast cancer. J Clin Oncol. 2009;27(16):2630–7.
Viale G, Giobbie-Hurder A, Regan MM, Coates AS, Mastropasqua MG, Dell'Orto P, Maiorano E, MacGrogan G, Braye SG, Ohlschlegel C, et al. Prognostic and predictive value of centrally reviewed Ki-67 labeling index in postmenopausal women with endocrine-responsive breast cancer: results from Breast International Group Trial 1–98 comparing adjuvant tamoxifen with letrozole. J Clin Oncol. 2008;26(34):5569–75.
Jones RL, Salter J, A'Hern R, Nerurkar A, Parton M, Reis-Filho JS, Smith IE, Dowsett M. The prognostic significance of Ki67 before and after neoadjuvant chemotherapy in breast cancer. Breast Cancer Res Treat. 2009;116(1):53–68.
Hannemann J, Oosterkamp HM, Bosch CAJ, Velds A, Wessels LFA, Loo C, Rutgers EJ, Rodenhuis S, van de Vijver MJ. Changes in gene expression associated with response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol. 2005;23(15):3331–42.
Mulligan JM, Hill LA, Deharo S, Irwin G, Boyle D, Keating KE, Raji OY, McDyer FA, O'Brien E, Bylesjo M, et al. Identification and validation of an anthracycline/cyclophosphamide-based chemotherapy response assay in breast cancer. J Natl Cancer Inst. 2014;106(1):djt335.
Welsh AW, Moeder CB, Kumar S, Gershkovich P, Alarid ET, Harigopal M, Haffty BG, Rimm DL. Standardization of estrogen receptor measurement in breast cancer suggests false-negative results are a function of threshold intensity rather than percentage of positive cells. J Clin Oncol. 2011;29(22):2978–84.
Dunbier AK, Anderson H, Ghazoui Z, Folkerd EJ, A'Hern R, Crowder RJ, Hoog J, Smith IE, Osin P, Nerurkar A, et al. Relationship between plasma estradiol levels and estrogen-responsive gene expression in estrogen receptor-positive breast cancer in postmenopausal women. J Clin Oncol. 2010;28(7):1161–7.
Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, Dowsett M, Fitzgibbons PL, Hanna WM, Langer A, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. Arch Pathol Lab Med. 2007;131(1):18–43.
Hatzis C, Sun H, Yao H, Hubbard RE, Meric-Bernstam F, Babiera GV, Wu Y, Pusztai L, Symmans WF. Effects of tissue handling on RNA integrity and microarray measurements from resected breast cancers. J Natl Cancer Inst. 2011;103(24):1871–83.
Meric-Bernstam F, Akcakanat A, Chen H, Sahin A, Tarco E, Carkaci S, Adrada BE, Singh G, Do KA, Garces ZM, et al. Influence of biospecimen variables on proteomic biomarkers in breast cancer. Clin Cancer Res. 2014;20(14):3870–83.
Purdie CA, Jordan LB, McCullough JB, Edwards SL, Cunningham J, Walsh M, Grant A, Pratt N, Thompson AM. HER2 assessment on core biopsy specimens using monoclonal antibody CB11 accurately determines HER2 status in breast carcinoma. Histopathology. 2010;56(6):702–7.
Detre S, Saclani Jotti G, Dowsett M. A "quickscore" method for immunohistochemical semiquantitation: validation for oestrogen receptor in breast carcinomas. J Clin Pathol. 1995;48(9):876–8.
Harvey JM, Clark GM, Osborne CK, Allred DC. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol. 1999;17(5):1474–81.
Werner M, Chott A, Fabiano A, Battifora H. Effect of formalin tissue fixation and processing on immunohistochemistry. Am J Surg Pathol. 2000;24(7):1016–9.
De Marzo AM, Fedor HH, Gage WR, Rubin MA. Inadequate formalin fixation decreases reliability of p27(Kip1) immunohistochemical staining: Probing optimal fixation time using high-density tissue microarrays. Hum Pathol. 2002;33(7):756–60.
Morrogh M, Andrade VP, Patil AJ, Qin LX, Mo QX, Sakr R, Arroyo CD, Brogi E, Morrow M, King TA. Differentially expressed genes in window trials are influenced by the wound-healing process: lessons learned from a pilot study with anastrozole. J Surg Res. 2012;176(1):121–32.
Iwamoto T, Booser D, Valero V, Murray JL, Koenig K, Esteva FJ, Ueno NT, Zhang J, Shi WW, Qi Y, et al. Estrogen Receptor (ER) mRNA and ER-related gene expression in breast cancers that are 1 % to 10 % ER-positive by immunohistochemistry. J Clin Oncol. 2012;30(7):729–34.
Loi S, Michiels S, Baselga J, Bartlett JM, Singhal SK, Sabine VS, Sims AH, Sahmoud T, Dixon JM, Piccart MJ, et al. PIK3CA genotype and a PIK3CA mutation-related gene signature and response to everolimus and letrozole in estrogen receptor positive breast cancer. PLoS One. 2013;8(1), e53292.
Heskamp S, Boerman OC, Molkenboer-Kuenen JD, Wauters CA, Strobbe LJ, Mandigers CM, Bult P, Oyen WJ, van der Graaf WT, van Laarhoven HW. Upregulation of IGF-1R expression during neoadjuvant therapy predicts poor outcome in breast cancer patients. PLoS One. 2015;10(2):e0117745.
Motamedolshariati M, Memar B, Aliakbaian M, et al. Accuracy of prognostic and predictive markers in core needle breast biopsies compared with excisional specimens. Breast Care (Basel). 2014;9(2):107–10.
Munch-Petersen HD, Rasmussen BB, Balslev E. Reliability of histological malignancy grade, ER and HER2 status on core needle biopsy vs surgical specimen in breast cancer. APMIS. 2014;122(9):750–4.
Loubeyre P, Bodmer A, Tille JC, et al. Concordance between core needle biopsy and surgical excision specimens for tumour hormone receptor profiling according to the 2011 St. Gallen Classification, in clinical practice. Breast J. 2013;19(6):605–10.
Dekker TJ, Smit VT, Hooijer GK, et al. Reliability of core needle biopsy for determining ER and HER2 status in breast cancer. Ann Oncol. 2013;24(4):931–7.
Greer LT, Rosman M, Mylander WC, et al. Does breast tumor heterogeneity necessitate further immunohistochemical staining on surgical specimens? J Am Coll Surg. 2013;216(2):239–51.
Lee AH, Key HP, Bell JA, et al. Concordance of HER2 status assessed on needle core biopsy and surgical specimens of invasive carcinoma of the breast. Histopathology. 2012;60(6):880–4.
Ricci MD, Calvano Filho CM, Oliveira Filho HR, et al. Analysis of the concordance rates between core needle biopsy and surgical excision in patients with breast cancer. Rev Assoc Med Bras. 2012;58(5):532–6.
Khoury T, Zakharia Y, Tan W, et al. Breast hormonal receptors test should be repeated on excisional biopsy after negative core needle biopsy. Breast J. 2011;17(2):180–6.
Lorgis V, Algros MP, Villanueva C, et al. Discordance in early breast cancer for tumour grade, estrogen receptor, progesteron receptors and human epidermal receptor-2 status between core needle biopsy and surgical excisional primary tumour. Breast. 2011;20(3):284–7.
Park SY, Kim KS, Lee TG, et al. The accuracy of preoperative core biopsy in determining histologic grade, hormone receptors, and human epidermal growth factor receptor 2 status in invasive breast cancer. Am J Surg. 2009;197(2):266–9.
Usami S, Moriya T, Amari M, et al. Reliability of prognostic factors in breast carcinoma determined by core needle biopsy. Jpn J Clin Oncol. 2007;37(4):250–5.
Cahill RA, Walsh D, Landers RJ, et al. Preoperative profiling of symptomatic breast cancer by diagnostic core biopsy. Ann Surg Oncol. 2006;13(1):45–51.
Burge CN, Chang HR, Apple SK. Do the histologic features and results of breast cancer biomarker studies differ between core biopsy and surgical excision specimens? Breast. 2006;15(2):167–72.
Hodi Z, Chakrabarti J, Lee AH, et al. The reliability of assessment of oestrogen receptor expression on needle core biopsy specimens of invasive carcinomas of the breast. J Clin Pathol. 2007;60(3):299–302.
Badoual C, Maruani A, Ghorra C, et al. Pathological prognostic factors of invasive breast carcinoma in ultrasound-guided large core biopsies-correlation with subsequent surgical excisions. Breast. 2005;14(1):22–7.
Usami S, Moriya T, Kasajima A, et al. Pathological aspects of core needle biopsy for non-palpable breast lesions. Breast Cancer. 2005;12(4):272–8.
Al Sarakbi W, Salhab M, Thomas V, et al. Is preoperative core biopsy accurate in determining the hormone receptor status in women with invasive breast cancer? Int Semin Surg Oncol. 2005;2:15.
Deshpande A, Garud T, Holt SD. Core biopsy as a tool in planning the management of invasive breast cancer. World J Surg Oncol. 2005;3(1):1.
O'Leary R, Hawkins K, Beazley JC, et al. Agreement between preoperative core needle biopsy and postoperative invasive breast cancer histopathology is not dependent on the amount of clinical material obtained. J Clin Pathol. 2004;57(2):193–5.
Andrade VP, Gobbi H. Accuracy of typing and grading invasive mammary carcinomas on core needle biopsy compared with the excisional specimen. Virchows Arch. 2004;445(6):597–602.
Harris GC, Denley HE, Pinder SE, et al. Correlation of histologic prognostic factors in core biopsies and therapeutic excisions of invasive breast carcinoma. Am J Surg Pathol. 2003;27(1):11–5.
Connor CS, Tawfik OW, Joyce AJ, et al. A comparison of prognostic tumor markers obtained on imageguided breast biopsies and final surgical specimens. Am J Surg. 2002;184(4):322–4.
McIntosh SA, Panchalingam L, Payne S, et al. Freehand core biopsy in breast cancer: an accurate predictor of tumour grade following neoadjuvant chemotherapy? Breast. 2002;11(6):496–500.
Sharifi S, Peterson MK, Baum JK, et al. Assessment of pathologic prognostic factors in breast core needle biopsies. Mod Pathol. 1999;12(10):941–5.
Gotzinger P, Gebhard B, Gnant M, et al. Value of punch biopsy in diagnosis of palpable breast tumors. A prospective analysis of 150 patients. Chirurg. 1998;69(10):1068–71.
Jacobs TW, Siziopikou KP, Prioleau JE, et al. Do prognostic marker studies on core needle biopsy specimens of breast carcinoma accurately reflect the marker status of the tumor? Mod Pathol. 1998;11(3):259–64.
Di Loreto C, Puglisi F, Rimondi G, et al. Large core biopsy for diagnostic and prognostic evaluation of invasive breast carcinomas. Eur J Cancer. 1996;32A(10):1693–700.
Dahlstrom JE, Jain S, Sutton T, et al. Diagnostic accuracy of stereotactic core biopsy in a mammographic breast cancer screening programme. Histopathology. 1996;28(5):421–7.
Baildam AD, Turnbull L, Howell A, et al. Extended role for needle biopsy in the management of carcinoma of the breast. Br J Surg. 1989;76(6):553–8.
Zidan A, Christie Brown JS, Peston D, et al. Oestrogen and progesterone receptor assessment in core biopsy specimens of breast carcinoma. J Clin Pathol. 1997;50(1):27–9.
Acknowledgements
The authors thank the patients who supported these studies.
Funding
SH and PGR were funded by Breast Cancer Research Scotland. The authors are grateful to the support of the Tayside Tissue Bank for support in tissue handling and storage.
Availability of data and materials
Gene expression array data will be provided for personal research purposes through the corresponding author; residual tissues from the studies may be applied for through the Tayside Tissue Bank, Dundee, Scotland.
Authors’ contributions
SH, PGR, SMT and AMT conceived and designed the studies; LBJ and CP provided expert pathology for the IHC; TI and LP provided microarray analytical support; SH and AMT wrote the manuscript; All authors read, edited and have approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent to publish
Not applicable.
Ethics approval and consent to participate
This study was approved by Tayside Research Ethics Committee of Ninewells Hospital and Medical School, Dundee, Scotland. DD1 9SY. Written informed consent to participate in the study was obtained from all participants.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Hadad, S.M., Jordan, L.B., Roy, P.G. et al. A prospective comparison of ER, PR, Ki67 and gene expression in paired sequential core biopsies of primary, untreated breast cancer. BMC Cancer 16, 745 (2016). https://doi.org/10.1186/s12885-016-2788-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-016-2788-x