Abstract
Background
The causes of infertility have remained an important challenge. The relationship between VDR gene polymorphisms and infertility has been reported, with controversial findings.
Objective and rationale
We aimed to determine this relationship by conducting a systematic review and meta-analysis.
Search methods
The study was started with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) declaration and the final draft was registered as a protocol in PROSPERO (ID: CRD42023416535). The international electronic databases including PubMed (Medline), Scopus, Web of Sciences, and Cumulative Index to Nursing and Allied Health Literature (CINHAL) were searched until January 30, 2023, by using appropriate keywords. The quality of the final studies was assessed using the NOS Checklist for case–control studies. The odds ratios (ORs) for each of the genetic models were pooled, and a subgroup analysis based on geographical region and types of infertility was carried out by the MetaGenyo online tool.
Outcomes
Case–control studies including 18 and 2 studies about infertility in women and men, respectively, and 4 miscarriage studies were entered into the meta-analysis. The VDR gene TaqI polymorphism was associated with infertility susceptibility in women in the allele contrast [OR = 1.2065, 95% CI (1.0846–1.3421); P = 0.0005], Recessive model [OR = 1.3836, 95% CI (1.1197–1.7096); P = 0.002], Dominant model [OR = 1.2146, 95% CI (0.0484–1.4072); P = 0.009], Homozygote [OR = 1.4596, 95% CI (1.1627–1.8325); P = 0.001], and TT vs. Tt [OR = 1.2853, 95% CI (1.0249–1.6117); P = 0.029. ApaI and FokI gene polymorphisms were found to be significantly protective SNPs against women and men infertility in the Dominant model [OR = 0.8379, 95% CI (0.7039- 0.9975); P = 0.046] and Recessive model [OR = 0.421, 95% CI (0.1821–0.9767); P = 0.043], respectively. Sub-group meta-analysis showed a protection association of ApaI in dominant [OR = 0.7738, 95% CI = 0.6249–0.9580; P = 0.018] and AA vs. aa [OR = 0.7404, 95 CI% (0.5860–0.9353) P = 0.011725] models in PCOS subgroup, however, a negative association with idiopathic infertility was found in AA vs. Aa [OR = 1.7063, 95% CI (1.1039–2.6375); P = 0.016187] and Aa vs. aa [OR = 0.6069, 95% CI (0.3761–0.9792); P = 0.040754]. TaqI SNP was significantly associated with infertility in the African population and BsmI was associated with the disease mostly in the Asian population.
Conclusion
This meta-analysis showed that the TaqI polymorphism may be linked to women’s infertility susceptibility. However, ApaI and FokI might be the protective SNPs against infertility in Women and men, respectively.
Similar content being viewed by others
Introduction
Infertility is a disease of the female or male reproductive system in which pregnancy does not occur after 12 months of regular unprotected sex [1]. This disease is a very common condition that affects between 48.5 and 186 million males and females worldwide, respectively. According to WHO, almost one out of six people of reproductive age experience infertility during their lifetime [2, 3]. Genetic, environmental, and some idiopathic factors are among the effective causes of infertility[4]. Male infertility is usually due to problems in the semen existence, the absence or low levels of sperm, or the abnormal shape and movement of sperm, and infertility in women is also caused by a range of abnormalities of the ovaries, uterus, fallopian tubes, the endocrine system, etc. [5]. Furthermore, Miscarriage is generally defined as the loss of a pregnancy before viability, which is considered the other complication of successful pregnancy [6]. It is estimated that 23 million miscarriages occur worldwide each year [7]. The short-term national economic cost of miscarriage in the UK was estimated at 471 million pounds annually in 2005 [8]. Physical consequences of miscarriage include bleeding or infection and psychological consequences such as increased risk of anxiety, depression, post-traumatic stress disorder, and suicide [9]. Its determinants include fetal genetic and chromosomal abnormalities, genital anatomy, endometrial pathology, hereditary thrombophilia, antiphospholipid syndrome, etc. Most of these factors are difficult to correct, but there are also controllable ones whose negative effects can be completely reduced before conception. These include nutritional deficiencies, including vitamin D (Vit D) deficiency [10, 11].
Vit D is a hormone that has a fundamental role in endocrine function, regulation of cell proliferation, and other metabolic pathways, such as pathways involved in the immune response [12]. Recent studies show the relationship between vitamin D deficiency and adverse pregnancy outcomes, including miscarriage [13,14,15]. Vit D is locally metabolized in the male reproductive system and the expression of Vitamin D receptor (VDR) has been shown in human testes and in ejaculated human sperms [16]. Studies have proven that Men who receive more diet and supplements produce sperm with less DNA damage [17]. Maternal Vit D deficiency is associated with many gynecological and obstetric diseases such as polycystic ovary syndrome, endometriosis, ovarian cancer, as well as gestational diabetes, which are all associated with reduced successful pregnancy. Preeclampsia and preterm labor are related, which can affect fertility [4, 18, 19]. Polycystic ovary syndrome (PCOS) is the most common endocrine metabolic disorder that affects 5 to 10% of women of reproductive age and is one of the common causes of ovulatory infertility [20]. The VDR gene is considered an important candidate gene for PCOS [21].
Since new research studies indicated the significance of vitamin D in the endocrine system and its relation not only with bone mineral density but also with certain cancers, autoimmune diseases, diabetes mellitus, depression, allergy, cardiovascular disease, pregnancy complications, infertility, and even frailty, vitamin D deficiency has just been identified as endemic to a variety of health consequences [22, 23]. The results of various studies taken together have shown that a variety of environmental and genetic factors influence vitamin D status variations. Studying the genetic basis of vitamin D metabolism, however, has brought to light the significance of multiple genes, including CG, DHCR1, CYP2R1, CYP24A1, and VDR [24]. By interacting with the vitamin D receptor (VDR), a member of the superfamily of steroid/thyroid hormone receptors, 1,25(OH)2D3, the active form of vitamin D, affects the transcriptional activation and repression of several target genes [25]. The VDR gene has many single-nucleotide polymorphisms (SNPs), which have been linked to a variety of physiological and pathological characteristics including different pregnancy complications in numerous populations [26,27,28]. VDR gene polymorphisms most likely have an impact on the expression and function of VDR [29].VDRs are found in the endometrium, placenta, decidual cells, ovarian granulosa cells, fallopian tube epithelium, pituitary gland, and hypothalamus. [30] Expression of the VDR in the placenta and decidua, which probably has an active role in the local autocrine and paracrine response, suggests that the local synthesis of Vit D potentially modulates placental function and fetal growth. Therefore, VDR gene function could be influenced by several factors such as genetic polymorphism that might related to susceptibility to fertility problems [31, 32]. The most intensively studied VDR polymorphisms are FokI (rs2228570), TaqI (rs731236), BsmI (rs1544410), and ApaI (rs7975232) variants. The association of these polymorphisms with different types of infertility complications including PCOS, endometriosis, miscarriage, etc. has been investigated in single studies, with conflicting results. Considering the above-mentioned observations, our meta-analysis study aimed to more powerfully and comprehensively assess the association between four VDR polymorphisms (rs2228570, rs1544410, rs7975232, rs731236) and infertility and miscarriage in different populations and geographical regions by conducting a systematic review.
Methods
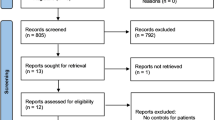
This systematic review and meta-analysis were conducted based on the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines (Fig. 1) [33]. The PROSPERO registration number and the published protocol were CRD42023416535.
Search strategy and screening process
For this meta-analysis, we accessed several international databases, including PubMed (Medline), Web of Science, and Scopus. These databases were searched for literature published up to January 2023, using specific search terms and their synonyms: "Infertility," "miscarriage," "VDR" or "vitamin D receptor," and "Polymorphism." Furthermore, we conducted a manual search within these databases, carefully examining the references of relevant studies, and also looked through grey literature to identify any additional related studies that might not have been captured through the initial database search. To ensure thoroughness and accuracy, the screening process was carried out independently by two authors (AM, MA). In cases where there were disagreements, they were resolved through discussion and consensus with a third author (YM). This rigorous approach helped maintain the integrity of the study selection process and ensured that all relevant studies were included for analysis.
Eligibility criteria
Eligible studies were limited to those [1] case–control studies whose main purpose was to determine the association between VDR gene polymorphisms ApaI, BsmI, TaqI, and FokI and the risk of infertility and miscarriage, (2) studies that have reported the frequencies of genotypes or alleles by comparing at least two groups, a group including Infertility or miscarriage against healthy groups, (3) and, studies report odds ratio (ORs) and 95% confidence interval (CIs). Exclusion criteria included (1) all other types of studies including cohorts, cross-sectional, case reports, case series, letters to the editor, reports, clinical trial studies, and review studies, (2) Case–control studies without reporting inclusion criteria, (3) Repetitive and non-English language studies.
Data extraction
The process of data extraction involved utilizing a structured form designed to gather essential information from each included study. This information encompassed various key aspects, such as the author's name, study location, publication date, ethnicity of the participants, mean age of the study population, sample size, genotyping methods employed, as well as the number of cases and controls pertaining to VDR gene polymorphisms.
Risk of Bias
The Newcastle–Ottawa Quality Assessment Scale (NOS) checklist was used with the purpose of methodological quality and risk evaluation of non-randomized studies in Meta-Analysis. The NOS consists of eight items divided into three categories: Selection of cases and controls, comparability of them, and Ascertainment of exposure. Items can be awarded a maximum of one star for each one within the two first categories and a maximum of two stars for Comparability. The scoring ranged from zero to nine. A score of 6 and above indicates the high quality of the study[34].
Statistical analysis
The control genotype distribution was assessed by the Hardy–Weinberg equilibrium (HWE) (p < 0.05 was considered meaningful). Calculation of ORs and 95% confidence intervals (CIs) in seven different genetic models was used to estimate its effect on a forest plot and the strength of the relationship between VDR polymorphisms and the risk of infertility and miscarriage. genetic models of polymorphisms are discussed in the following: TaqI —allele contrast (t vs. T), recessive model (tt vs. Tt + TT), dominant model (tt + Tt vs. TT), over dominant (Tt vs. TT + tt), tt vs. TT model, TT vs. Tt model, Tt vs. tt model; FokI — allele contrast (f vs. F), recessive model (ff vs. Ff + FF), dominant model (ff + Ff vs. FF), over-dominant model (Ff vs. FF + ff), ff vs. FF model, FF vs. Ff model, Ff vs. ff model; ApaI —allele contrast (a vs. A), recessive model (aa vs. Aa + AA), dominant model (aa + Aa vs. AA), over dominant model (Aa vs. AA + aa), aa vs. AA model, AA vs. Aa model, Aa vs. aa model; BsmI —allele contrast (b vs. B), recessive model (bb vs. Bb + BB), dominant model (bb + Bb vs. BB), over dominant model (Bb vs. BB + bb), bb vs. BB model, BB vs. Bb model, Bb vs. bb model. Also, heterogeneity between studies was assessed by Q Cochrane tests and I2. To test Publication biases, funnel plots and Egger’s test (p < 0.05) were used. All statistical analyses were done using MetaGenyo; a web tool to conduct a meta-analysis of genetic association studies [35]. The forest plot and funnel plot pertaining to all examined polymorphisms are depicted in (Figs. 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15 and 16).
Results
Description of Studies
Out of a total of 6060 and 2194 relevant citations on infertility and miscarriage, respectively, 5930 references remained after eliminating duplicates. Following title and abstract screening, and full-text review, 24 studies meeting the search criteria were identified, comprising 3424 cases and 3697 controls (refer to Tables 1, 2 and 3). Notably, the total cases and controls were categorized based on the methodology of the studies rather than individual SNP analysis. Among these studies, 18 investigated the association of VDR genetic polymorphisms with female infertility, 2 with male infertility, and 4 with miscarriage. Tables 4, 5, and 6 summarizes the characteristics and genotype frequencies of the included studies.
Association between VDR Genetic Polymorphisms and Infertility in Women
FokI (rs2228570) SNP
Fourteen studies involving 5,210 participants reported on the association between FokI SNP and female infertility. While a protective association was observed in the FF vs. Ff model (OR = 0.87, 95% CI = 0.76–1.00, P = 0.05), no significant association was found in other genetic models.
BsmI (rs1544410) SNP
Thirteen studies examined the association between BsmI SNP and infertility, with no significant correlation found in any of the genetic models assessed.
TaqI (rs731236) SNP
Eleven studies focused on TaqI SNP, reporting a positive association in some genetic models, including Allele contrast and Recessive model, but not in others.
ApaI (rs7975232) SNP
Twelve case–control studies evaluated the ApaI SNP, showing a protective association in the Dominant model and Aa vs. aa model, but no significant association in other genetic models.
Association between VDR Genetic Polymorphisms and Infertility in Men
FokI (rs2228570) SNP
Two studies involving 404 participants investigated the association between FokI SNP and male infertility, revealing a protective association in the Recessive model.
Association between VDR Genetic Polymorphisms and Miscarriage
Four studies examined the association between VDR genetic polymorphisms and miscarriage, evaluating TaqI, ApaI, FokI, and BsmI SNPs. Characteristics of these studies are summarized in Tables 7,8 and 9.
Heterogeneity, Publication Bias, and Sensitivity Analysis
Heterogeneity was observed in certain genetic models, particularly in the TaqI SNP. Egger's tests revealed no publication bias. The results of sensitivity analysis are presented in relevant charts.
Discussion
This meta-analysis suggests that vitamin D receptor gene variations may play a role in infertility risk and outcome. The TaqI polymorphism may increase the susceptibility to infertility in women, possibly by affecting the implantation and placentation processes. The ApaI and FokI polymorphisms may have protective effects against infertility in women and men, respectively, possibly by modulating the immune system and the hormonal balance. These findings may have implications for the diagnosis, prevention, and treatment of infertility, as well as for the understanding of the molecular mechanisms of reproductive health. In the development of pregnancy problems, genetic variables have grown increasingly relevant. Previous research has linked VDR gene variations to infertility in women and men due to PCOS, endometriosis, preeclampsia, idiopathic infertility, and other causes [36, 37]. Serum 25-hydroxyvitamin D [25 (OH) D] has been found to inhibit VDR-mediated pathogenesis by modulating target gene expression [38]. The VDR gene is a potential gene for infertility because it controls several genes that participate in diverse molecular and cellular processes [39]. Recurrent miscarriages (RM), which occur at a rate of 1 to 3% of female reproductive age, are another major medical, social, and psychological complication associated with pregnancy. Although numerous pathways for the development of RM have already been discovered, the underlying causes of around 50% of patients remain unexplained [13, 14]. Nevertheless, the multifaceted etiology of this problem, including immune system irregularities and vitamin D inadequacy, has been recognized for some time. As a result, it seems that altered metabolism of the VD/VDR complex via immune response modulation might be significant in the pathogenesis of both spontaneous abortion and RM [40]. Furthermore, VDR is a receptor with a pleiotropic action on human cells. remarkably, the presence of polymorphic variations in the VDR gene may affect VDR activity [41]. Regarding the evaluation of the connection between VDR gene polymorphisms and infertility/recurrent miscarriage in numerous single studies in different populations, the findings are contradictory. Given the volume of data accumulated and the ambiguous role of VDR in the etiology of infertility/recurrent miscarriage in general, we decided to conduct a comprehensive meta-analysis of any published research on the association between the most studied VDR polymorphisms and any infertility/recurrent miscarriage.
The present study included a total of 22 articles and showed that the VDR gene TaqI polymorphism was associated with infertility susceptibility in women. ApaI and FokI gene polymorphisms were found to be significantly protective SNPs against women's and men's infertility. The published studies related to the association of selected VDR SNPs and recurrent miscarriage were not enough for meta-analysis, therefore, a systematic review was alone performed. The findings were consistent with prior research and may give an entirely novel biomarker in infertility/recurrent miscarriage with diverse etiologies [20, 42,43,44,45]. A subgroup analysis was also undertaken to investigate the possible significance of patient ethnicity or infertility etiology on the association between VDR polymorphisms and the risk of infertility. TaqI SNP was shown to be significantly connected with infertility in Africans, while BsmI was found to be associated with the disease mostly in Asians. This finding could be explained by genetic differences between ethnic groupings. Furthermore, due to the procedure of natural selection, functional variations in various groups may differ [26]. Furthermore, VDR ApaI (rs7975232) was found to be associated with infertility susceptibility in the PCOS subgroup, however, a protection association with idiopathic infertility was found.
VDR gene polymorphism could contribute to the pathophysiology of infertility by influencing gene expression and mRNA stability, and hence the cellular and molecular processes associated with infertility etiology. Nevertheless, these polymorphisms are mostly nonfunctional, linkage disequilibrium with another undiscovered functional variant of the VDR gene appears to be the most likely explanation for the observed association. We meta-analyzed the VDR gene TaqI, BsmI, FokI, ApaI polymorphisms, and women/men infertility for the first time.
The FokI SNP is the only VDR polymorphism leading to a VDR protein with a different structure. Furthermore, it is the only SNP that is not linked to any other VDR polymorphism, implying that it plays a distinct function [46]. The polymorphism, which is a C to T alteration, is located at the 5' end of the gene. This alteration results in a protein of a different size, a 424 amino acid (aa) variant encoded by the major allele form (ACG) and a 427 aa variant expressed by the minor allele form (ATG). The variations are thought to be functionally relevant, with the 424 aa VDR variant having higher transcriptional activity and being associated with lower circulating 25(OH) D levels than the 427 aa variant [46, 47]. Moreover, Yan et al. showed that women with RPL have lower levels of VDR expression in chorionic villi, decidua, and serum compared with normal pregnant women [48]. It has previously been suggested that CC genotype / 424 aa VDR variant has a higher frequency in women with RPL, which leads to lower circulating 25(OH) D levels, respectively. Several studies have demonstrated that high Vitamin D levels might protect against a variety of illnesses, including infertility and recurrent miscarriage. The idea has been suggested in several research that greater pre-diagnosis plasma levels of 25-hydroxy vitamin D, the predominant circulating form of vitamin D, is related to a significant decrease in pregnancy problems such as PCOS, endometriosis, infertility, and recurrent pregnancy loss [49,50,51,52]. Furthermore, comprehensive reviews and meta-analyses revealed a substantial reduction in total pregnancy outcomes in Vitamin D-deficient patients [53, 54]. Our finding revealed a marginally significant association of FokI SNP with infertility under the FF vs. Ff genetic model (OR = 0.8763, 95% CI [0.7651–1.0036], P = 0.05). This indicates that the FokI f allele might be a risk factor for infertility, future studies with larger sample sizes and considering other confounder variables still need to confirm these findings though.
The functional evaluation of the three significant non-coding VDR SNPs (Bsml, TaqI, and ApaI) examined in this meta-analysis revealed contradictory findings from prior studies regarding their biological implications. Even if these SNPs are nonfunctional, the impacts identified in this meta-analysis and other studies could be driven by other, actually important SNPs in significant LD located elsewhere in the VDR gene. Some studies aimed at characterizing differences in VDR expression for SNPs in the 3' end of the VDR gene found that the Bsml-ApaI-TaqI haplotype BAt (rs1544410-A/rs7975232-A/rs731236-C) had higher levels of VDR mRNA expression than the baT (rs1544410-G/rs7975232-C/rs731236-T). These SNPs could be implicated in gene expression control, specifically by mRNA stability modulation. To be more specific, the existence of the TaqI G allele improves VDR mRNA stability and half-life, leading to an increased VDR synthesis and therefore directly altering vitamin D levels and consequently subsequent effects of vitamin D [55, 56].
A significant association was found between ApaI and infertility in the present meta-analysis. We observed a borderline and a significant protective association for the Aa vs. aa model (OR = 0.83, P = 0.05) and the dominant model (OR = 0.84, P < 0.05), however, no significant association was reported in other genetic model contrasts. These findings show that individuals who inherited ApaI SNP in a dominant form might be more protected against infertility. This polymorphism is in strong linkage disequilibrium with the poly(A) microsatellite located in the 3′ untranslated region [45] of the VDR gene, which appears to influence VDR messenger RNA stability and VDR translational activity (9). Sub-group analysis, however, showed a protective association against infertility in the PCOS subgroup under dominant (AA + Aa vs. aa), over-dominant, (Aa vs. AA + aa, AA vs. aa, and Aa vs. aa genetic models and a susceptibility association under the recessive genetic model in idiopathic infertility sub-group.
In our study, we noted a higher frequency of the genotype containing a mutated t allele of TaqI polymorphism. Interestingly, TaqI polymorphism was the only SNP that showed significant association with infertility overall and based on the etiology, excluding Over dominant genetic model. Our results showed that TaqI polymorphism may increase susceptibility to infertility under the allele contrast, recessive, dominant, homozygote, TT vs. Tt, Tt vs. tt genetic models. This indicates the If Taq t allele is a protective factor for infertility, then the infertility chance of patients with Taq t allele will be lower than that of patients with Taq T allele (OR > 1, P < 0.05). These data suggested the role of these genetic variants might be attributed with infertility due to the influence on the VDR function and consequently disturbed vitamin D metabolism or might be due to the LD with other VDR SNPs. These results suggest the special role of maternal setting genetic variants of the VDR gene in the etiology of this pregnancy complication. However, further research is required to determine what exactly FokI is acting as a marker for infertility.
As ~ 50% of patients with recurrent pregnancy loss (RPL) do not have a definite etiology, we further aimed to perform the meta-analysis of the association between VDR polymorphisms and recurrent miscarriage. The potential association of VDR polymorphisms with the etiology of recurrent miscarriages has been indicated in several studies [31, 45, 57]. Although with conflicting results, most of them suggested VDR SNPs association with RM in women. A study reported lower expression of VDR in trophoblastic, decidua, and serum villi in the RM group compared to the control, suggesting that impaired VDR expression in the first trimester of pregnancy may be associated with the occurrence of RM [48]. Accordingly, it could be suggested that VDR SNPs might be involved in the susceptibility and protection against RPL through influence on the VDR mRNA expression level and stability or due to the LD with other SNPs. In our study, we only found the association of FokI, and RPL in more than two studies, therefore the meta-analysis was performed for FokI polymorphism. Our data showed that FokI is significantly associated with a lower risk of RPL in allele contrast 9OR = 1.23, P = 0.034), recessive model (OR = 1.47, P = 0.010), and FF vs. Ff (OR = 1.46, OR = 0.016) genetic models. This indicates that carriers of FokI SNP might be more protected against RPL, however, it is required to be studied in larger sample sizes and to examine the exact functional effect of this SNP on the RPL etiology.
Because of racial differences, evidence of disease occurrence is not always accurate. This shows that various races have distinct effects on genetic background [58]. Therefore, based on subgroup analysis of different races, it can be found that the same polymorphisms in disease susceptibility in different populations play different roles. In our study, subgroup analysis suggested that the VDR gene BsmI polymorphism was significantly associated with susceptibility to infertility for the comparison of (AA vs. aa), (AA vs. Aa), and recessive model, and was protective SNP in the over-dominant genetic model in Asian population. For VDR gene TaqI polymorphism, it was significantly associated with susceptibility to infertility under the comparison of allele contrast (A vs. a), recessive model (AA vs. Aa + aa), dominant model (AA + Aa vs. aa), over-dominant (Aa vs. AA + aa), AA vs. aa, AA vs. Aa, Aa vs. aa genetic models in African and Asian population. However, for VDR gene ApaI polymorphism, it was protectively associated with infertility under dominant model (AA + Aa vs. aa), over-dominant (Aa vs. AA + aa), AA vs. aa, AA vs. Aa, Aa vs. aa genetic models and a susceptibility association was observed under recessive model (aa vs. Aa + AA) in Asian. FokI polymorphism was not significantly associated with infertility under any genetic models in any geographic population. The opposite association in different populations for an SNP in subgroup analysis might be due to ethnic differences. Of course, it also may be the difference in results caused by the insufficient number of studies included. We certainly need more and better research to get more reliable results.
Our study contains certain characteristics linked to study design that can help to strengthen the conclusions. The criteria for study selection were stringent, and such an exact selection guaranteed the right degree of analysis. Both groups (patients and controls) had participants who were similar in terms of age, ethnic background, and area of residence, reducing the impact of known confounders. In the genetic models, statistical power was adequate for genotype and allele frequencies of reported gene polymorphisms, as well as relationships between individual VDR polymorphisms and the probability of infertility/RPL. A drawback of this research is that we did not have original data, so we were unable to control for other factors such as circulating vitamin D levels, sun exposure, aspirin/NSAID use, stage disease, calcium, and vitamin D intake. The main drawback of the current study is the relatively small sample size and a lack of enough publications on the association of VDR SNPs and RPL. Finally, only four single nucleotide variations of the VDR gene were studied in this study, while, there are several more genetic variations that influence VD metabolism.
The limitation lies in the incapacity to explore diverse age groups through subgroup analyses, relying on the specified age range of 20 to 40 years in the primary studies. This constraint was underscored within the study's delineation of limitations regarding subgroup analyses grounded on age. Also, this study relied on secondary data sources, limiting our ability to control for potential confounding factors, including circulating vitamin D levels, sun exposure, aspirin/NSAID use, stage of disease, calcium, and vitamin D intake. The relatively small sample size in the current study reduced both statistical power and the generalizability of the results. Furthermore, insufficient publications on the association of VDR SNPs and RPL hindered comparison with previous studies. The examination in this study was confined to four single nucleotide variations of the VDR gene—FokI, BsmI, ApaI, and TaqI. However, numerous other genetic variations, such as CYP2R1, CYP27B1, CYP24A1, and GC, influence vitamin D metabolism. Consequently, our findings may not fully capture the genetic effects of vitamin D on RPL.
Conclusion
Some comparisons revealed heterogeneity, but it was somewhat addressed by ethnicity-based subgroup analysis. According to our findings, VDR ApaI and FokI can have a role in infertility/recurrent miscarriage. These SNPs might be utilized to assess the risk of infertility/recurrent miscarriage. The observed relationships should be replicated in a bigger meta-analysis. Furthermore, expression studies are essential for fully comprehending the function of VDR polymorphisms in the etiology of infertility/recurrent miscarriage. Finally, investigations should be conducted to determine whether nutritional therapies such as vitamin D can provide a possible response to the hereditary propensity. Finally, our findings imply that VDR FokI and ApaI polymorphisms may be linked to infertility/recurrent miscarriage. However, more research with a larger sample size and considering other confounding factors is required in the future to reach a conclusive conclusion.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Starc A, Trampuš M, Pavan Jukić D, Grgas-Bile C, Jukić T, Polona Mivšek A. Infertility and sexual dysfunctions: a systematic literature review. Acta Clin Croat. 2019;58(3.):508–15.
2023 WI. Infertility 2023. Available from: https://www.who.int/news-room/fact-sheets/detail/infertility.
Djurovic J, Stamenkovic G, Todorovic J, Aleksic N, Stojkovic O. Polymorphisms and haplotypes in VDR gene are associated with female idiopathic infertility. Hum Fertil. 2020;23(2):101–10.
Aghajafari F, Nagulesapillai T, Ronksley PE, Tough SC, O’Beirne M, Rabi DM. Association between maternal serum 25-hydroxyvitamin D level and pregnancy and neonatal outcomes: systematic review and meta-analysis of observational studies. Bmj. 2013;346.
2018 WICoD. International Classification of Diseases 2018. Available from: https://www.who.int/standards/classifications/classification-of-diseases.
Quenby S, Gallos ID, Dhillon-Smith RK, Podesek M, Stephenson MD, Fisher J, et al. Miscarriage matters: the epidemiological, physical, psychological, and economic costs of early pregnancy loss. Lancet. 2021;397(10285):1658–67.
Badawy A, Inany H, Mosbah A, Abulatta M. Luteal phase clomiphene citrate for ovulation induction in women with polycystic ovary syndrome: a novel protocol. Fertil Steril. 2009;91(3):838–41.
Petrou S, Trinder J, Brocklehurst P, Smith L. Economic evaluation of alternative management methods of first-trimester miscarriage based on results from the MIST trial. BJOG. 2006;113(8):879–89.
Farren J, Jalmbrant M, Falconieri N, Mitchell-Jones N, Bobdiwala S, Al-Memar M, et al. Posttraumatic stress, anxiety and depression following miscarriage and ectopic pregnancy: a multicenter, prospective, cohort study. American Journal of Obstetrics and Gynecology. 2020;222(4):367. e1-. e22.
Palacios C, Gonzalez L. Is vitamin D deficiency a major global public health problem? J Steroid Biochem Mol Biol. 2014;144:138–45.
Petrushkina AA, Pigarova EA, Rozhinskaya LY. The prevalence of vitamin D deficiency in Russian Federation. Osteoporosis and bone diseases. 2019;21(3):15–20.
Froicu M, Weaver V, Wynn TA, McDowell MA, Welsh JE, Cantorna MT. A crucial role for the vitamin D receptor in experimental inflammatory bowel diseases. Mol Endocrinol. 2003;17(12):2386–92.
Chan S, Susarla R, Canovas D, Vasilopoulou E, Ohizua O, McCabe C, et al. Vitamin D promotes human extravillous trophoblast invasion in vitro. Placenta. 2015;36(4):403–9.
Cyprian F, Lefkou E, Varoudi K, Girardi G. Immunomodulatory effects of vitamin D in pregnancy and beyond. Front Immunol. 2019;10:2739.
Hou H, Zhang J, Chen D, Deng F, Morse A, Qiu X, et al. Altered decidual and placental catabolism of vitamin D may contribute to the aetiology of spontaneous miscarriage. Placenta. 2020;92:1–8.
Merke J, Hügel U, Ritz E. Nuclear testicular 1, 25-dihydroxyvitamin D3 receptors in Sertoli cells and seminiferous tubules of adult rodents. Biochem Biophys Res Commun. 1985;127(1):303–9.
Hazouri M, Rizk F. Vitamin D Levels in Serum, Vitamin D Receptor Polymorphisms and Semen Quality Correlations in Lebanon: A Pilot Cross-Sectional Study. Univers J Public Health. 2014;2(4):118–124.
Liu N, Hewison M, Vitamin D. the placenta and pregnancy. Arch Biochem Biophys. 2012;523(1):37–47.
Colonese F, Laganà AS, Colonese E, Sofo V, Salmeri FM, Granese R, et al. The pleiotropic effects of vitamin D in gynaecological and obstetric diseases: an overview on a hot topic. Biomed Res Int. 2015;2015:986281.
Dasgupta S, Dutta J, Annamaneni S, Kudugunti N, Battini MR. Association of vitamin D receptor gene polymorphisms with polycystic ovary syndrome among Indian women. Indian J Med Res. 2015;142(3):276.
Bagheri M, Rad IA, Jazani NH, Nanbakhsh F. Vitamin D receptor TaqI gene variant in exon 9 and polycystic ovary syndrome risk. Int J Fertil Steril. 2013;7(2):116.
Bouillon R, Norman AW, Lips P. Vitamin D deficiency. N Engl J Med. 2007;357(19):1980–1.
Holick MF, Chen TC. Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr. 2008;87(4):1080S–S1086.
Dastani Z, Li R, Richards B. Genetic regulation of vitamin D levels. Calcif Tissue Int. 2013;92:106–17.
Deeb KK, Trump DL, Johnson CS. Vitamin D signalling pathways in cancer: potential for anticancer therapeutics. Nat Rev Cancer. 2007;7(9):684–700.
Ranjzad F, Mahban A, Irani Shemirani A, Mahmoudi T, Vahedi M, Nikzamir A, et al. Influence of gene variants related to calcium homeostasis on biochemical parameters of women with polycystic ovary syndrome. J Assist Reprod Genet. 2011;28:225–32.
Wehr E, Trummer O, Giuliani A, Gruber H-J, Pieber TR, Obermayer-Pietsch B. Vitamin D-associated polymorphisms are related to insulin resistance and vitamin D deficiency in polycystic ovary syndrome. Eur J Endocrinol. 2011;164(5):741–9.
Zadeh-Vakili A, Tehrani FR, Daneshpour MS, Zarkesh M, Saadat N, Azizi F. Genetic polymorphism of vitamin D receptor gene affects the phenotype of PCOS. Gene. 2013;515(1):193–6.
Bai Y, Yu Y, Yu B, Ge J, Ji J, Lu H, et al. Association of vitamin D receptor polymorphisms with the risk of prostate cancer in the Han population of Southern China. BMC Med Genet. 2009;10(1):1–8.
Irani M, Merhi Z. Role of vitamin D in ovarian physiology and its implication in reproduction: a systematic review. Fertility and sterility. 2014;102(2):460–8. e3.
Wolski H, Kurzawińska G, Ożarowski M, Mrozikiewicz AE, Drews K, Karpiński TM, et al. Vitamin D receptor gene polymorphisms and haplotypes in the etiology of recurrent miscarriages. Sci Rep. 2021;11(1):4646.
Valdivielso JM, Fernandez E. Vitamin D receptor polymorphisms and diseases. Clin Chim Acta. 2006;371(1–2):1–12.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Annals of internal medicine. 2009;151(4):W–65–W–94.
Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–5.
Martorell-Marugan J, Toro-Dominguez D, Alarcon-Riquelme ME, Carmona-Saez P. MetaGenyo: a web tool for meta-analysis of genetic association studies. BMC Bioinformatics. 2017;18(1):1–6.
Vilarino FL, Bianco B, Lerner TG, Teles JS, Mafra FA, Christofolini DM, et al. Analysis of vitamin D receptor gene polymorphisms in women with and without endometriosis. Hum Immunol. 2011;72(4):359–63.
Nandi A, Sinha N, Ong E, Sonmez H, Poretsky L. Is there a role for vitamin D in human reproduction? Horm Mol Biol Clin Invest. 2016;25(1):15–28.
Mahmoudi T, Majidzadeh-A K, Farahani H, Mirakhorli M, Dabiri R, Nobakht H, et al. Association of vitamin D receptor gene variants with polycystic ovary syndrome: A case control study. International Journal of Reproductive BioMedicine. 2015;13(12):793.
Shahrokhi SZ, Ghaffari F, Kazerouni F. Role of vitamin D in female reproduction. Clin Chim Acta. 2016;455:33–8.
Gonçalves DR, Braga A, Braga J, Marinho A. Recurrent pregnancy loss and vitamin D: A review of the literature. Am J Reprod Immunol. 2018;80(5): e13022.
Abouzid M, Karazniewicz-Lada M, Glowka F. Genetic determinants of vitamin D-related disorders; focus on vitamin D receptor. Curr Drug Metab. 2018;19(12):1042–52.
Szczepańska M, Mostowska A, Wirstlein P, Skrzypczak J, Misztal M, Jagodziński PP. Polymorphic variants in vitamin D signaling pathway genes and the risk of endometriosis-associated infertility. Mol Med Rep. 2017;12(5):7109–15.
Reginatto MW, Pizarro BM, Antunes RA, Mancebo AC, Hoffmann L, Fernandes P, et al. Vitamin D receptor TaqI polymorphism is associated with reduced follicle number in women utilizing assisted reproductive technologies. Front Endocrinol. 2018;9:252.
Bhakat R, Chandra L, Saxena A, Sarda A, Krishnamurthy K, Yadav P. Evaluation of metabolic syndrome and vitamin D receptor gene polymorphism in male factor infertility. Indian J Clin Biochem. 2017;32:468–72.
Barišić A, Pereza N, Hodžić A, Krpina MG, Ostojić S, Peterlin B. Genetic variation in the maternal vitamin D receptor FokI gene as a risk factor for recurrent pregnancy loss. J Matern Fetal Neonatal Med. 2021;34(14):2221–6.
Uitterlinden AG, Fang Y, Van Meurs JB, Pols HA, Van Leeuwen JP. Genetics and biology of vitamin D receptor polymorphisms. Gene. 2004;338(2):143–56.
Uitterlinden AG, Fang Y, van Meurs JB, van Leeuwen H, Pols HA. Vitamin D receptor gene polymorphisms in relation to Vitamin D related disease states. J Steroid Biochem Mol Biol. 2004;89:187–93.
Yan X, Wang L, Yan C, Zhang X, Hui L, Sheng Q, et al. Decreased expression of the vitamin D receptor in women with recurrent pregnancy loss. Arch Biochem Biophys. 2016;606:128–33.
Zhao H, Wei X, Yang X. A novel update on vitamin D in recurrent pregnancy loss. Mol Med Rep. 2021;23(5):1–8.
Khalifa AA, ELazony RH, AL-klbash MM. The Role Of Vitamin D In Polycystic Ovary Syndrome. J Pharm Negat Results. 2022:2094–110.
Cito G, Cocci A, Micelli E, Gabutti A, Russo GI, Coccia ME, et al. Vitamin D and male fertility: an updated review. The world journal of men’s health. 2020;38(2):164.
Kahlon BK, Simon-Collins M, Nylander E, Segars J, Singh B. A systematic review of vitamin D and endometriosis-role in pathophysiology, diagnosis, treatment, and prevention. F&S Rev. 2022;4(1):1–4.
Mulligan ML, Felton SK, Riek AE, Bernal-Mizrachi C. Implications of vitamin D deficiency in pregnancy and lactation. Am J Obstet Gynecol. 2010;202(5):429 e1–Jagodziński PP.
Tamblyn JA, Pilarski NS, Markland AD, Marson EJ, Devall A, Hewison M, et al. Vitamin D and miscarriage: a systematic review and meta-analysis. Fertil Steril. 2022;118(1):111–22.
Rosenfeld T, Salem H, Altarescu G, Grisaru-Granovsky S, Tevet A, Birk R. Maternal–fetal vitamin D receptor polymorphisms significantly associated with preterm birth. Arch Gynecol Obstet. 2017;296:215–22.
Al-Daghri NM, Mohammed AK, Al-Attas OS, Ansari MGA, Wani K, Hussain SD, et al. Vitamin D receptor gene polymorphisms modify cardiometabolic response to vitamin D supplementation in T2DM patients. Sci Rep. 2017;7(1):8280.
Radzinsky V, Ramazanova F, Khamoshina M, Azova M, Orazov M, Orazmuradov A. Vitamin D insufficiency as a risk factor for reproductive losses in miscarriage. Gynecol Endocrinol. 2021;37(sup1):8–12.
Hirschhorn JN, Lohmueller K, Byrne E, Hirschhorn K. A comprehensive review of genetic association studies. Genet Med. 2002;4(2):45–61.
Acknowledgements
Not applicable.
Funding
No specific funding was sought for the study.
Author information
Authors and Affiliations
Contributions
AM, MA, and AA wrote the paper, and, along with YM, contributed to the design and analysis of the data, while SA and MGh gathered data and assisted in writing the manuscript. YM and AA critically reviewed it, YM gave final approval to the published version. All authors have read and given their approval to the manuscript. Every writer should have made significant input in the creation and design of the work; SA, MGh, and MA should have been involved in collecting, analyzing, and interpreting data, as well as developing new software for the project; AM must have prepared the initial draft and AA, YA should have made substantial revisions to it. YA and AA have given their approval to the version that was submitted (as well as any significantly modified versions that include the author's contributions to the study).
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study was reviewed and approved by the Research Ethics Committees of Kurdistan University of Medical Sciences Medicine. The project was found to be by the ethical principles and the national norms and standards for conducting Medical Research in Iran. Consent to participate was not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Moradkhani, A., Azami, M., Assadi, S. et al. Association of vitamin D receptor genetic polymorphisms with the risk of infertility: a systematic review and meta-analysis. BMC Pregnancy Childbirth 24, 398 (2024). https://doi.org/10.1186/s12884-024-06590-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12884-024-06590-0