Abstract
Background
Lamotrigine has become one of the most commonly prescribed antiseizure medications (ASM) in epileptic women during pregnancy and therefore requires regular updates regarding its safety. The aim of this study was to estimate the association between in utero exposure to lamotrigine monotherapy and the occurrence of neurodevelopmental outcomes.
Methods
All comparative studies assessing the occurrence of neurodevelopmental outcomes after epilepsy-indicated lamotrigine monotherapy exposure during pregnancy were searched. First, references were identified through a snowballing approach, then, through electronic databases (Medline and Embase) from 2015 to June 2022. One investigator evaluated study eligibility and extracted data and a second independent investigator reviewed the meta-analysis (MA). A systematic review and random-effects model approach were performed using a collaborative WEB-based meta-analysis platform (metaPreg.org) with a registered protocol (osf.io/u4gva).
Results
Overall, 18 studies were included. For outcomes reported by at least 4 studies, the pooled odds ratios and 95% confidence interval obtained with the number of exposed (N1) and unexposed children (N0) included were: neurodevelopmental disorders as a whole 0.84 [0.66;1.06] (N1 = 5,271; N0 = 22,230); language disorders or delay 1.16 [0.67;2.00] (N1 = 313; N0 = 506); diagnosis or risk of ASD 0.97 [0.61;1.53] (N1 = at least 5,262; N0 = 33,313); diagnosis or risk of ADHD 1.14 [0.75;1.72] (N1 = at least 113; N0 = 11,530) and psychomotor developmental disorders or delay 2.68 [1.29–5.56] (N1 = 163; N0 = 220). The MA of cognitive outcomes included less than 4 studies and retrieved a significant association for infants exposed to lamotrigine younger than 3 years old but not in the older age groups.
Conclusion
Prenatal exposure to lamotrigine monotherapy is not found to be statistically associated with neurodevelopmental disorders as a whole, language disorders or delay, diagnosis or risk of ASD and diagnosis or risk of ADHD. However, the MA found an increased risk of psychomotor developmental disorders or delay and cognitive developmental delay in less than 3 years old children. Nevertheless, these findings were based exclusively on observational studies presenting biases and on a limited number of included children. More studies should assess neurodevelopmental outcomes in children prenatally exposed to lamotrigine.
Similar content being viewed by others

Background
There is a growing interest for neurodevelopmental consequences after in utero exposure to antiseizure medications (ASM). However, long-term outcomes are rarely addressed in pregnancy safety studies [1]. Although a higher occurrence of adverse neurodevelopmental outcomes has been identified for valproate [1, 2] and more recently for topiramate [3], the risks for other ASM remain unclear or have not yet been assessed [1, 4]. In the last few decades, these specific concerns, as well as the risk of teratogenicity, has led to changes in prescription practices from older to newer ASM. Lamotrigine has thus been increasingly prescribed in pregnant women worldwide and is now the most frequently used ASM along with levetiracetam [5, 6]. Although there is extensive data on the malformative risk associated with lamotrigine use [7,8,9,10], there is still a lack of evidence regarding neurodevelopmental outcomes in the scientific literature.
In 2017, for the first time, a network meta-analysis (MA) [2] raised concerns about in utero exposure to lamotrigine and the risk of autism diagnoses. Since the release of this network MA, a large number of new studies investigating neurodevelopmental outcomes have been published [3, 11,12,13,14,15,16,17,18]. These concerns therefore need to be reassessed.
The purpose of the present work was to update the knowledge on the neurodevelopmental consequences of prenatal exposure to lamotrigine monotherapy in women with epilepsy by means of a systematic review and MA.
Methods
A systematic review and MA were conducted to assess the association between in utero exposure to lamotrigine monotherapy and the occurrence of neurodevelopmental disorders. This study was reported in accordance with the standards of the Cochrane collaboration [19, 20] using MOOSE (Meta-analyses Of Observational Studies in Epidemiology) and PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines when appropriate.
Data management and analyses were conducted using metaPreg (metaPreg.org), a proprietary collaborative WEB-based MA platform. The master protocol was established before starting the study, was registered in open science framework (osf.io/u4gva), is available on the metaPreg website (http://metapreg.org/doc/protocol.pdf) and was published in a peer-reviewed journal [21].
Study identification
A therapeutic class approach was used in order to improve comprehensiveness. All drugs of the antiepileptic class were searched to also identify studies that included lamotrigine but that did not index it individually. The relevant studies were identified by a two steps process: (i) through a snowballing approach to identify relevant papers based on the reference lists of published meta-analyses and/or systematic reviews; (ii) with the search of two electronic databases (Medline and Embase) starting from the last publication search date of the most recent MA [2] (i.e. 2015) until June 2022 (Supplement S1).
Criteria for considering studies
All studies with a non-treated comparator group, assessing the association between lamotrigine monotherapy use in pregnant women with epilepsy and neurodevelopmental outcomes in offspring were eligible. Studies assessing several antiseizure medications, without non-treated comparator, were excluded because there is no antiseizure medication with sufficient reassuring data to be used as an active comparator.
Types of studies
Prospective cohort studies, historical cohort studies (also known as retrospective cohort studies), case–control studies, and randomized clinical trials were included. Studies were included regardless of publication status or language of publication. In case of iterative studies using the same database, only the most recent publication was included. In case of overlapping data (different publications using the same dataset, on the same study period, to assess the same outcome), only the one with the largest sample size or with a methodology that provided a better consideration of the confounding factors was kept. If a new study overlaps partially the previous one, the both were included only if the majority of the information is not common, i.e. the new study overlaps less than 50% of previous study period.
Types of exposure
The exposure was restricted to monotherapy in order to clearly identify the contribution of lamotrigine and avoid confounding by other ASM. Secondly, only pregnancies exposed to lamotrigine for an epilepsy indication were included to maintain a relative homogeneity in terms of confounding factors (notably comorbidities) and in the doses used, which may differ according to the indication; when the indication was not specified, the study was not included. No selection was made on the period of exposure during pregnancy, because no specifically relevant exposure period has been previously identified.
Types of outcomes
The neurodevelopmental outcomes of interest were: neuro-developmental disorders considered as a whole by authors (i.e. including several cognitive and behavioral disorders as a whole); language disorders or delay; psychomotor developmental disorders or delay; diagnosis or risk of attention deficit / hyperactivity disorder (ADHD); diagnosis or risk of autism spectrum disorder (ASD); cognitive developmental delay (at < 3 years old, 3–6 years old, and > 6 years old); severe cognitive developmental delay (at 3–6 years old and > 6 years old); and learning disorders. The MA for each outcome of interest are presented but the sensitivity analyses, risk-of-bias assessment, and publication bias assessment were only performed for the outcomes reported by at least four studies.
Since neurodevelopmental disorders cover a wide range of outcomes assessed by numerous scales and tools, a correspondence matrix was developed with the help of a child psychiatrist and a psychiatrist to aggregate the different measurement tools available with as much clinical relevance as possible (Supplement S2).
If the same neurodevelopmental outcome was assessed for several ages in the same children, the results obtained at the oldest age were preferred (and thus used for the MA) because the disorder could be considered as better established [22, 23]. Moreover, if the same outcome was assessed using different scales, the results obtained with the most reliable one were used (for example, a confirmed clinical diagnosis > tests performed by practitioners > parent-reported screening tools).
Study selection
Study selection was a two-stage process. First, the abstracts of all the studies identified in the above search were screened by one reviewer (AP) assisted by automation tools based on artificial intelligence (metaPreg.org) [24]. Then, the full-text reports of potentially relevant studies were assessed by the same reviewer (AP), to establish whether they fulfilled the inclusion criteria. In case of doubt regarding the inclusion of a study, the matter was discussed with the scientific directors of the project (JC, CP, and MC) until agreement was reached. The process of study selection was documented and reported using a PRISMA flow diagram. The feasibility and acceptance of the semi-automated process and single-screening approach were assessed and globally, it was shown that this process reduces the time required for a MA without altering expert confidence in the methodological and scientific rigor [24, 25].
Data extraction and analyses
Information regarding study description, methods, and results were extracted from the included studies using a standardized electronic data collection form on a WEB-based collaborative MA platform (metaPreg.org) (Supplement S3). Authors were contacted if information was missing or unclear.
When available, the adjusted effect measures (odds ratio (OR), risk ratio, or hazard ratio) from the included studies were used for the analyses. When not available, the crude ORs were used. If the study did not report any OR, they were calculated with the raw data provided by the authors. Cognitive assessments scored with dichotomous criteria were preferred, otherwise, continuous scales were considered and transformed to an OR according to the formula recommended by the Cochrane handbook [26].
Some studies considered different comparator groups and provided estimates for each. In the main analysis, the type of control group was chosen in the following preferred order: (i) pregnant women with epilepsy or history of epilepsy not exposed to ASM during pregnancy (but that could have received treatment prior to pregnancy), referred to as “unexposed, women with epilepsy”; (ii) pregnant women not exposed to ASM but whose health status (presence or absence of disease) was unspecified or not known, called “unexposed (general population or not otherwise specified (NOS))”; and (iii) pregnant non-epileptic women not exposed to ASM, defined as “unexposed, disease free”.
Three sensitivity analyses according to (i) the type of study (cohort or case-control), (ii) the type of comparison group (“unexposed, women with epilepsy” or “unexposed, disease free or unspecified”), and (iii) adjustment performed by the included studies (Yes/No) were undertaken to investigate sources of heterogeneity for the outcomes reported by at least four studies.
After completing the MA, a quality control process was conducted by a second independent reviewer (CP) who checked every item, including missing details. Disagreements were discussed among the biocurators until resolution or during a project meeting.
Assessment of risk of bias in included studies
For outcomes reported by at least four studies, the risk of bias of the included studies was assessed and presented at an outcome-level using the Cochrane Risk of Bias Tool for Non-Randomized Studies of Interventions (ROBINS-I) [27]. The ROBINS-I tool signaling questions were adapted to observational studies evaluating medicine safety in pregnancy. Six types of bias were considered: (a) selection bias, (b) confounding bias, (c) bias in classification of exposure, (d) bias due to missing data, (e) bias in measurement outcomes, and (f) bias in selection of reported results. The ROBINS-I item on bias due to deviations from intended interventions was considered to be specific to randomized clinical trials and not applicable to observational studies. For confounding bias, four levels of risk were considered: low, moderate, serious, and critical. For the other biases, only three levels of risk were considered (low, moderate, and critical) as no situation where a degree of fineness between critical and serious was identified. For each bias type, an additional unclear category was available, when the data reported was insufficient to allow assignment to the aforementioned categories.
One biocurator (AP) performed the assessment and if any doubts occurred, they were resolved by consensus during a multidisciplinary team meeting. The studies were not excluded on the grounds of risk of bias.
Statistical analysis
The data from observational studies were used to perform random-effects MA. The summary effect size with a pooled OR and its 95% confidence interval (95% CI) was estimated using the inverse variance method based on the DerSimonian and Laird random-effects model. The MA was performed by using only the summary data and no attempt was made to obtain individual patient data. Forest plots were produced using the meta package of the R language for outcomes reported by at least four studies.
The assessment of heterogeneity was performed by means of the I-squared statistic [28] and tau-squared test. The random-effects model was selected a priori, whatever the heterogeneity, in order to consider both within-study and between-study variation by incorporating the heterogeneity of effects into the overall analyses. The publication bias and small study effect were assessed using the funnel plot for outcomes reported by at least four studies and using the Egger’s test [29] when at least ten studies were included. The trim and fill method was used to determine the number of missing studies and to adjust for publication bias [30].
All the data used in this work are stored in the collaborative WEB-based MA platform (metaPreg.org) and available on request.
Results
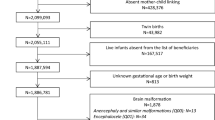
Overall, 744 records were screened, 493 of which were identified through the literature search of electronic databases and 251 from other sources (previously published MA and websites). Of the 294 full-text reports assessed for eligibility, 18 studies [3, 11,12,13,14,15,16,17,18, 31,32,33,34,35,36,37,38,39] concerned neurodevelopmental disorders after in utero exposure to lamotrigine monotherapy in women with epilepsy and were included in the MA (Fig. 1).
The other full-text reports mainly documented other outcomes, other indications, an ASM other than lamotrigine, or were duplicates (Supplement S4). The details of the 18 included studies are available in Table 1.
Among the considered outcomes, the following were reported by at least four studies: overall neurodevelopmental disorders (defined as several cognitive and behavioral disorders as a whole, without distinction between the disorders), language disorders or delay, psychomotor developmental disorders or delay, diagnosis or risk of ASD, and diagnosis or risk of ADHD. The other outcomes were reported by less than four studies: cognitive developmental delay (at < 3 years old, 3–6 years old, and > 6 years old); severe cognitive developmental delay (at 3–6 years old and > 6 years old); and learning disorders.
Meta-analyses of neurodevelopmental disorders reported by at least four studies
An overview of the MA results for each outcome reported by at least four studies are presented in Table 2.
The risk of neurodevelopmental disorders considered as a whole by authors (i.e. including several cognitive and behavioral disorders as a whole) was reported in five studies [3, 12, 34, 37, 39]. The MA did not show a statistically significant association (pooled OR 0.84, 95% CI [0.66;1.06]; I² = 0%; tau²=0; n = 5,271 and 22,230 exposed and unexposed children respectively; Fig. 2a). The trim and fill method reported a very similar OR (Supplement S5a). A sensitivity analysis on the type of study could not be performed as all studies were cohorts (n = 5). The majority of the included studies used unexposed women with epilepsy as comparator (n = 4) and did not perform any adjustment (n = 4) (Supplement S6a).
The risk of language disorders or delay was assessed in seven studies [13,14,15,16,17, 31, 37]. There was no statistically significant increase in the occurrence of language disorders or delay (pooled OR 1.16, 95% CI [0.67;2.00]; I²=58%; tau²=0.29; n = 313 and 506 exposed and unexposed children respectively; Fig. 2b). After trim and fill, the pooled OR was similar (Supplement S5b). In the sensitivity analyses, when the type of control was restricted to untreated epileptic women, the results were consistent with the pooled OR (0.96, 95% [CI 0.51;1.80]; k = 2; I²=0) of the main analysis. The sensitivity analysis on the type of study could not be performed (n = 7 cohorts). Only one study performed an adjustment, but two of the remaining six studies performed a matching (Supplement S6b).
Four studies [13, 17, 37, 39] reported the risk for psychomotor developmental disorders or delay for which the MA found a statistically significant association (pooled OR 2.68, 95% CI [1.29;5.56]; I²=9%; tau²=0.07; n = 163 and 220 exposed and unexposed children respectively; Fig. 2c). Publication bias could not be assessed with Egger’s test and the trim and fill method was not applied because the funnel plot appeared symmetrical, meaning no publication bias was detected (Supplement S5c). The sensitivity analyses according to adjustment and type of studies could not be performed as all studies were homogenous. Although no results were adjusted, one study did use matching. In the sensitivity analysis on the type of comparator, a loss of statistical significance was observed in the sub-analysis using the comparator “unexposed, women with epilepsy” (n = 1 study, pooled OR 2.68, 95% CI [0.10;68.88]; I²=NA; Supplement S6c).
The diagnosis or risk of ADHD was reported in four studies [18, 37,38,39]. The pooled OR did not reach statistical significance (pooled OR 1.14, 95% CI [0.75;1.72]; I²=0%; tau²=0; n = at least 113 and 11,530 exposed and unexposed children respectively; Fig. 2d). The funnel plot did not lead to a trim and fill; no publication bias was detected (Supplement S5d). The sensitivity analysis according to the type of studies could not be performed. A majority of the included studies used unexposed women with epilepsy as comparator (n = 3) and no adjustment (n = 3); the pooled ORs were similar to that of the main analysis (Supplement S6d).
Five studies [3, 11, 13, 18, 39] addressed the diagnosis or risk of ASD. The MA showed no statistically significant association (pooled OR 0.97, 95% CI [0.61;1.53]; I²=30%; tau²=0.08; n = at least 5262 and 33,313 exposed and unexposed children respectively; Fig. 2e). No publication bias was highlighted by the funnel plot (Supplement S5e.). All included studies were cohorts meaning this parameter was not contributive to the sensitivity analysis. The majority of the included studies used unexposed women with epilepsy as comparator (n = 4); the pooled OR remained similar to that of the main analysis. Two studies performed an adjustment and among the three studies that did not, one performed a matching (Supplement S6e). Lastly, the pooled OR remained similar to that of the main after exclusion of the 2 studies with a partial overlapping with Bjørk et al. 2022 (Wiggs et al. 2020 and Bjørk et al. 2018): pooled OR 0.81, 95% CI [0.59;1.11] (I2 = 0% n = 5,186 exposed; 21,743 unexposed children).
The risk-of-bias was assessed using the ROBINS-I tool at an outcome-level. Overall, the confounding bias was frequently rated as critical/serious or unclear and other types of bias were considered low or moderate in at least 50% of the included studies (Supplement S7).
Meta-analyses of neurodevelopmental disorders reported by less than four studies
An overview of the MA results for each outcome reported by less than four studies are presented in Table 2.
The cognitive developmental delay in infants under 3 years of age was investigated in two studies [17, 32]. In the MA including a total of 42 exposed infants, a statistically significant increase was obtained (pooled OR 3.42, 95% CI [1.17;9.98]; I²=0%; tau²=0; n = 42 and 86 exposed and unexposed children respectively). In older children, the risk was not statistically increased according to the MA carried out on 79 in utero exposed children aged 3–6 years old in three studies [15, 33, 37] (pooled OR 3.39, 95% CI [0.56;20.57]; I²=68%; tau²=1.70; n = 79 and 146 exposed and unexposed children respectively) and on 113 in utero exposed children older than 6 years of age in three studies [13, 31, 36] (pooled OR 0.75, 95% CI [0.41;1.37]; I²=0%; tau²=0; n = 113 and 124 exposed and unexposed children respectively). Two studies [3, 33] specifically evaluated severe cognitive developmental delay in 3–6 years old children and more than 6 years old children. For both, no statistically significant increase was obtained.
Learning disorders were assessed by two studies [13, 35]. A total of 125 in utero exposed children were evaluated, and the pooled OR was not statistically significant (pooled OR 1.45, 95% CI [0.82;2.54]; I²=0%; tau²=0; n = 125 and 224 exposed and unexposed children respectively).
Discussion
The present systematic review and MA mainly assessed 5 outcomes investigated by at least 4 studies. Prenatal exposure to lamotrigine monotherapy in children born to women with epilepsy is not found to be statistically associated with neurodevelopmental disorders as a whole, language disorders or delay, diagnosis or risk of ASD and diagnosis or risk of ADHD, with a number of exposures ranging from 113 to 5271, depending on the outcome. On the contrary, the MA found that psychomotor developmental disorders were significantly associated with in utero exposure to lamotrigine monotherapy used for epilepsy. Cognitive developmental disorders and learning disorders have also been assessed but by fewer studies, and the MA reported a statistically significant increase of cognitive developmental disorders in prenatally lamotrigine-exposed infants younger than 3 years old but no significant association in the older age groups.
Psychomotor developmental disorders or delay
The present MA retrieved a statistically significant association between psychomotor developmental disorders or delay and prenatal exposure to lamotrigine. To our knowledge, no other MA in the literature has reported this association. The 2017 network MA [2] reported a non-statistically significant pooled OR lower than the estimate herein (1.86 95%CI [0.72;4.76]; I² unknown; k unknown). Although the risk estimates remained similar in the sensitivity analyses according to the type of control group performed herein, the associations did not remain statistically significant, likely due to a loss of statistical power.
Interestingly, among the two studies who reported a significantly increased OR for psychomotor developmental disorders or delay, one study [37] showed only low or moderate risk for every type of bias assessed. The other study [17] was the only one to assess the risk of psychomotor developmental disorders or delay in very young infants (7 months old versus at least 4 years old in the other studies). This is intriguing as the sensitivity for detecting psychomotor developmental disorders or delay is known to increase with age [41]. As a child grows, he/she develops more complex skills and disorders and delays may thus appear in areas for which they were previously in line with their peers [16]. The study by Videman et al. [17] may thus reveal the most severe cases that can occur at younger ages. Nevertheless, a longer follow-up of the 7 months old infants might have allowed the authors to report a more constituted and accurate diagnosis [16]. Finally, it is important to note that only 163 in utero exposed children were assessed overall in the four included studies, further underlying the need for additional investigations to be carried out in order to clarify the association between psychomotor developmental disorders or delay and prenatal exposure to lamotrigine.
Cognitive developmental delay
A total of 42 in utero exposed children were assessed in the two studies included in the MA. A cognitive developmental delay in infants younger than 3 years old was found to be significantly increased in lamotrigine-exposed pregnancies compared to controls. However, no significant association was found in the older age groups. A similar finding was reported in a study by Meador et al. [42], in which mean cognitive scores were rated by assessors using the differential ability scales (DAS) in children followed between the ages of 2, 3, 4, 5, and 6 years: Intelligence quotient (IQ) measures of children exposed to lamotrigine appeared to improve over time. When parents are informed of a cognitive delay diagnosis in their young child, they can be referred to intervention programs [42]. Eventually, children will then catch up and reach their age-standards as they grow, which is conceivable if the delay was not too severe. Unfortunately, the present analysis cannot corroborate whether the less than 3 years old children were severely delayed. Nevertheless, two studies [3, 33] did not report a statistically increased risk of severe delay in older children. The possibility of an incidental finding cannot be ruled out given that only 42 in utero exposed children less than 3 years old were evaluated.
Autism spectrum disorder
In a previously published network MA [2], lamotrigine was associated with an increased occurrence of autism (traits and diagnosis combined) but no other neurodevelopmental defect. The same conclusion was not observed herein perhaps because of the methodological differences between the two MA protocols, notably the inclusion of polytherapies and the exclusion of outcomes reported as continuous variables in the MA published by Veroniki et al. in 2017 [2]. Moreover, since the release of this network MA, a large number of new studies investigating ASD were published [3, 11, 13, 18] and included in the MA herein (four out of the five studies included in the present MA were published after the publication of the network MA [2]).
Strengths and limitations
Overall, the interpretation of the results was limited by the biases highlighted with the ROBINS-I tool. Confounding factors were not usually given enough consideration even though numerous prenatal, perinatal, and postnatal characteristics are known risk factors for neurodevelopmental disorders such as underlying genetic component, parental IQ, prematurity, socio-economic status, maternal age, etc. [31, 43,44,45]. These were either partially or not at all considered and may have thus impacted the findings. Furthermore, some countries don’t provide a basic healthcare system and that can generate disparities between countries in their ability to detect specific neurodevelopmental outcomes. This was taken into account by downgrading the risk-of-bias rating for selection bias and bias in outcome measurement.
Moreover, because no antiseizure medication has sufficient data regarding safety on neurodevelopmental outcomes after prenatal exposure, we chose to not consider an active comparator, excluding studies that compare antiseizure medications with each other. Nevertheless, the results obtained for lamotrigine should be considered in light with results for other antiseizure medications.
Because of the large number of scales and questionnaires measuring neurodevelopmental outcomes reported in the literature, we chose to build a neurodevelopmental correspondence matrix by aggregating different measurement tools. This was done in collaboration with psychiatrists (LJ and MN) to ensure clinical relevance. If the measurement tools considered for a single type of neurodevelopmental outcome had been too heterogeneous, this would have invalidated any clinical interpretation by oversimplifying the data and not taking into account possible subtleties. Conversely, if the correspondence matrix had been too discriminating, it would not have been possible to aggregate enough data. Nevertheless, the combination of data obtained in children of different ages and/or with different scales could introduce heterogeneity that should be discussed if necessary. Importantly, the developed matrix was found to be reliable and valuable for the classification of disorders assessed by tests in the present study. The use of the matrix was also a strength as it allowed to discard measurement tools that were identified as too weak or incomplete to fully assess the neurodevelopmental outcome of interest. This means that assessments obtained with tools deemed inadequate or insufficient were not included in the MA, thus avoiding dilution of the evidence collected with less reliable information.
Another strength of this MA was the exclusion of studies assessing lamotrigine for the treatment of other indications than epilepsy (e.g., bipolar disorders). This clinical homogeneity may have contributed to better capture baseline risks associated with neurodevelopment, therefore limiting confounding factors. Since the present work aimed to assess the potential functional deficits triggered by lamotrigine, only monotherapy interventions were included. This allowed the MA results to not be biased by the potential or proven neurodevelopmental impact of other ASM.
Neurodevelopmental outcomes are rarely addressed in the literature [1] partly because of the extensive follow-up needed. This leads to small sample sizes in studies that limit the ability to detect associations. Therefore, using an MA approach was a strength to study neurodevelopmental outcomes. In addition, the MA herein is currently the most up-to-date and therefore provides the latest state of the art in this field. Finally, the outcomes reported by at least four studies were not subject to publication bias according to the funnel plots.
Implication for clinical practice
In case of pregnancy in a woman with epilepsy, and preferably when a pregnancy is planned, the benefit-risk of an ASM treatment must be re-evaluated. Although lamotrigine is increasingly prescribed [4, 46], the present MA underlines the fact that there is still limited evidence regarding its impact on neurodevelopmental outcomes in children prenatally exposed to lamotrigine monotherapy. Larger studies assessing children with long-term follow-up are required to closely monitor neurodevelopmental outcomes. Given the high use of lamotrigine and the emerging evidence regarding its impact on neurodevelopmental outcomes, the results of the present MA call for special attention to be directed towards these children.
Conclusion
Prenatal exposure to lamotrigine monotherapy in children born to women with epilepsy is not found to be statistically associated with neurodevelopmental disorders as a whole, language disorders or delay, diagnosis or risk of ASD and diagnosis or risk of ADHD. On the contrary, the MA found an increased risk of psychomotor developmental disorders or delay and cognitive developmental delay in less than 3 years old children (but not in the older age groups) after in utero exposure to lamotrigine monotherapy. Nevertheless, these findings were based exclusively on observational studies presenting biases and were limited by the small number of children included. There is still limited evidence regarding the impact of prenatal exposure to lamotrigine on neurodevelopmental outcomes. More studies should assess the risk of neurodevelopmental outcomes in children prenatally exposed to lamotrigine.
Availability of data and materials
The datasets generated and/or analysed during the current study are available in the metaPreg repository at metapreg.org, and are available from the corresponding author on reasonable request.
Abbreviations
- ADHD:
-
Attention Deficit / Hyperactivity Disorder
- ASD:
-
Autism Spectrum Disorder
- ASM:
-
Antiseizure Medications
- BMI:
-
Body Mass Index
- DAS:
-
Differential Ability Scales
- IQ:
-
Intelligence Quotient
- MA:
-
Meta-analysis
- NOS:
-
Not Otherwise Specified
- OR:
-
Odd Ratio
- WWE:
-
Women With Epilepsy
References
Bromley R, Weston J, Adab N, Greenhalgh J, Sanniti A, McKay AJ, et al. Treatment for epilepsy in pregnancy: neurodevelopmental outcomes in the child. Cochrane Database Syst Rev. 2014;2014(10):CD010236. https://doi.org/10.1002/14651858.CD010236.pub2. Cité 5 Août 2020.
Veroniki AA, Rios P, Cogo E, Straus SE, Finkelstein Y, Kealey R et al. Comparative safety of antiepileptic drugs for neurological development in children exposed during pregnancy and breast feeding: a systematic review and network meta-analysis. BMJ Open. 2017;7(7). Disponible sur: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5642793/. Cité 4 Sept 2020.
Bjørk MH, Zoega H, Leinonen MK, Cohen JM, Dreier JW, Furu K, et al. Association of prenatal exposure to antiseizure medication with risk of autism and intellectual disability. JAMA Neurol. 2022. Disponible sur: https://jamanetwork.com/journals/jamaneurology/fullarticle/2793003. Cité 1 Juin 2022.
Knight R, Wittkowski A, Bromley RL. Neurodevelopmental outcomes in children exposed to newer antiseizure medications: a systematic review. Epilepsia. 2021;62(8):1765–79.
Cohen JM, Cesta CE, Furu K, Einarsdóttir K, Gissler M, Havard A, et al. Prevalence trends and individual patterns of antiepileptic drug use in pregnancy 2006–2016: a study in the five nordic countries, United States, and Australia. Pharmacoepidemiol Drug Saf. 2020;29(8):913–22.
Wen X, Meador KJ, Hartzema A. Antiepileptic drug use by pregnant women enrolled in Florida Medicaid. Neurology. 2015;84(9):944–50.
Campbell E, Kennedy F, Russell A, Smithson WH, Parsons L, Morrison PJ, et al. Malformation risks of antiepileptic drug monotherapies in pregnancy: updated results from the UK and Ireland Epilepsy and pregnancy registers. J Neurol Neurosurg Psychiatry. 2014;85(9):1029–34.
Hernández-Díaz S, Smith CR, Shen A, Mittendorf R, Hauser WA, Yerby M, et al. Comparative safety of antiepileptic drugs during pregnancy. Neurol. 2012;78(21):1692–9.
Tomson T, Battino D, Bonizzoni E, Craig J, Lindhout D, Perucca E, et al. Comparative risk of major congenital malformations with eight different antiepileptic drugs: a prospective cohort study of the EURAP registry. Lancet Neurol. 2018;17(6):530–8.
Vajda FJE, Graham JE, Hitchcock AA, Lander CM, O’Brien TJ, Eadie MJ. Antiepileptic drugs and foetal malformation: analysis of 20 years of data in a pregnancy register. Seizure. 2019;65:6–11.
Bjørk M, Riedel B, Spigset O, Veiby G, Kolstad E, Daltveit AK, et al. Association of Folic Acid Supplementation During Pregnancy With the Risk of Autistic Traits in Children Exposed to Antiepileptic Drugs In Utero. JAMA Neurol. 2018;75(2):160.
Charlton RA, McGrogan A, Snowball J, Yates LM, Wood A, Clayton-Smith J, et al. Sensitivity of the UK Clinical Practice Research Datalink to Detect Neurodevelopmental effects of Medicine exposure in Utero: comparative analysis of an antiepileptic drug-exposed cohort. Drug Saf. 2017;40(5):387–97.
Cohen-Israel M, Berger I, Martonovich EY, Klinger G, Stahl B, Linder N. Short- and long-term complications of in utero exposure to lamotrigine: complications of in utero lamotrigine. Br J Clin Pharmacol. 2018;84(1):189–94.
Husebye ESN, Gilhus NE, Spigset O, Daltveit AK, Bjørk MH. Language impairment in children aged 5 and 8 years after antiepileptic drug exposure in utero – the Norwegian mother and child cohort study. Eur J Neurol. 2020;27(4):667–75.
Kasradze S, Gogatishvili N, Lomidze G, Ediberidze T, Lazariashvili M, Khomeriki K, et al. Cognitive functions in children exposed to antiepileptic drugs in utero - study in Georgia. Epilepsy Behav. 2017;66:105–12.
Meador KJ, Cohen MJ, Loring DW, May RC, Brown C, Robalino CP, et al. Two-year-old cognitive outcomes in children of pregnant women with Epilepsy in the maternal outcomes and Neurodevelopmental effects of antiepileptic drugs study. JAMA Neurol. 2021;78(8):927.
Videman M, Stjerna S, Roivainen R, Nybo T, Vanhatalo S, Gaily E, et al. Evidence for spared attention to faces in 7-month-old infants after prenatal exposure to antiepileptic drugs. Epilepsy Behav. 2016;64:62–8.
Wiggs KK, Rickert ME, Sujan AC, Quinn PD, Larsson H, Lichtenstein P, et al. Antiseizure medication use during pregnancy and risk of ASD and ADHD in children. Neurol. 2020;95(24):e3232–3240.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of Observational studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–12.
Picot C, Ajiji P, Jurek L, Nourredine M, Massardier J, Peron A, et al. Risk of drug use during pregnancy: master protocol for living systematic reviews and meta-analyses performed in the metaPreg project. Syst Rev. 2023;12(1):101.
Benedetto L, Cucinotta F, Maggio R, Germanò E, De Raco R, Alquino A, et al. One-year Follow-Up Diagnostic Stability of Autism Spectrum Disorder Diagnosis in a clinical sample of children and toddlers. Brain Sci. 2021;11(1):37.
Jansen R, Maljaars J, Zink I, Steyaert J, Noens I. The complexity of early diagnostic decision making: a follow-up study of young children with language difficulties. Autism Dev Lang Impair. 2021;6:2396941520984894.
Ajiji P, Cottin J, Picot C, Uzunali A, Ripoche E, Cucherat M, et al. Feasibility study and evaluation of expert opinion on the semi-automated meta-analysis and the conventional meta-analysis. Eur J Clin Pharmacol. 2022;78(7):1177–84.
Waffenschmidt S, Sieben W, Jakubeit T, Knelangen M, Overesch I, Bühn S, et al. Increasing the efficiency of study selection for systematic reviews using prioritization tools and a single-screening approach. Syst Rev. 2023;14(1):161.
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA. Cochrane handbook for systematic reviews of interventions version 6.4 (updated August 2023). Cochrane; 2023. Available from: https://www.training.cochrane.org/handbook. Disponible sur: https://training.cochrane.org/handbook. Cité 6 Sept 2022.
Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919.
Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58.
Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34.
Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455–63.
Baker GA, Bromley RL, Briggs M, Cheyne CP, Cohen MJ, Garcia-Finana M, et al. IQ at 6 years after in utero exposure to antiepileptic drugs: a controlled cohort study. Neurol. 2015;84(4):382–90.
Bromley RL, Mawer G, Love J, Kelly J, Purdy L, McEwan L, et al. Early cognitive development in children born to women with epilepsy: a prospective report. Epilepsia. 2010;51(10):2058–65.
Cummings C, Stewart M, Stevenson M, Morrow J, Nelson J. Neurodevelopment of children exposed in utero to lamotrigine, sodium valproate and carbamazepine. Arch Dis Child. 2011;96(7):643–7.
Dean J, Robertson Z, Reid V, Wang QD, Hailey H, Moore S, et al. Fetal anticonvulsant syndromes and polymorphisms in MTHFR, MTR, and MTRR. Am J Med Genet A. 2007;143A(19):1.
Elkjær LS, Bech BH, Sun Y, Laursen TM, Christensen J. Association Between Prenatal Valproate Exposure and Performance on Standardized Language and Mathematics Tests in School-aged Children. JAMA Neurol. 2018;75(6):663.
Gopinath N, Muneer AK, Unnikrishnan S, Varma RP, Thomas SV. Children (10–12 years age) of women with epilepsy have lower intelligence, attention and memory: observations from a prospective cohort case control study. Epilepsy Res Nov. 2015;117:58–62.
Rihtman T, Parush S, Ornoy A. Developmental outcomes at preschool age after fetal exposure to valproic acid and lamotrigine: cognitive, motor, sensory and behavioral function. Reprod Toxicol. 2013;41:115–25.
Veiby G, Daltveit AK, Schjølberg S, Stoltenberg C, Øyen AS, Vollset SE, et al. Exposure to antiepileptic drugs in utero and child development. Epilepsia. 2013;54(8):1462–72.
Bromley RL, Mawer GE, Briggs M, Cheyne C, Clayton-Smith J, Garcia-Finana M, et al. The prevalence of neurodevelopmental disorders in children prenatally exposed to antiepileptic drugs. J Neurol Neurosurg Psychiatry. 2013;84(6):637–43.
Bromley RL, Mawer G, Clayton-Smith J, Baker GA. Liverpool and Manchester Neurodevelopment Group. Autism spectrum disorders following in utero exposure to antiepileptic drugs. Neurol. 2008;71(23):1923–4.
Wide K, Winbladh B, Tomson T, Sars-Zimmer K, Berggren E. Psychomotor development and minor anomalies in children exposed to antiepileptic drugs in utero: a prospective population-based study. Dev Med Child Neurol. 2000;42(2):87–92.
Meador KJ, Baker GA, Browning N, Cohen MJ, Bromley RL, Clayton-Smith J, et al. Fetal antiepileptic drug exposure and cognitive outcomes at age 6 years (NEAD study): a prospective observational study. Lancet Neurol. 2013;12(3):244–52.
Cénat JM, Blais-Rochette C, Morse C, Vandette MP, Noorishad PG, Kogan C, et al. Prevalence and risk factors Associated with Attention-Deficit/Hyperactivity Disorder among US Black Individuals: a systematic review and Meta-analysis. JAMA Psychiatry. 2021;78(1):21–8.
Cogley C, O’Reilly H, Bramham J, Downes M. A systematic review of the risk factors for Autism Spectrum Disorder in Children Born Preterm. Child Psychiatry Hum Dev. 2021;52(5):841–55.
Wang C, Geng H, Liu W, Zhang G. Prenatal, perinatal, and postnatal factors associated with autism: a meta-analysis. Med (Baltimore). 2017;96(18):e6696.
Meador KJ, Penovich P, Baker GA, Pennell PB, Bromfield E, Pack A, et al. Antiepileptic drug use in women of childbearing age. Epilepsy Behav Juill. 2009;15(3):339–43.
Acknowledgements
We thank Véréna Landel (Direction de la Recherche en Santé, Hospices Civils de Lyon) for her help in manuscript preparation.
Funding
This research received a specific grant from the French National Agency for the Safety of Medicines and Health Products. The funding body did not influence the design of the study and collection, analysis and interpretation of data and the writing of the manuscript.
Author information
Authors and Affiliations
Contributions
MC, JC, CP, PA and AP for the study protocol; LJ, MN, CP and AP for building the neurodevelopmental correspondence matrix; CP for data reviewing; AP for screening eligibility, data extraction and risk-of-bias assessment. All authors were involved in the analysis and interpretation of the data and read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable. Patient consent for publication is not required because this work, involving meta-analysis of previously published studies, is exempt from direct ethical review board approval.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Peron, A., Picot, C., Jurek, L. et al. Neurodevelopmental outcomes after prenatal exposure to lamotrigine monotherapy in women with epilepsy: a systematic review and meta-analysis. BMC Pregnancy Childbirth 24, 103 (2024). https://doi.org/10.1186/s12884-023-06242-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12884-023-06242-9