Abstract
Introduction
Pregabalin is an antiepileptic drug frequently prescribed to pregnant women. Risks of adverse birth and postnatal neurodevelopmental outcomes following prenatal exposure to pregabalin are uncertain.
Objective
To investigate the association between prenatal exposure to pregabalin and the risks of adverse birth and postnatal neurodevelopmental outcomes.
Methods
This study was conducted using population-based registries in Denmark, Finland, Norway, and Sweden (2005–2016). We compared pregabalin exposure against no exposure to antiepileptics and against active comparators lamotrigine and duloxetine. We obtained pooled propensity score-adjusted estimates of association using fixed-effect and Mantel–Haenszel (MH) meta-analyses.
Results
The total number of pregabalin-exposed births was 325/666,139 (0.05%) in Denmark, 965/643,088 (0.15%) in Finland, 307/657,451 (0.05%) in Norway, and 1275/1,152,002 (0.11%) in Sweden. The adjusted prevalence ratios (aPRs) with 95% confidence interval (CI) following pregabalin exposure versus no exposure were 1.14 (0.98–1.34) for major congenital malformations and 1.72 (1.02–2.91) for stillbirth, which attenuated to 1.25 (0.74–2.11) in MH meta-analysis. For the remaining birth outcomes, the aPRs were close to or attenuated toward unity in analyses using active comparators. Adjusted hazard ratios (95% CI) contrasting prenatal pregabalin exposure versus no exposure were 1.29 (1.03–1.63) for ADHD and attenuated when using active comparators, 0.98 (0.67–1.42) for autism spectrum disorders, and 1.00 (0.78–1.29) for intellectual disability.
Conclusions
Prenatal exposure to pregabalin was not associated with low birth weight, preterm birth, small for gestational age, low Apgar score, microcephaly, autism spectrum disorders, or intellectual disability. On the basis of the upper value of the 95% confidence interval, increased risks greater than 1.8 were unlikely for any major congenital malformation and ADHD. For stillbirth and most groups of specific major congenital malformations, the estimates attenuated in MH meta-analysis.
Similar content being viewed by others
Prenatal pregabalin exposure compared with no exposure showed a 1.1-fold association (95% CI 0.98–1.32) with the prevalence of any major congenital malformation among live and stillborn offspring, and a 1.7-fold association (95% CI 1.02–2.91) with stillbirth; the association was attenuated in Mantel–Haenszel meta-analysis for the latter outcome. |
We found no association between prenatal pregabalin exposure and risks of birth outcomes other than major congenital malformations or stillbirth. |
Prenatal exposure to pregabalin compared with no exposure to pregabalin and active comparators showed a 1.3-fold association (95% CI 1.03–1.63) with attention deficit/hyperactivity disorder; the association was attenuated in the analyses using active comparators and Mantel–Haenszel meta-analysis. There was no evidence of an association with other examined neurodevelopmental postnatal outcomes. |
1 Introduction
Pregabalin is an antiepileptic drug (AED) prescribed to approximately 0.5 per 1000 pregnant women in Europe, with increasing use after 2010 [2, 3]. Approved indications for pregabalin treatment in the European Union (EU) are epilepsy, neuropathic pain, and generalized anxiety disorder (GAD) [4]. In the general population in the UK (2004–2009), pregabalin was most frequently prescribed for neuropathic pain (18–98%) and least frequently for epilepsy (4–6%) [5]. Similarly in Sweden (2005–2009), indication for pregabalin treatment was primarily neuropathic pain (36%), while GAD (3.6%) and epilepsy (1.3%) were an indication less frequently [6].
A study using data from European Network of Teratology Information Services (ENTIS) collected in 2004–2013 and based on 164 pregabalin-exposed versus unexposed infants reported an associated threefold increased risk of any major non-chromosomal congenital malformation [7]. Limitations of that study included lack of data on specific malformations, selection and detection bias inherent in the data source due to participants’ self-referral, and imprecise estimates [8]. A combined study on the basis of 477 pregabalin-exposed pregnancies resulting in a live birth among Medicaid beneficiaries (2000–2010), 174 privately insured (MarketScan) women with pregabalin-exposed births in the USA, and the earlier study conducted by ENTIS [7], showed pooled risk ratio (RR) of 1.33 [95% confidence interval (CI) 0.83–2.15] for an association between any first-trimester exposure to pregabalin and major congenital malformations when compared with no exposure to pregabalin or other AEDs [9]. First-trimester pregabalin monotherapy compared with no AED use resulted in pooled RR 1.02 (95% CI 0.69–1.51) [9]. However, a French cohort study (2011–2015) on the basis of 1671 pregnancies with exposure to pregabalin monotherapy versus no AED exposure showed a nearly sixfold increased risk of coarctation of aorta [10]. Several other studies suggested an association between in-pregnancy exposure to pregabalin versus lamotrigine or versus no exposure to pregabalin and adverse pregnancy and birth outcomes [11, 12]. The investigation of prenatal exposure to antiepileptics and postnatal neurodevelopmental outcomes showed no association comparing prenatal pregabalin exposure versus lamotrigine [13] and versus no exposure to antiepileptics [14]. However, in the study by Blotière and colleagues [13] the median ages of children in the cohort were below the average age of detection for many of the neurodevelopmental diagnoses, and studies replicating this result were lacking. Confounding by indication is a major concern since most of the earlier studies compared exposure to pregabalin with no exposure to AED.
The aim of this study was to examine the association between prenatal exposure to pregabalin and the risks of major congenital malformations, other adverse birth outcomes, and postnatal neurodevelopmental outcomes, compared in four separate analyses with active comparators (lamotrigine, duloxetine, and lamotrigine and/or duloxetine) and with no exposure to pregabalin, other AED, or active comparators. This non-interventional study was a post-authorization safety (PAS) study conducted as a commitment to the European Medicines Agency (EUPAS27339).
2 Methods
2.1 Setting and Study Design
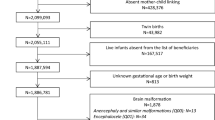
We performed a population-based study using all births identified in the respective medical birth registers from 1 January 2005 to 31 December 2015 in Denmark, Finland, and Norway and from 1 July 2006 to 31 December 2016 in Sweden. In all countries we excluded births with exposure to medications known as potentially teratogenic during the first pregnancy trimester and births of a fetus with a chromosomal abnormality diagnosis (Supplementary Table 1); in Norway, stillbirths before week 22 were excluded. Data were obtained from the national health databases, including birth registries, patient registries, psychiatric registries, and prescription registries, and linked at secure servers of the participating countries [15,16,17,18,19,20,21,22]. For the birth outcomes, only children surviving until birth could be analyzed, and therefore, prevalence design was used [23]. Cohort design was used for the postnatal outcomes by following children from birth until the earliest outcome of interest, death, or emigration [23].
2.2 Exposure
In main analyses, we defined first-trimester pregabalin exposure as at least one outpatient dispensing of pregabalin from last menstrual period (LMP) − 90 days to LMP + 97 days (both dates inclusive) and any-trimester pregabalin exposure as at least one dispensing of pregabalin from LMP − 90 days to the day before date of birth. In sensitivity analyses, we defined monotherapy pregabalin exposure as at least one dispensing of pregabalin and no dispensing of any other AED or active comparators during the specified period (first trimester or any trimester). We used first-trimester pregabalin exposure for major congenital malformations outcome and any-trimester pregabalin exposure for the remaining outcomes.
2.3 Comparators
Pregnancies without dispensing of pregabalin, any other AED, and any of the active comparators formed the cohort with no exposure to AED.
The active comparator drugs were lamotrigine and duloxetine, based on their safety profile during pregnancy.[24,25,26] Similarly to pregabalin, lamotrigine is indicated for epilepsy, while duloxetine is indicated for GAD and neuropathic pain. In the main analyses, we defined births exposed to lamotrigine, duloxetine, and either lamotrigine, duloxetine, or both combined (lamotrigine and/or duloxetine) when at least one prescription was redeemed in the first pregnancy trimester for major congenital malformation investigation or any pregnancy trimester for the remaining outcome investigation. In the sensitivity analyses, prenatal exposure to active comparators was defined as at least one dispensing of lamotrigine alone, duloxetine alone, or both duloxetine and lamotrigine and no dispensing of any other antiepileptic drugs. For analyses that used lamotrigine as the comparator, pregnancies exposed to both pregabalin and lamotrigine were excluded. For analyses that used duloxetine as the comparator, pregnancies exposed to both pregabalin and duloxetine were excluded.
2.4 Outcomes
Birth outcomes were major congenital malformations, stillbirth, low birth weight, preterm birth, being small for gestational age (SGA), low Apgar score at 5 min, and microcephaly (Supplementary Table 2). Major congenital malformations were divided into overall and specific according to the EUROCAT classification using International Classification of Diseases, 10th revision (ICD-10) codes [27, 28] and were ascertained among live and stillbirths from the date of birth until 1 year of age to allow for delay in detection of diagnoses using the medical birth registry in Norway and the national patient registries in Denmark, Finland, and Sweden [28]. We ascertained stillbirths from the national medical birth registries.
We classified infants’ weight at birth below 2500 g as low birth weight and preterm birth as deliveries before 37 weeks of gestation. The newborns were classified as SGA if the birth weight was less than two standard deviations (SD) of sex- and completed gestational week-specific mean according to country-specific standards [29,30,31]. Apgar score at 5 min below 7 was defined as low.
Postnatal neurodevelopmental outcomes were attention deficit/hyperactivity disorder (ADHD), autism spectrum disorder (ASD), and intellectual disability. We identified ADHD, ASD, and intellectual disability on the basis of the diagnostic records at the inpatient or outpatient clinic specialist visit. For ADHD, we additionally used a proxy via dispensed ADHD medication (Supplementary Table 2).
2.5 Covariables
Data on all covariables were available for at least 12 months before the end of the earliest identified pregnancy in each participating country, with the exception of Norway, where data from the national patient registry were not available before 2008. We ascertained maternal pre-LMP comorbidities using diagnostic codes from the national patient registries recorded according to the International Classification of Diseases, 10th revision (ICD-10) in all countries, except for identification of congenital malformations in Finland, which were recorded according to the International Classification of Diseases, 9th revision (ICD-9). We ascertained maternal pre-LMP medication use from the national prescription registers using the Anatomical Therapeutic Chemical (ATC) codes (Supplementary Tables 1 and 2).
The potential confounders we identified were the calendar year of delivery, age in years at LMP, marital/cohabiting status, smoking during pregnancy, obesity [body mass index (BMI) ≥ 30 kg/m2 before pregnancy or a diagnosis of obesity], single or multiple gestation, comorbidity history, indicators of maternal healthcare utilization in the 12 months pre-LMP (number of inpatient and specialized outpatient encounters) and maternal medication use history defined by at least one dispensing in pregnancy (Supplementary Table 2).
We reported cesarean delivery, children’s sex distributions and distributions of potential indications for pregabalin use inferred by recorded diagnoses in the 12 months pre-LMP period without adjusting for them in the analyses.
2.6 Statistical Analyses
We conducted analyses separately in each country based on a common protocol, which was registered in the EU PAS register before the start of the data collection (EUPAS27339). We reported characteristics of pregnancies and births according to the exposure status. For the birth outcomes, we computed crude and adjusted prevalence ratios (aPRs) with 95% CIs using robust cluster-adjusted or generalized estimating equation approach for offspring with prenatal exposure to pregabalin versus comparators using log-binomial regression. For postnatal neurodevelopmental outcomes, we computed crude and adjusted hazard ratios (HRs) with robust cluster-adjusted 95% CIs using Cox proportional-hazards regression and followed live born children for a minimum of 1 year and a maximum of 10 years in Denmark and Finland, and 12 years in Norway and Sweden, until the earliest of event of interest, emigration (except Norway), death, or the study end (31 December 2017 in Sweden and Norway; 31 December 2016 in Denmark and Finland).
We used propensity score (PS) fine stratification to adjust for confounding [9, 32]. We computed PS using logistic regression as the probability of being exposed to pregabalin versus each of comparator drugs, conditional on the prespecified covariables, separately for first-trimester and any-trimester exposure [9, 32,33,34,35]. We fitted a distinct PS model for each country and contrast. We trimmed the non-overlapping areas of PS distribution and defined PS fine strata on the basis of the PS distribution among births prenatally exposed to pregabalin. Further, we classified the comparators into fine strata on the basis of their PS values and assigned stratum-specific weights to comparator births while assigning the weight of 1 to all births prenatally exposed to pregabalin. Finally, we performed weighted regression analyses to computed PS-adjusted PRs and HRs.
For every outcome, we pooled country-specific relative risk estimates in a meta-analysis with fixed effects for different countries [36]. Since several analyses in the individual countries showed zero pregabalin-exposed events, we conducted a post-hoc analysis using Mantel–Haenszel (MH) pooling allowing to retain information from strata with no pregabalin-exposed events. Missing data were relatively rare (< 5%) except for smoking status and BMI in Norway. We did not impute missing values. Data management and analyses were conducted using SAS version 9.3 or later and R version 3.1.1 or later.
2.7 Ethics
The study received all required approvals or was reported to the national data authority, according to local requirements of the participating Nordic countries. This study was reported to the Danish Data Protection Agency [1] through registration at Aarhus University (record number 2016-051-000001, sequential number 544). In Finland, the study received approval by Ethics Committee of the Hospital District of Helsinki and Uusimaa (HUS/887/2018). In Norway, the study received approval by Regional Committee for Medical and Health Research Ethics (2017/1507/REK vest) and by the Norwegian Data Protection Authority (17/01659-2/CDG). In Sweden, the study received approval by the Regional Ethical Review Board in Stockholm (reference numbers 2015/1826-31/2, 2017/2238-32, and 2018/1790-32).
3 Results
The total number of pregabalin-exposed from all pregnancies ending in a live birth or stillbirth was 325/666,139 (0.05%) in Denmark, 965/643,088 (0.15%) in Finland, 307/657,451 (0.05%) in Norway, and 1275/1,152,002 (0.11%) in Sweden (Supplementary Figs. 1–4). The distribution of potential indications for pregabalin use differed by country, with GAD being the most prevalent potential indication in Finland (21.7%), Norway (8.5%), and Sweden (43.6%) and neuropathic pain in Denmark (11.7%) (Table 1 and Supplementary Table 4). Prevalence of maternal smoking among offspring with exposure to pregabalin, lamotrigine, duloxetine, and no exposure to AED was 28–40%, 14–28%, 17–28%, and 6–15%, respectively. Most of the comorbidities and medication use were markedly more prevalent among offspring with prenatal exposure to pregabalin versus no exposure to AED. Covariable distribution among offspring with prenatal exposure to pregabalin was more similar to that among offspring with exposure to the active comparators than those with no AED exposure (Supplementary Table 5). However, there were appreciable differences in the distribution of pre-pregnancy history of epilepsy, neuropathic pain, GAD, depression, and alcohol and drug abuse or dependence, as well as antidepressant, hypnotic, and antipsychotic use among offspring with prenatal exposure to pregabalin versus active comparators (Table 1, Supplementary Table 5).
The PS distribution among pregabalin-exposed and unexposed or active comparators largely overlapped. The trimming across all conducted analyses resulted in elimination of, at most, three pregabalin-exposed observations. Among pregabalin-unexposed and active comparators cohorts, trimming resulted in post-trimming population size of 74% of that before trimming for the contrasts of pregabalin-exposed versus lamotrigine-exposed. For contrasts with other comparators, post-trimming populations constituted 80–98% of the pre-trimming populations.
3.1 Major Congenital Malformations
The pooled prevalence of any major congenital malformation among births with first-trimester exposure to pregabalin was 6%, while among comparators it varied between 4% for offspring with no AED exposure and 5% for the offspring with prenatal first-trimester lamotrigine and/or duloxetine exposure. The MH pooled aPR (95% CI) for any major congenital malformation among births with first-trimester exposure to pregabalin versus offspring with no AED exposure, versus lamotrigine, versus duloxetine, and versus lamotrigine and/or duloxetine was 1.13 (0.98–1.32), 1.27 (1.04–1.55), 1.42 (1.12–1.79), and 1.27 (1.05–1.53), respectively (Table 2). The MH pooled aPR for eye malformations was 1.88 (95% CI 1.01–3.49) among offspring with first-trimester exposure to pregabalin versus offspring with no exposure to AED. In analyses of offspring with exposure to pregabalin versus lamotrigine, the following MH pooled aPRs (95% CIs) were observed: 3.20 (0.86–12.00) for malformations of the nervous system, 2.53 (1.09–5.86) for urinary, and 1.94 (0.97–3.89) for genital organs. In analyses of offspring with exposure to pregabalin versus duloxetine, MH pooled aPRs (95% CIs) were 2.04 (0.86–4.87) for urinary organs malformations and 2.69 (1.09–6.65) for genital malformations. In analyses of offspring with exposure to pregabalin versus lamotrigine and/or duloxetine, MH pooled aPR (95% CI) for genital malformations was 2.03 (1.06–3.87). The aPR for all site-specific congenital malformations were based on small number of events leading to imprecise and several nonreportable estimates (Table 2).
3.2 Other Birth Outcomes
For stillbirth, the pooled prevalence among births with any-trimester exposure to pregabalin was 0.5%, while among active comparators it varied between 0.3 and 0.4%. For low birth weight, the pooled prevalence among births with exposure to pregabalin was 6.3%, while among comparators it varied between 4.0% and 6.3%. For preterm birth, the pooled prevalence among births with exposure to pregabalin was 8.0%, while it varied between 5.0% among AED unexposed and 8.4% among active comparators. For SGA, the pooled prevalence among births with exposure to pregabalin was 4.6%, while it was 4.0% among comparators. For low Apgar score, the pooled prevalence among births with exposure to pregabalin was 2.9%, while it varied between 1.3% among AED unexposed and 2.2% among active comparators. For microcephaly, the pooled prevalence among births with exposure to pregabalin was 2.5%, while it was 2% among active comparators.
For stillbirth, MH pooled aPR (95% CI) was 1.25 (0.74–2.11) among offspring with any-trimester exposure to pregabalin versus AED unexposed, 1.30 (0.69–2.47) versus lamotrigine, 1.11 (0.44–2.82) versus duloxetine, and 1.93 (0.98–3.78) versus lamotrigine and/or duloxetine (Table 3). For preterm birth, SGA, and low Apgar score, MH pooled aPRs were 1.12 (0.99–1.27), 1.10 (0.90–1.36), and 1.16 (0.94–1.44), respectively, for offspring with exposure to pregabalin versus no exposure to AED; the estimates attenuated toward the null value in the analyses using active comparators. For the remaining adverse birth outcomes (low birth weight and microcephaly), the aPRs were close to unity in the comparisons of offspring prenatally exposed to pregabalin versus AED unexposed or versus active comparators (Table 3).
3.3 Postnatal Neurodevelopmental Outcomes
For ADHD, the pooled incidence rate was 48 per 10,000 person-years among births with any-trimester exposure to pregabalin, while among comparators it varied between 20 per 10,000 person-years among offspring with no exposure to AED and 30–35 per 10,000 person-years among active comparators. For ASD, the pooled incidence rate was 17 per 10,000 person-years among births with any-trimester exposure to pregabalin, while among comparators it varied between 20 per 10,000 person-years among offspring with no exposure to AED and 30–35 per 10,000 person-years among active comparators. For intellectual disability, the pooled incidence rate was 30 per 10,000 person-years among births with any-trimester exposure to pregabalin, while among comparators it varied between 25 per 10,000 person-years among offspring with no exposure to AED and 43 per 10,000 person-years among active comparators.
The MH pooled aHR (95% CI) for ADHD was 1.22 (0.97–1.54) in the analyses of offspring with any-trimester exposure to pregabalin versus no exposure to AED, 1.06 (0.80–1.42) in the comparison with lamotrigine, and 1.04 (0.74–1.45) in the comparison with duloxetine. There was no association between prenatal exposure to pregabalin and ASD or intellectual disability outcomes across all pooled analyses (Table 4). The results of the analyses restricted to pregabalin monotherapy showed findings similar to the main analyses for all outcomes (Supplementary Table 6).
4 Discussion
4.1 Key Results
In this registry-based study in four Nordic countries, after adjusting for maternal characteristics and concomitant medications during pregnancy, the risk of major congenital malformations among live and stillborn offspring prenatally exposed to pregabalin was increased 1.3–1.4-fold in comparison with lamotrigine and duloxetine, respectively; however, estimates were imprecise and absolute prevalences were small. Additionally, the comparison with no exposure to AED resulted in an estimate close to the null value [PR (95% CI) 1.13 (0.98–1.32)] suggesting residual confounding in the contrasts of pregabalin exposure with active comparators. We found 1.1–1.9-fold increased risk of stillbirth following prenatal exposure to pregabalin versus no exposure to AED and versus active comparators; however, estimates were based on a small number of events. There was a 1.2–1.3-fold increased risk of SGA and ADHD among offspring with prenatal exposure to pregabalin versus no exposure to AED. Of note, these associations attenuated toward unity in analyses using active comparators and at least partly can be explained by residual confounding. We found no evidence of an association between prenatal exposure to pregabalin and increased risk of low birth weight, SGA, preterm birth, low Apgar score at 5 min, microcephaly, ASD, or intellectual disability.
4.2 Major Congenital Malformations and Other Adverse Birth Outcomes
Results of this study are in line with one previous US study using US Medicaid Analytic eXtract data [9], which showed the relative risk of major congenital malformations among offspring with exposure to pregabalin versus no AED exposure was 1.16 (95% CI 0.81–1.67). We found an up to 1.4-fold increased risk of any major congenital malformation among offspring with exposure to pregabalin versus active comparators, while the increased risk was less pronounced in the comparison with no exposure to AED. The use of active comparators is desirable in pharmacoepidemiologic studies to reduce the confounding by indication. Active comparators used in this study have indications overlapping with those of pregabalin, but are not identical to it, and thus may not fully eliminate confounding by indication.
We cannot fully rule out an association between prenatal exposure to pregabalin and increased risk of eye, urinary, and genital malformations. We performed post hoc analyses in Sweden, where the increased risk of eye malformations was found. The aPR (95% CI) of eye malformations was 2.71 (1.22–6.02) at 1 year of follow-up, 1.12 (0.53–2.34) at 2 years, 1.13 (0.56–2.25) at 3 years, 1.09 (0.55–2.18) at 4 years, and 1.19 (0.62–2.28) at 5 years of follow-up. Surveillance bias due to differential healthcare follow-up for offspring with exposure to pregabalin versus no exposure to AED resulting in earlier diagnoses of eye or other specific malformations among those exposed could explain this finding. Several earlier studies found an increased risk of SGA following prenatal exposure to pregabalin [11, 12]. However, in this study the magnitude of the associations between pregabalin and adverse birth outcomes, in general, was small and the estimates were imprecise. On the basis of the upper limit of the 95% CI, the MH meta-analyses ruled out the associations for prenatal exposure to pregabalin versus no AED exposure greater than 1.4–2.6 for most groups of specific major congenital malformations.
Animal studies on developmental toxicity after fetal exposure to pregabalin are not consistent, with at least one study reporting no teratogenic effect in rats or rabbits even at high doses [37], and one study showing teratogenic effect in rats at therapeutic doses [38].
4.3 Postnatal Neurodevelopmental Outcomes
We found a 1.2-fold association between prenatal exposure to pregabalin and ADHD when compared with no exposure to AED. Despite adjusting for a large number of maternal and pregnancy-related characteristics, we cannot rule out confounding by indication. After propensity score adjustment, several potential confounding factors remained unbalanced for the contrast of offspring with exposure to pregabalin versus no exposure to AED. These factors were the use of analgesics, antipsychotics, antidepressants, and a history of depression and other neurological disorders. This highlights the potential of confounding by indication for this contrast. While prenatal exposure to valproate is associated with 1.5-fold increased risk of ADHD [39], in the present study, analyses using active comparators resulted in attenuated association with ADHD in accordance with another Nordic study, which similarly found no association between pregabalin and neurodevelopmental disorders in offspring [14]. The validity of neurodevelopmental outcomes is, in general, reasonably high in the Nordic registries and few false positives are expected [15, 40,41,42]. For example, in the Danish registry capturing psychiatric diagnoses, the positive predictive values for ADHD and autism coding were 87% [43] and 94% [44], respectively.
Moreover, the mean children’s age at the neurodevelopmental outcomes is similar across participating countries. For example, the mean age at ADHD diagnosis is 8 years in Denmark [45], 7.5 years in Finland [46], 10.5 years in Norway [47], and 12 years in Sweden [48]. In this study, the mean age at ADHD diagnosis was 8 years in all exposure groups in Denmark and 6–7 years in Finland, Norway, and Sweden (Supplementary Table 7). The mean age at other neurodevelopmental diagnoses in this study was similar to previously published data, including the mean age of 4–6 years at intellectual disability diagnosis, and 6–7 years for ASD diagnosis [45, 46].
4.4 Strengths and Limitations
We used population-based healthcare registries from the Nordic countries with free universal health care and exact linkage between the maternal and the offspring records. The data in the Nordic registries are collected routinely and prospectively, reducing the risk of selection bias. The present study did not evaluate the cumulative dose of prenatal exposure to pregabalin or active comparators. Except for major congenital malformations, the timing of exposure in pregnancy and duration of the exposure was also not evaluated. The non-differential misclassification of exposure is expected to bias the study results toward the null. Although misclassification of exposure is still possible since the true intake of pregabalin and comparators is unknown, filling of prescriptions of medicines represent a better proxy of drug intake than issued prescriptions, minimizing non-compliance in drug pick-up. Additionally, for drugs used chronically, there is a good level of agreement between general practice data and dispensing records [49].
The clinician evaluating the child for the neurodevelopmental outcome may be aware of the offspring’s status regarding prenatal exposure to pregabalin or active comparators. Although such detection bias cannot be ruled out in the present study, it is unlikely to explain the results suggesting no association between prenatal exposure to pregabalin and ASD and intellectual disability, and may in part explain the results suggesting an association with ADHD. Children with neurodevelopmental conditions experiencing subdiagnostic symptoms may be missed in the present study. It is likely that the population of children with clinically detectable neurodevelopmental conditions represents a more severe spectrum of the neurodevelopmental conditions.
Although we were able to adjust for numerous potential confounders using PS fine stratification [9, 32], residual confounding by indication cannot be ruled out. Indication for the use of medication is not directly available from the dispensing data. The diagnoses of epilepsy, neuropathic pain, and GAD were collected from the hospital registries as a proxy for the potential indication. At the baseline, offspring with prenatal exposure to pregabalin differed considerably from AED-unexposed and offspring with prenatal exposure to active comparator drugs. These differences included uneven distribution of indication for prescribed AED, maternal history of psychiatric disorders including depression, and other neurological disorders. The history of analgesics use was more prevalent among offspring with prenatal exposure to pregabalin versus lamotrigine, and folic acid use was less prevalent among offspring with prenatal exposure to pregabalin versus lamotrigine. These important measured differences among offspring with prenatal exposure to pregabalin versus comparators indicate the possibility of residual and unmeasured confounding, including confounding by familial history of neurodevelopmental outcomes.
Another possibility for residual confounding in this study is differential underdiagnosis of neuropsychiatric disorders, e.g., ADHD, among women with pregabalin-exposed pregnancies. Women with ADHD [50] are more likely to be smokers, to have a higher BMI, and to have a history of anxiety than women from the general population. It is possible that offspring with pregabalin exposure had a higher proportion of mothers with undiagnosed ADHD [50]. Given heritability of ADHD [51], children of mothers with ADHD may be at a higher risk of developing ADHD during the follow-up. This may at least partly explain non-null association between prenatal exposure to pregabalin and ADHD found in this study. Differential underreporting of smoking in pregnancy by exposure status in the Nordic Birth Registries cannot be ruled out and could contribute to residual confounding; however, the proportion of false-positive smokers in both pregabalin-exposed and comparators is expected to be small [52, 53].
Any major congenital malformation was a composite outcome, which allowed increased statistical efficiency, however, it does not warrant a causal interpretation. The estimates for specific major congenital malformations and stillbirth had low precision due to the low number of accrued events, and showed no pattern of an increased risk for any group of specific major congenital malformations among births with prenatal exposure to pregabalin versus comparators. Although we cannot rule out the differential misclassification of malformations in respect to offspring exposure to pregabalin, the outcomes in this study have high validity and the proportion of false positives is small [15].
5 Conclusions
We found no evidence of an association between prenatal exposure to pregabalin and low birth weight, preterm birth, SGA, low Apgar score, and microcephaly when compared with no exposure to antiepileptic drugs or with active comparators. Similarly to the results of another large Nordic study, this study did not find evidence for an association between prenatal pregabalin exposure any time in pregnancy and increased risks of autism spectrum disorders or intellectual disability when compared with no exposure to antiepileptic drugs or with active comparators. Analyses of site-specific congenital malformations and stillbirths gained imprecise estimates due to low number of events, and the results did not suggest a pattern of specificity for congenital malformations. On the basis of the upper values of the 95% confidence intervals, increased risks in excess of 1.8 were unlikely for any major congenital malformation outcome and ADHD when compared with no exposure to antiepileptic drugs or with active comparators. Similarly, based on the upper values of the 95% confidence intervals, for stillbirth and most groups of specific major congenital malformations, increased risks in excess of 2.6 were unlikely in comparisons of prenatal exposure to pregabalin versus no AED exposure.
References
Datatilsynet. Datatilsynet/the danish data protection agency. Available from: http://www.datatilsynet.dk/english
Hurault-Delarue C, Morris JK, Charlton R, Gini R, Loane M, Pierini A, et al. Prescription of antiepileptic medicines including valproate in pregnant women: a study in three European countries. Pharmacoepidemiol Drug Saf. 2019;28:1510–8.
Daugaard CA, Sun Y, Dreier JW, Christensen J. Use of antiepileptic drugs in women of fertile age. Dan Med J. 2019;66(8):A5563.
EMA. Lyrica. European Medicines Agency. 2018. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/lyrica
Asomaning K, Abramsky S, Liu Q, Zhou X, Sobel RE, Watt S. Pregabalin prescriptions in the United Kingdom: a drug utilisation study of The Health Improvement Network (THIN) primary care database. Int J Clin Pract. 2016;70:380–8.
Wettermark B, Brandt L, Kieler H, Bodén R. Pregabalin is increasingly prescribed for neuropathic pain, generalised anxiety disorder and epilepsy but many patients discontinue treatment. Int J Clin Pract. 2014;68:104–10.
Winterfeld U, Merlob P, Baud D, Rousson V, Panchaud A, Rothuizen LE, et al. Pregnancy outcome following maternal exposure to pregabalin may call for concern. Neurology. 2016;86:2251–7 (Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology).
Ehrenstein V, Sørensen HT, Bakketeig LS, Pedersen L. Medical databases in studies of drug teratogenicity: methodological issues. Clin Epidemiol. 2010;2:37–43.
Patorno E, Bateman BT, Huybrechts KF, MacDonald SC, Cohen JM, Desai RJ, et al. Pregabalin use early in pregnancy and the risk of major congenital malformations. Neurology. 2017;88:2020–5.
Blotière P-O, Raguideau F, Weill A, Elefant E, Perthus I, Goulet V, et al. Risks of 23 specific malformations associated with prenatal exposure to 10 antiepileptic drugs. Neurology. 2019;93:e167–80 (Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology).
Margulis AV, Hernandez-Diaz S, McElrath T, et al. Relation of in-utero exposure to antiepileptic drugs to pregnancy duration and size at birth. PLOS ONE. Public Library of Science; 2019;14:e0214180.
Mostacci B, Poluzzi E, D’Alessandro R, Cocchi G, Tinuper P. Adverse pregnancy outcomes in women exposed to gabapentin and pregabalin: data from a population-based study. J Neurol Neurosurg Psychiatry. BMJ Publishing Group Ltd; 2018;89:223–224.).
Blotière P-O, Miranda S, Weill A, Mikaeloff Y, Peyre H, Ramus F, et al. Risk of early neurodevelopmental outcomes associated with prenatal exposure to the antiepileptic drugs most commonly used during pregnancy: a French nationwide population-based cohort study. BMJ Open. 2020;10: e034829 (British Medical Journal Publishing Group).
Bjørk M-H, Zoega H, Leinonen MK, Cohen JM, Dreier JW, Furu K, et al. Association of prenatal exposure to antiseizure medication with risk of autism and intellectual disability. JAMA Neurol. 2022. https://doi.org/10.1001/jamaneurol.2022.1269.
Laugesen K, Ludvigsson JF, Schmidt M, Gissler M, Valdimarsdottir UA, Lunde A, et al. Nordic health registry-based research: a review of health care systems and key registries. CLEP Dove Press. 2021;13:533–54.
Schmidt M, Pedersen L, Sorensen HT. The Danish Civil Registration System as a tool in epidemiology. Eur J Epidemiol. 2014;29:541–9.
Schmidt M, Schmidt SA, Sandegaard JL, Ehrenstein V, Pedersen L, Sorensen HT. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449–90.
Mors O, Perto GP, Mortensen PB. The Danish psychiatric central research register. Scand J Public Health. 2011;39:54–7.
Wettermark B, Zoëga H, Furu K, Korhonen M, Hallas J, Nørgaard M, et al. The Nordic prescription databases as a resource for pharmacoepidemiological research—a literature review. Pharmacoepidemiol Drug Saf. 2013;22:691–9.
Ludvigsson JF, Otterblad-Olausson P, Pettersson BU, Ekbom A. The Swedish personal identity number: possibilities and pitfalls in healthcare and medical research. Eur J Epidemiol. 2009;24:659–67.
Cnattingius S, Ericson A, Gunnarskog J, Källén B. A quality study of a medical birth registry. Scandinavian J Soc Med. 1990;18:143–8 (SAGE Publications).
Irgens LM. The Medical Birth Registry of Norway. Epidemiological research and surveillance throughout 30 years. Acta Obstet Gynecol Scand. 2000;79:435–9.
Lash TL, Rothman KJ, VanderWeele TJ, Haneuse S. Modern epidemiology. 4th ed. Wolters Kluwer; 2020.
Moore JL, Aggarwal P. Lamotrigine use in pregnancy. Expert opinion on pharmacotherapy. Taylor Francis. 2012;13:1213–6.
Lassen D, Ennis ZN, Damkier P. First-trimester pregnancy exposure to venlafaxine or duloxetine and risk of major congenital malformations: a systematic review. Basic Clin Pharmacol Toxicol. 2016;118:32–6.
Huybrechts KF, Bateman BT, Pawar A, Bessette LG, Mogun H, Levin R, et al. Maternal and fetal outcomes following exposure to duloxetine in pregnancy: cohort study. BMJ. 2020;368:m237 (British Medical Journal Publishing Group).
Kinsner-Ovaskainen A, Lanzoni M, Garne E, Loane M, Morris J, Neville A, et al. A sustainable solution for the activities of the European network for surveillance of congenital anomalies: EUROCAT as part of the EU Platform on Rare Diseases Registration. Eur J Med Genet. 2018;61:513–7.
EUROCAT, University of Ulster. EUROCAT Guide 1.4: Instruction for the registration of congenital anomalies. EUROCAT Central Registry; 2013. Available from: https://eu-rd-platform.jrc.ec.europa.eu/sites/default/files/Full_Guide_1_4_version_28_DEC2018.pdf.
Sankilampi U, Hannila M-L, Saari A, Gissler M, Dunkel L. New population-based references for birth weight, length, and head circumference in singletons and twins from 23 to 43 gestation weeks. Ann Med. 2013;45:446–54.
Glinianaia SV, Skjærven R, Magnus P. Birthweight percentiles by gestational age in multiple births. Acta Obstet Gynecol Scand. 2000;79:450–8.
Maršál K, Persson P-H, Larsen T, Lilja H, Selbing A, Sultan B. Intrauterine growth curves based on ultrasonically estimated foetal weights. Acta Paediatr. 1996;85:843–8.
Desai RJ, Rothman KJ, Bateman BT, Hernandez-Diaz S, Huybrechts KF. A Propensity-score-based fine stratification approach for confounding adjustment when exposure is infrequent. Epidemiology. 2017;28:249–57.
Williamson E, Morley R, Lucas A, Carpenter J. Propensity scores: from naïve enthusiasm to intuitive understanding. Stat Methods Med Res. 2012;21:273–93 (SAGE Publications Ltd STM).
Wyss R, Girman CJ, LoCasale RJ, Alan Brookhart M, Stürmer T. Variable selection for propensity score models when estimating treatment effects on multiple outcomes: a simulation study. Pharmacoepidemiol Drug Saf. 2013;22:77–85.
Conover MM, Rothman KJ, Stürmer T, Ellis AR, Poole C, Jonsson FM. Propensity score trimming mitigates bias due to covariate measurement error in inverse probability of treatment weighted analyses: a plasmode simulation. Stat Med. 2021;40:2101–12.
Viale L, Allotey J, Cheong-See F, Arroyo-Manzano D, Mccorry D, Bagary M, et al. Epilepsy in pregnancy and reproductive outcomes: a systematic review and meta-analysis. Lancet. 2015;386:1845–52.
Morse DC. Embryo-fetal developmental toxicity studies with pregabalin in mice and rabbits. Birth Defects Res B. 2016;107:85–93.
Singh KP, Gupta K. Teratogenic effects of third-generation antiepileptic drug, pregabalin: an in vivo study. Curr Drug Saf. 2018;13:113–21.
Christensen J, Pedersen L, Sun Y, Dreier JW, Brikell I, Dalsgaard S. Association of prenatal exposure to valproate and other antiepileptic drugs with risk for attention-deficit/hyperactivity disorder in offspring. JAMA Netw Open. 2019;2: e186606.
Ludvigsson JF, Andersson E, Ekbom A, Feychting M, Kim J-L, Reuterwall C, et al. External review and validation of the Swedish national inpatient register. BMC Public Health. 2011;11:450.
Nesvåg R, Jönsson EG, Bakken IJ, Knudsen GP, Bjella TD, Reichborn-Kjennerud T, et al. The quality of severe mental disorder diagnoses in a national health registry as compared to research diagnoses based on structured interview. BMC Psychiatry. 2017;17:93.
Sund R. Quality of the Finnish Hospital Discharge Register: a systematic review. Scand J Public Health. 2012;40:505–15 (Sage Publications, Ltd.).
Mohr-Jensen C, Vinkel Koch S, Briciet Lauritsen M, Steinhausen H-C. The validity and reliability of the diagnosis of hyperkinetic disorders in the Danish Psychiatric Central Research Registry. Eur Psychiatry. 2016;35:16–24.
Lauritsen MB, Jørgensen M, Madsen KM, Lemcke S, Toft S, Grove J, et al. Validity of childhood autism in the Danish psychiatric central register: findings from a cohort sample born 1990–1999. J Autism Dev Disord. 2010;40:139–48.
Dalsgaard S, Thorsteinsson E, Trabjerg BB, Schullehner J, Plana-Ripoll O, Brikell I, et al. Incidence rates and cumulative incidences of the full spectrum of diagnosed mental disorders in childhood and adolescence. JAMA Psychiat. 2020;77:155–64 (American Medical Association).
Rocco I, Corso B, Bonati M, Minicuci N. Time of onset and/or diagnosis of ADHD in European children: a systematic review. BMC Psychiatry. 2021;21:575.
Duric NS, Elgen I. Characteristics of Norwegian children suffering from ADHD symptoms: ADHD and primary health care. Psychiatry Res. 2011;188:402–5.
Bahmanyar S, Sundström A, Kaijser M, von Knorring A-L, Kieler H. Pharmacological treatment and demographic characteristics of pediatric patients with Attention Deficit Hyperactivity Disorder, Sweden. Eur Neuropsychopharmacol. 2013;23:1732–8.
Johannesdottir SA, Horváth-Puhó E, Ehrenstein V, Schmidt, Pedersen L, Sørensen H. Existing data sources for clinical epidemiology: the Danish National Database of Reimbursed Prescriptions. CLEP. 2012;303-313.
Nörby U, Winbladh B, Källén K. Perinatal outcomes after treatment with ADHD medication during pregnancy. Pediatrics. 2017;140: e20170747.
Faraone SV, Larsson H. Genetics of attention deficit hyperactivity disorder. Mol Psychiatry. 2019;24:562–75.
Clemmensen KKB, Lynge E, Clemmensen IH. Nationwide tobacco surveys and sales data in Denmark from 1920 to 2010. Dan Med J. 2012;59:5.
Grøtvedt L, Egeland GM, Kvalvik LG, Madsen C. Evaluation of incomplete maternal smoking data using machine learning algorithms: a study from the Medical Birth Registry of Norway. BMC Pregnancy Childbirth. 2020;20:710.
Acknowledgements
We thank Pia Vattulainen (EPID Research Oy part of IQVIA) for her expertise in statistical analyses of Finnish registry-based data.
Funding
Open access funding provided by Royal Danish Library.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The study was funded by Pfizer Ltd through institutional research funding to and administered by Aarhus University. EPID Research Oy part of IQVIA was responsible for the study conducted in Finland funded by Pfizer Inc. through an Outcomes Research Services Agreement.
Conflicts of Interest
The funder contributed to the design of the study, the interpretation, reporting of results, or writing of the manuscript. All final decisions were made by the principal investigator.
Ethics Approval
The study received all required approvals or was reported to the national data authority according to local requirements of the participating Nordic countries. This study was reported to the Danish Data Protection Agency [1] through registration at Aarhus University (record number 2016-051-000001, sequential number 544). In Finland, the study received approval by Ethics Committee of the Hospital District of Helsinki and Uusimaa (HUS/887/2018). In Norway, the study received approval by Regional Committee for Medical and Health Research Ethics (2017/1507/REK vest) and by the Norwegian Data Protection Authority (17/01659-2/CDG). In Sweden, the study received approval by the Regional Ethical Review Board in Stockholm (reference numbers 2015/1826-31/2, 2017/2238-32, and 2018/1790-32).
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Availability of Data and Material
In the four Nordic countries participating in this study, the pseudonymized data were stored on and accessed via secure servers. For legal reasons and to protect individuals whose data were analyzed, the datasets used in this study will not be made public.
Code Availability
The code producing the datasets used in this study will not be made public to protect potential individual-level data.
Author Contribution
All authors contributed substantially to the study’s conception, design, and interpretation of results, and critically revised the manuscript for intellectual content. GT, VE, KA, AKD, KH, FH, HK, IO, JR, and LS conceptualized the study. SKS, MR, AL, and PK analyzed the data. ED and GT drafted the manuscript. All authors read and approved the final manuscript for submission.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Dudukina, E., Szépligeti, S.K., Karlsson, P. et al. Prenatal exposure to pregabalin, birth outcomes and neurodevelopment – a population-based cohort study in four Nordic countries. Drug Saf 46, 661–675 (2023). https://doi.org/10.1007/s40264-023-01307-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40264-023-01307-2