Abstract
Background
Growing evidence suggests low and high maternal hemoglobin (Hb) concentrations may have adverse consequences for maternal and child health. There remain questions on specific Hb thresholds to define anemia and high Hb as well as how cutoffs may vary by anemia etiology and timing of assessment.
Methods
We conducted an updated systematic review (using PubMed and Cochrane Review) on low (< 110 g/L) and high (≥ 130 g/L) maternal Hb concentrations and associations with a range of maternal and infant health outcomes. We examined associations by timing of Hb assessment (preconception; first, second, and third trimesters, as well as at any time point in pregnancy), varying cutoffs used for defining low and high hemoglobin concentrations and performed stratified analyses by iron-deficiency anemia. We conducted meta-analyses to obtain odds ratios (OR) and 95% confidence intervals.
Results
The updated systematic review included 148 studies. Low maternal Hb at any time point in pregnancy was associated with: low birthweight, LBW (OR (95% CI) 1.28 (1.22–1.35)), very low birthweight, VLBW (2.15 (1.47–3.13)), preterm birth, PTB (1.35 (1.29–1.42)), small-for-gestational age, SGA (1.11 (1.02–1.19)), stillbirth 1.43 (1.24–1.65)), perinatal mortality (1.75 (1.28–2.39)), neonatal mortality (1.25 (1.16–1.34), postpartum hemorrhage (1.69 (1.45–1.97)), transfusion (3.68 (2.58–5.26)), pre-eclampsia (1.57 (1.23–2.01)), and prenatal depression (1.44 (1.24–1.68)). For maternal mortality, the OR was higher for Hb < 90 (4.83 (2.17–10.74)) than for Hb < 100 (2.87 (1.08–7.67)). High maternal Hb was associated with: VLBW (1.35 (1.16–1.57)), PTB (1.12 (1.00-1.25)), SGA (1.17 (1.09–1.25)), stillbirth (1.32 (1.09–1.60)), maternal mortality (2.01 (1.12–3.61)), gestational diabetes (1.71 (1.19–2.46)), and pre-eclampsia (1.34 (1.16–1.56)). Stronger associations were noted earlier in pregnancy for low Hb and adverse birth outcomes while the role of timing of high Hb was inconsistent. Lower Hb cutoffs were associated with greater odds of poor outcomes; for high Hb, data were too limited to identify patterns. Information on anemia etiology was limited; relationships did not vary by iron-deficiency anemia.
Conclusion
Both low and high maternal Hb concentrations during pregnancy are strong predictors of adverse maternal and infant health outcomes. Additional research is needed to establish healthy reference ranges and design effective interventions to optimize maternal Hb during pregnancy.
Similar content being viewed by others
Background
Anemia remains a persistent global health problem, impacting over 269 million children, 571 million women of reproductive age and 32 million pregnant women [1]. While iron deficiency is considered one of the leading causes of anemia, the etiology of anemia, including the role of other micronutrient deficiencies, infections, inflammation or hemoglobinopathies, may vary widely by setting [1]. Anemia has adverse health and developmental outcomes across the lifespan including increased risk of hemorrhaging during birth; delayed child growth, cognition, and motor development; reduced physical work capacity in adults; and elevated morbidity and mortality among older populations [2]. Given the significance of the public health problem, there have been global calls to reduce anemia, particularly among the most vulnerable and high-risk population groups [3]. In 2020, the prevalence of anemia among pregnant and nonpregnant women was included as a key indicator for the UN Sustainable Development Goals (SDGs) [4]. The World Health Assembly called for a 50% reduction in anemia prevalence among women of reproductive age (15–49 years) by 2025, although this may be extended to 2030 [3, 5]. However, there has been insufficient progress and the prevalence of anemia has remained high among women of reproductive age (31% in 2000 to 30% in 2019) and pregnant women (41% in 2000 to 36% in 2019) [1]. Much of the progress has been in reducing severe and moderate anemia, with limited change in the prevalence of mild anemia.
A critical component of tracking progress towards global targets is having an agreed upon definition of anemia by the global community. WHO Hb cutoffs for defining anemia are currently being re-examined [6]. The WHO anemia cutoffs are based on limited data, in particular for pregnant women, and lack global representativeness and are widely acknowledged as outdated as they do not account for gestational age-specific changes in plasma volume expansion. Current overall anemia cutoffs are defined as < 110 g/L during pregnancy, [7] or trimester-specific anemia cutoffs (first trimester: < 110 g/L; second trimester: < 105 g/L; third trimester: < 110 g/L) [8, 9]. Across the literature, there is often inconsistency in the range of Hb cutoffs used to define low and high Hb thresholds, which impacts the prevalence of the problem and likelihood of detecting relationships with adverse maternal and child outcomes [10]. Studies using multiple Hb cutoffs have reported that in some cases only lower cutoff values were associated with adverse outcomes [11,12,13,14]. Few studies have evaluated the long-term health impact of maternal Hb concentrations [15]. An exception is a recent analysis of data from the Fetal Growth Longitudinal Study of the INTERGROWTH-21st Project, a study of prospective, population-based data from Brazil, China, India, Italy, Kenya, Oman, the United Kingdom and the United States [16]. This analysis provides maternal Hb normative centiles during pregnancy associated with positive birth outcomes as well as adequate child growth and development across the first 1000 days. The results suggest Hb cutoffs during pregnancy that are lower than currently recommended by the WHO [16]. However, key knowledge gaps remain on the optimal thresholds of maternal Hb concentration related to both maternal and infant health, and whether those thresholds vary by anemia etiology or timing of measurement. In response to the WHO call to re-evaluate the evidence on hemoglobin thresholds to define anemia, we previously conducted a systematic review on maternal Hb concentrations and maternal and infant health outcomes including studies through October 2018 [15]. Since then, significant new research has been reported on outcomes of interest, such as maternal depression and maternal mortality, that were not included in the previous meta-analysis due to a paucity of studies.
The objective of this study was to conduct an updated systematic review and meta-analysis to examine the associations of low and high maternal Hb concentrations during pregnancy with adverse maternal and infant health outcomes. Analyses were stratified by timing of Hb assessment (preconception, first, second and third trimesters), Hb cutoff category, and etiology of anemia (iron-deficiency anemia/non-iron deficiency anemia).
Methods
Search Strategy
Our search criteria included studies of associations between maternal anemia or Hb concentrations, measured during pregnancy or preconception, and adverse maternal and infant health outcomes, as previously defined [15]. Maternal outcomes included blood transfusion, gestational diabetes, maternal mortality, postpartum hemorrhage, prenatal depression, postpartum depression, and preeclampsia. Infant outcomes included low birth weight (LBW; <2500 g), very low birth weight (VLBW; <1500 g), neonatal mortality, perinatal mortality, preterm birth (PTB; <37 weeks), small-for-gestational age (SGA), and stillbirth. Cochrane Review and PubMed were searched with no restrictions for study population, language, or date, building directly from our prior systematic review [15]. In addition, references from prior reviews were also reviewed to identify additional studies. To be eligible for inclusion, studies had to report associations between maternal Hb concentrations or anemia (defined by Hb cutoff) assessed during preconception or pregnancy, and at least one of the adverse outcomes. Only peer-reviewed studies adjusting for one or more confounders were included. Publication dates for the studies in the meta-analysis (including both prior review [15] and updated search combined) ranged from January 1990 to April 2021 and included a total sample size of 13,839,327 women across 148 studies.
Data extraction and quality assessment
Reviews for title and abstract screening, full text review, and data extraction were conducted using Covidence systematic review software by two team members. Conflicts were resolved by a third team member (for title and abstract review) or team committee (for full text). Key information extracted from manuscript included study design, year, gestational age/trimester at time of assessment, Hb concentration cutoffs, adverse outcome measures and adjusted measures of association with 95% confidence intervals. To ensure data quality, 10% of data extractions were conducted in duplicate. Authors were contacted for missing information when possible.
Data management and analyses
Data were stratified by low Hb (< 110 g/L) and high Hb (≥ 130 g/L) and by timing of Hb measurement: preconception, first (≤ 13 weeks), second (14–26 weeks), and third (≥ 27 weeks) trimesters. The overall pregnancy estimates for low (< 110 g/L) and high Hb (≥ 130 g/L) included Hb concentrations measured at any time point during pregnancy (including studies with and without gestational age data). The Hb cutoffs used to define anemia varied across studies; thus, we created standard cutoffs to conduct various analyses (≤ 70, ≤ 80, ≤90, ≤ 100, ≤110, ≥ 130, ≥140, ≥ 150, and ≥ 160 g/L) to create summary estimates across Hb concentrations. Each category was cumulative whereby the estimates reported for the ≥ 130 g/L category include measurements for all studies using an Hb cutoff of ≥ 130, including all studies in the ≥ 140, ≥150, and ≥ 160 g/L categories. The reference group varied across studies and was either a cutoff (e.g. ≥110 g/L) or reference range (e.g. 110–119 g/L). Separate meta-analyses were conducted for iron deficiency anemia (IDA) and non-IDA for LBW, PTB, and SGA. All analyses were conducted using STATA version 14.2 (Stata Corp. 2015, College Station, TX). Values reported as odds ratio (OR) and 95% confidence intervals with significance indicated at P < 0.05. Q- and I2 values over 50% indicated heterogeneity and a Q-statistic P-value of > 0.10 indicated substantial heterogeneity. Given the significant heterogeneity, we used random effect models and the inverse-variance method for weighting for the meta-analyses.
Results
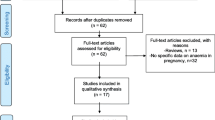
The systematic review identified 9874 studies, of which 57 duplicates were removed (See Supplementary Fig. 1, Additional File 1). After screening abstracts for eligibility, we conducted full text reviews on 1142 studies and excluded 994 based on inclusion and exclusion criteria (n = 743), inability to contact the study author (n = 38), unable to translate (n = 9), inability to retrieve the full text document (n = 26), study result duplication (n = 1), inclusion of other statistical measures incompatible for meta-analysis (n = 61), other outcomes not included in review or insufficient for meta-analysis (n = 90) or conducted among high risk populations (n = 26). In total, we included 148 studies in the meta-analysis (See Supplementary Fig. 1, Additional File 1) [11, 17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163].
Study characteristics
Among eligible studies, 43 were prospective cohorts, 50 were retrospective cohorts, 36 were case-control, and 19 were cross-sectional studies. This included an additional 53 new studies, including data from 32 new countries from the prior review [15]. As illustrated in Fig. 1 our combined updated review includes data from a total of 69 countries. Across the included countries there was variation in data available with China, Ethiopia, and India having over 10 studies and countries such as Afghanistan, Mozambique, and Nicaragua having only 1–2 studies and many countries none. The sample sizes from included studies ranged from 124 to 2,869,415 and the total sample size across all studies was 13,839,327 women. There was insufficient data to conduct meta-analyses on long-term outcomes. Limited data was available on preconception Hb concentrations and birth outcomes.
Global distribution of sites used in included studies (Analysis of the data was done using the rworldmap package v1.3-6 (1): https://www.rdocumentation.org/packages/rworldmap/versions/1.3-6 )
Infant health outcomes
Birth outcomes with sufficient data for meta-analysis included: (1) LBW (n = 61); (2) PTB (n = 63); (3) SGA (n = 37); (4) stillbirth (n = 27); (5) perinatal mortality (n = 13); and (6) neonatal mortality (n = 10).
Figure 2 provides an overview of associations between maternal Hb and infant health outcomes, which are further described in Table 1 and Table 2 for low and high maternal Hb and stratified by Hb cutoff and timing.
Low maternal hb and infant outcomes
Overall estimates of low Hb (< 110 g/L) during pregnancy and the relationship to LBW, VLBW, PTB, SGA, stillbirth, perinatal mortality, and neonatal mortality are illustrated in Fig. 2a. Low maternal Hb was significantly associated with odds of LBW (OR (95% CI) 1.28 (1.22–1.35)), VLBW (OR (95% CI) 2.15 (1.47–3.13)), PTB (OR (95% CI) 1.35 (1.29–1.42)), SGA (OR (95% CI) 1.11 (1.02–1.19)), stillbirth (OR (95% CI) 1.43 (1.24–1.65)), perinatal mortality (OR (95% CI) 1.75 (1.28–2.39)), and neonatal mortality (OR (95% CI) 1.25 (1.16–1.34)).
By timing
Summary estimates were constructed for low maternal Hb (< 110 g/L) and the odds of LBW, VLBW, PTB, SGA, stillbirth, perinatal mortality, and neonatal mortality by timing at preconception, 1st trimester, 2nd trimester, and 3rd trimester (Table 1). The strongest associations between low maternal Hb and LBW and SGA were observed during the preconception period (OR (95% CI) 1.72 (1.31–2.26)); OR (95% CI) 1.79 (1.39–2.31)), respectively), while relationships were nonsignificant for PTB and stillbirth during this time. During the first trimester, low maternal Hb was associated with increased odds of the following adverse birth outcomes: LBW (OR (95% CI) 1.31 (1.16–1.49)), VLBW (OR (95% CI) 3.21 (1.06–9.71)), PTB (OR (95% CI) 1.22 (1.15–1.31)), SGA (OR (95% CI) 1.12 (1.04–1.21)) and stillbirth (OR (95% CI) 1.54 (1.12–2.12)). During the second trimester, relationships between low maternal Hb and infant outcomes were significant for PTB (OR (95% CI) 1.37 (1.15–1.64)) and stillbirth (OR (95% CI) 2.22 (1.36–3.65)) but not for the other outcomes. During the third trimester, low maternal Hb was associated with increased odds of the following adverse birth outcomes: LBW (OR (95% CI) 1.45 (1.23–1.70)), VLBW (OR (95% CI) 2.20 (1.43–3.38)) and PTB (OR (95% CI) 1.49 (1.30–1.71)) but decreased odds of SGA (OR (95% CI) 0.89 (0.80–0.98)). Data to examine perinatal and neonatal morality by timing were limited; relationships within each time period were non-significant.
By cutoff
Summary estimates were created for low Hb and the odds of LBW, VLBW, PTB, SGA, stillbirth, perinatal mortality, and neonatal mortality by Hb concentration cutoff: ≤70, ≤ 80, ≤90, ≤ 100, and ≤ 110 g/L (Table 1). The odds of poor birth outcomes generally increased as Hb concentration decreased across cutoffs, with strongest associations at the lowest cutoff of ≤ 70 g/L for LBW (OR (95% CI) 2.06 (1.63–2.61)), PTB ≤ 70 g/L: (OR (95% CI) 2.08 (1.67–2.59)), stillbirth ≤ 70 g/L: (OR (95% CI) 2.85 (1.66–4.90)), perinatal mortality ≤ 70 g/L (OR (95% CI) 4.41 (2.21–8.81)) and neonatal mortality ≤ 70 g/L: (OR (95% CI) 1.83 (1.52–2.19)). The odds of VLBW were highest at ≤ 90 g/L (OR (95% CI) 4.95 (1.60-15.29)); however, limited data existed at lower cutoffs. Odds of SGA were similar across Hb cutoffs ranging from OR (95% CI) 1.31 (1.14–1.51) for ≤ 80 g/L to OR (95% CI) 1.11 (1.03–1.19) for ≤ 110 g/L.
By etiology
Limited information was available to examine the role of etiology of anemia (IDA vs. non-IDA) with regard to birth outcomes (See Supplementary Tables 2, Additional File 1). From the data available, neither IDA (by itself) or non-IDA (by itself) during pregnancy was significantly associated with LBW, SGA, or PTB.
High maternal hb and infant outcomes
Overall estimates of high Hb (≥ 130 g/L) during pregnancy and relationship to LBW, VLBW, PTB, SGA, stillbirth, perinatal mortality, and neonatal mortality are illustrated in Fig. 2b. High maternal Hb was significantly associated with odds of VLBW (OR (95% CI) 1.35 (1.16–1.57)), PTB (OR (95% CI) 1.12 (1.00-1.25)), SGA (OR (95% CI) 1.17 (1.09–1.25)) and stillbirth (OR (95% CI) 1.32 (1.09–1.60)).
By timing
When data were sufficient, summary estimates were constructed for high maternal Hb (≥ 130 g/L) and the odds of LBW, VLBW, PTB, SGA, stillbirth, perinatal mortality, and neonatal mortality by timing at preconception, 1st trimester, 2nd trimester, and 3rd trimester (Table 2). During preconception, maternal high Hb was not associated with any of the infant health outcomes. During the first trimester, high maternal Hb was associated with increased odds of VLBW (OR (95% CI) 1.35 (1.16–1.57)) and stillbirth (OR (95% CI) 1.23 (1.03–1.47)) but not LBW, PTB, SGA, or neonatal mortality. During the second trimester, high maternal Hb was associated with increased odds of LBW (OR (95% CI) 1.40 (1.02–1.93)) and SGA (OR (95% CI) 1.27 (1.08–1.49)) but not PTB, stillbirth or perinatal mortality. During the third trimester, high maternal Hb was associated with increased odds of stillbirth (OR (95% CI) 2.31 (1.30–4.10)) but decreased odds of LBW (OR (95% CI) 0.58 (0.51–0.66)).
By cutoff
Summary estimates were created for high Hb and the odds of LBW, VLBW, PTB, SGA, stillbirth, perinatal mortality, and neonatal mortality by Hb concentration cutoff: ≥130, ≥ 140, ≥150, and ≥ 160 g/L (Table 2). Overall, there were limited data to evaluate different cutoffs and patterns were not clear. The exception was stillbirth, for which the odds doubled when shifting from a cutoff of ≥ 130 g/L (OR (95% CI) 1.32 (1.09–1.60)) to a cutoff of ≥ 140 g/L (OR (95% CI) 2.30 (1.38–3.85)).
Maternal outcomes
Maternal outcomes with sufficient data for meta-analysis included: (1) post-partum hemorrhage (n = 14); (2) transfusion (n = 10); (3) pre-eclampsia (n = 11); (4) gestational diabetes (n = 7); (5) prenatal depression (n = 3); (6) postpartum depression (n = 3) and (7) maternal mortality (n = 3). Figure 3 provides an overview of associations of maternal Hb and maternal health outcomes; which is further described in Tables 3 and 4 for low and high maternal Hb and stratified by Hb cutoff and timing.
Low maternal hb and maternal outcomes
Data across time points and cutoffs were combined to construct summary estimates of the relationship of low Hb (< 110 g/L) to postpartum hemorrhage, maternal mortality, transfusion, gestational diabetes, pre-eclampsia, postnatal depression, and postpartum depression (Fig. 3a). Low maternal Hb was significantly associated with odds of postpartum hemorrhage (OR (95% CI) 1.69 (1.45–1.97)), transfusion (OR (95% CI) 3.68 (2.58–5.26)), pre-eclampsia (OR (95% CI) 1.57 (1.23–2.01)), and prenatal depression (OR (95% CI) 1.44 (1.24–1.68)). We also conducted a sensitivity analysis with revised definition of postpartum blood loss by ≥ 1000 mL and the results remained consistent (OR (95% CI) 1.87 (1.15–3.06)), Figure S2.
By timing
Limited data were available to examine the role of timing with regard to low maternal Hb and maternal outcomes. During preconception, only one study reported a (non-significant) association with gestational diabetes and no data were available for other outcomes. During the first trimester, data were only available for pre-eclampsia and gestational diabetes and relationships were nonsignificant. During the second trimester, low maternal Hb increased the odds of post-partum hemorrhage (OR (95% CI) 1.43 (1.01–2.03)) but reduced the odds of pre-eclampsia (OR (95% CI) 0.50 (0.29–0.87)). During the third trimester, low maternal Hb was associated with increased odds of post-partum hemorrhage (OR (95% CI) 1.58 (1.40–1.79)), and transfusion (OR (95% CI) 6.15 (3.70-10.23)).
By cutoff
Summary estimates were constructed for low Hb and the odds of postpartum hemorrhage, transfusion, pre-eclampsia, gestational diabetes, postpartum depression, prenatal depression, and maternal mortality by Hb concentration cutoff (Table 3). The odds of postpartum hemorrhage, transfusion and pre-eclampsia increased as Hb concentration decreased with a 1.8 to 11.8-fold increase in ORs when shifting from a cutoff of ≤ 110 g/L to a cutoff of ≤ 70 g/L. For maternal mortality, while the overall results for ≤ 110 g/L were non-significant, at lower cutoffs of ≤ 100 (OR (95% CI) 2.87 (1.08–7.67)), and ≤ 90 (OR (95% CI) 4.83 (2.17–10.74)) relationships were significant.
High maternal hb and maternal outcomes
Data across time points and cutoffs were combined to construct summary estimates of the relationship of high Hb (≥ 130 g/L) to postpartum hemorrhage, maternal mortality, transfusion, gestational diabetes, pre-eclampsia, prenatal depression, and postpartum depression (Fig. 3b). When all data were combined, high Hb (≥ 130 g/L) was significantly associated with odds of maternal mortality (OR (95% CI) 2.01 (1.12–3.61)), gestational diabetes (OR (95% CI) 1.71 (1.19–2.46)), and pre-eclampsia (OR (95% CI) 1.34 (1.16–1.56)).
By timing
Limited data were available to examine associations of high maternal Hb with maternal outcomes. During preconception, high maternal Hb was associated with increased odds of gestational diabetes (OR (95% CI) 1.24 (1.11–1.38)) but data were not available for other outcomes. During the first trimester, high maternal Hb was associated with increased odds of gestational diabetes (OR (95% CI) 1.73 (1.10–2.71)). During the second and third trimester, high maternal Hb was associated with increased odds of pre-eclampsia (OR (95% CI) 1.27 (1.18–1.36)).
By cutoff
Summary estimates were created for the relationship of high Hb to post-partum hemorrhage, transfusion, pre-eclampsia, gestational diabetes, postpartum depression, prenatal depression, and maternal mortality by Hb concentration cutoff: ≥130, ≥ 140, and ≥ 150 g/L (Table 4). The odds of post-partum hemorrhage were similar and non-significant for the first two of these Hb cutoffs (≥ 130 g/L: (OR (95% CI) 1.00 (0.95–1.05)); ≥140 g/L (OR (95% CI) 1.00 (0.87–1.14)). The odds of pre-eclampsia were highest when Hb concentration was ≥ 150 g/L (OR (95% CI) 2.38 (1.20–4.69)) compared to a cutoff of ≥ 130 (OR (95% CI) 1.34 (1.16–1.56)). For gestational diabetes, ORs were significant for Hb ≥ 130 g/L (OR (95% CI) 1.71 (1.19–2.46)) and ≥ 140 g/L (OR (95% CI) 2.10 (1.65–2.68)). Data were limited for other outcomes.
Discussion
Our updated systemic review provides new insights into the critical role of optimal Hb concentrations during preconception and pregnancy. Unique aspects of this review are the dual focus on low and high Hb concentrations and consideration of a range of both maternal and infant health outcomes. During pregnancy, low maternal Hb was associated with increased odds of poor birth outcomes (LBW, VLBW, PTB, SGA, stillbirth, perinatal and neonatal mortality) and adverse maternal outcomes (post-partum hemorrhage, preeclampsia, prenatal depression, blood-transfusion and maternal mortality). Likewise, high maternal Hb was associated with increased odds of poor birth outcomes (VLBW, PTB, SGA, stillbirth) and adverse maternal outcomes (preeclampsia, gestational diabetes and maternal mortality). Reported associations varied by trimester of pregnancy and Hb cutoff values but not anemia etiology.
This review builds on our prior review [15] with the addition of 53 new studies for a total 148 studies and includes new outcomes including maternal depression and maternal mortality in the meta-analyses. This review expands upon prior reviews on maternal anemia and adverse birth outcomes [10, 164,165,166,167,168,169,170]. Low maternal Hb during pregnancy, depending on the timing of assessment and cutoff used, was associated with up to nearly a 5-fold increased risk of poor birth outcomes. This review highlights the importance of early prevention and treatment of anemia, before many women even seek antenatal care. The strongest associations with low maternal Hb were noted during the preconception period (for LBW and SGA) and during the first trimester (for VLBW). Throughout pregnancy, low maternal Hb concentrations remained an important predictor of poor birth outcomes. One exception was that low maternal Hb during the third trimester was associated with a reduced risk of SGA. The reason for this association is unclear and could be spurious finding or related to failure of plasma volume expansion [171]. Overall, lower Hb cutoffs were associated with greater risks of adverse outcomes in a dose-response pattern.
The mechanisms underlying the association of low maternal Hb with birth outcomes are complex and multifactorial and may include nutritional deficiencies (e.g., iron, vitamin A, folic acid, or vitamin B12 deficiency), infectious causes (e.g., malaria, schistosomiasis, hookworm infection, HIV), hemoglobinopathies (sickle cell anemia, thalassemia), and inflammation [172]. Iron deficiency has been reported to contribute to up to 75% of all types of anemia during pregnancy [172]. Iron deficiency results from insufficient dietary intake coupled with increased systemic demand, impaired absorption, or blood loss. The prevalence of iron deficiency varies geographically, with higher prevalence in low-income countries. Across pregnancy, there are changes in iron requirements and iron absorption, with decreases in requirements the first trimester followed by a nearly three-fold increase in the third trimester due to increased maternal red blood cell mass expansion, placental demand, and fetal growth [173, 174]. IDA is associated with lower oxygen delivery to the tissues, fatigue, increased risk of infection, and cardiac failure in severe cases [175]. Among offspring, IDA is associated with poor perinatal outcomes including LBW, intrauterine growth restriction, PTB, neonatal anemia. Although iron deficiency has been largely attributed to nutritional causes (e.g., insufficient iron intake or poor iron absorption), several non-nutritional causes may be important to consider as well. Inflammation (due to infectious causes or low-grade inflammation observed in individuals with overweight or obesity) may also impact iron uptake and metabolism via increased hepcidin levels, resulting in anemia of inflammation despite sufficient iron stores [173]. Furthermore, although outside of the scope of the present review, it is important to consider the interplay between hemoglobinopathies and iron deficiency. Recent studies report that thalassemia carriers have altered iron metabolism and erythropoiesis [176,177,178]. Within our review, relationships between maternal Hb and birth outcomes did not vary by anemia etiology; this is likely attributable to the lack of information across included studies with respect to prevalence of iron deficiency and merits further examination.
Maternal low Hb concentrations were also associated with a range of adverse maternal outcomes. For several outcomes, results were consistent with those of prior reviews [15] (postpartum hemorrhage, transfusion, and pre-eclampsia), but some outcomes were new to this review (prenatal and post-partum depression and maternal mortality). Maternal low Hb during pregnancy was associated with a 44% increased risk of prenatal depression, however, there is insufficient information to understand how this association varies by timing of Hb assessment or cutoff used. Overall, associations between maternal low Hb and maternal mortality were non-significant; however, when lower cutoffs of ≤ 100 g/L and ≤ 90 g/L were used, maternal Hb was associated with nearly a 3 to 5-fold increase in maternal mortality. Data are lacking on the importance of timing of maternal Hb assessment and maternal mortality.
Much of the existing literature has focused on low maternal Hb during pregnancy; however, our review demonstrates that high maternal Hb concentrations during this time are likewise associated with up to a 2-fold increased odds of adverse infant outcomes (VLBW, PTB, SGA and stillbirth) and maternal outcomes (pre-eclampsia, gestational diabetes and maternal mortality). There are several potential mechanisms to consider when reflecting on the maternal health and birth outcomes associated with high Hb concentrations. Plasma volume expansion, which occurs rapidly in the second and third trimester of pregnancy, is a normal physiological process and facilitates the transfer of nutrients to the fetus. High Hb concentrations may be a result of inadequate plasma volume expansion, which has been reported as a risk factor for both SGA and pre-eclampsia [179]. While mechanisms are unclear and there is the potential for reverse causality, there is evidence that abnormal placental vascularization may trigger a pathway of increased production of vasconstrictive agents, reduced plasma volume expansion, and pre-eclampsia [180, 181]. There is increasing attention to the possibility that pre-eclampsia may be a result of a dysfunctional maternal cardiovascular system that is unable to manage plasma volume expansion [182, 183]. High Hb concentrations may also be a result of higher iron status. While higher iron status is generally viewed favorably during pregnancy, iron is a pro-oxidant that can result in DNA damage of placenta cells and interfere with metabolic processes related to glucose metabolism if the body is experiencing iron overload [184,185,186]. High iron status during pregnancy has been associated with SGA and gestational diabetes in previous studies [34, 187].
Key strengths of this review are the inclusion of both ends of the spectrum for Hb concentrations and the range of adverse outcomes for both the mother and infant. Stratification of results by Hb cutoff and timing of assessment also adds further depth and understanding to these complex relationships. Associations between low Hb and adverse outcomes tend to be stronger earlier in pregnancy while associations between high Hb and adverse outcomes were inconsistent across time points. Mixed findings in the second trimester may point to a need to further examine optimal cutoffs during this period.
Our study is one of the largest systematic reviews to date on this topic, including data collected in 69 different countries over a 30-year time period. As such there is high heterogeneity in our analysis driven by differences in definitions and measurement of exposure (maternal Hb) and outcomes as well as potential true differences by context, perhaps driven by variable etiology of anemia and high Hb. It is important to note that this review examines observational associations that could be confounded by underlying socioeconomic, health or environmental factors and thus cannot establish causality. Furthermore, there are inherent limitations of using dichotomous characterizations of Hb rather than understanding risk across a range of continuous values of Hb [188, 189]. In addition, where data were available we stratified by trimester of pregnancy; however, a closer examination of maternal Hb by week of gestation and application of the new gestational age-specific centiles for maternal hemoglobin concentrations from the INTERGROWTH study would be ideal [16]. Future work with pooled individual level data and standardized adjustment for known confounding factors may allow for greater clarity on associations of hemoglobin level and increased risk of adverse outcomes. Depending on the prevalence of the adverse outcomes, future reporting of relative risk may be preferable [190]. This review is limited by the data available. Notably, there is a lack of data on the etiology of low or high Hb and many gaps remain in understanding the role of timing and cutoffs across preconception and pregnancy. Studies that reported data using multiple Hb cutoffs or time points were more heavily weighted in the overall estimates. Hb is a convenient and widely used biomarker in nutrition and health research, however it remains unclear if the associations with adverse outcomes are being driven by direct alterations in functional Hb for essential functions or by the indirect underlying causes of anemia (e.g. iron deficiency vs. hemoglobinopathy) or high Hb (e.g. excess iron vs. failure of plasma volume expansion). Further research is needed to better understand the implications of the etiology of anemia for redefining optimal Hb cutoffs during pregnancy and optimizing public health programs for women.
Conclusion
Both low and high hemoglobin concentrations were associated with adverse maternal and infant health outcomes. Our work builds on prior evidence on the importance of maintaining a healthy maternal Hb concentration from preconception to delivery. Further research is needed to define cutoffs for both anemia and high Hb and implement effective interventions to optimize healthy Hb ranges during pregnancy.
Data Availability
All data and materials are available upon request (Melissa Young, melissa.young@emory.edu).
Abbreviations
- HB:
-
Hemoglobin
- LBW:
-
Low birthweight
- PTB:
-
Preterm birth
- SGA:
-
Small-for-gestational age
- VLBW:
-
Very low birthweight
- WHO:
-
World Health Organization
References
Stevens GA, Paciorek CJ, Flores-Urrutia MC, Borghi E, Namaste S, Wirth JP, Suchdev PS, Ezzati M, Rohner F, Flaxman SR, et al. National, regional, and global estimates of anaemia by severity in women and children for 2000–19: a pooled analysis of population-representative data. The Lancet Global Health. 2022;10:e627–39.
Nutritional anaemias. Tools for effective prevention and control. Geneva: World Health Organization; 2017. Licence: CC BY-NC-SA 3.0 IGO.
WHO. Global nutrition targets 2025: anaemia policy brief (WHO/NMH/NHD/14.4). Geneva: World Health Organization; 2014.
UNGA. Global indicator framework for the Sustainable Development Goals and targets of the 2030 Agenda for Sustainable Development. March, 2020. https://unstats.un.org/sdgs/indicators/Global%20Indicator%20Framework%20after%202020%20review_Eng.pdf (accessed April 28th, 2022).
WHO, UNICEF. WHO/UNICEF discussion paper. The extension of the 2025 maternal, infant and young child nutrition targets to 2030. June, 2019. https://data.unicef.org/wp-content/uploads/2021/05/UNICEF-WHO-discussion-paper-extension-targets-2030.pdf (accessed April 28, 2022).
Garcia-Casal MN, Pasricha S-R, Sharma AJ, Peña-Rosas JP. Use and interpretation of hemoglobin concentrations for assessing anemia status in individuals and populations: results from a WHO technical meeting. Ann N Y Acad Sci. 2019;1450(1):5–14.
WHO. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. Vitamin and Mineral Nutrition Information System. Geneva, World Health Organization, 2011 (WHO/NMH/NHD/MNM/11.1 https://www.who.int/vmnis/indicators/haemoglobin.pdf, accessed 28 August 2017).
WHO. WHO recommendations on antenatal care for a positive pregnancy experience. Geneva, World Health Organization., ; 2016 Accessed Dec 13, 2016 http://www.who.int/nutrition/publications/guidelines/antenatalcare-pregnancy-positive-experience/en/.
Centers for Disease Control and Prevention. Recommendations to Prevent and Control Iron Deficiency in the United States. MMWR 1998; 47 (RR-3); 1–36.
Dewey KG, Oaks BM. U-shaped curve for risk associated with maternal hemoglobin, iron status, or iron supplementation. Am J Clin Nutr. 2017;106(Suppl 6):1694s–702.
Maghsoudlou S, Cnattingius S, Stephansson O, Aarabi M, Semnani S, Montgomery SM, Bahmanyar S. Maternal haemoglobin concentrations before and during pregnancy and stillbirth risk: a population-based case-control study. BMC Pregnancy Childbirth. 2016;16(1):135.
Chang SC, O’Brien KO, Nathanson MS, Mancini J, Witter FR. Hemoglobin concentrations influence birth outcomes in pregnant african-american adolescents. J Nutr. 2003;133(7):2348–55.
Zhang Q, Ananth CV, Li Z, Smulian JC. Maternal anaemia and preterm birth: a prospective cohort study. Int J Epidemiol. 2009;38(5):1380–9.
Zhang Q, Ananth CV, Rhoads GG, Li Z. The impact of maternal anemia on perinatal mortality: a population-based, prospective cohort study in China. Ann Epidemiol. 2009;19(11):793–9.
Young MF, Oaks BM, Tandon S, Martorell R, Dewey KG, Wendt AS. Maternal hemoglobin concentrations across pregnancy and maternal and child health: a systematic review and meta-analysis. Ann N Y Acad Sci. 2019;1450(1):47–68.
Ohuma EO, Young MF, Martorell R, Ismail LC, Pena-Rosas JP, Purwar M, Garcia-Casal MN, Gravett MG, de Onis M, Wu Q et al. International values for haemoglobin distributions in healthy pregnant women.EClinicalMedicine2020,29–30:100660.
Zenebe A, Eshetu B, Gebremedhin S. Association between maternal HIV infection and birthweight in a tertiary hospital in southern Ethiopia: retrospective cohort study. Ital J Pediatr. 2020;46(1):70.
Yuniati T, Judistiani RTD, Natalia YA, Irianti S, Madjid TH, Ghozali M, Sribudiani Y, Indrati AR, Abdulah R, Setiabudiawan B. First trimester maternal vitamin D, ferritin, hemoglobin level and their associations with neonatal birthweight: result from cohort study on vitamin D status and its impact during pregnancy and childhood in Indonesia. J neonatal-perinatal Med. 2020;13(1):63–9.
Symington EA, Baumgartner J, Malan L, Wise AJ, Ricci C, Zandberg L, Smuts CM. Maternal iron-deficiency is associated with premature birth and higher birth weight despite routine antenatal iron supplementation in an urban south african setting: the NuPED prospective study. PLoS ONE. 2019;14(9):e0221299.
Sun CF, Liu H, Hao YH, Hu HT, Zhou ZY, Zou KX, Liu XM, Sheng JZ, Ding GL, Huang HF. Association between gestational anemia in different trimesters and neonatal outcomes: a retrospective longitudinal cohort study. World J Pediatr. 2021;17(2):197–204.
Sun CC, Chou HH, Chuang LL. Trends and risk factors of stillbirth in Taiwan 2006–2013: a population-based study. Arch Gynecol Obstet. 2019;299(4):961–7.
Soysal S, Sarioz A, Anik Ilhan G, Kocagoz A, Dizi A, Gursoy I, Celik I, Ozmen D. Evaluation of late adolescent pregnancies: is late adolescence a risk factor for preterm labor? J Matern Fetal Neonatal Med. 2019;32(5):851–6.
Smith C, Teng F, Branch E, Chu S, Joseph KS. Maternal and perinatal morbidity and mortality Associated with Anemia in pregnancy. Obstet Gynecol. 2019;134(6):1234–44.
Shankar H, Kumar N, Sandhir R, Singh MP, Mittal S, Adhikari T, Tarique M, Kaur P, Radhika MS, Kumar A, et al. Association of dietary intake below recommendations and micronutrient deficiencies during pregnancy and low birthweight. J Perinat Med. 2019;47(7):724–31.
Sari IM, Adisasmita AC, Prasetyo S, Amelia D, Purnamasari R. Effect of premature rupture of membranes on preterm labor: a case-control study in Cilegon, Indonesia. Epidemiol Health. 2020;42:e2020025.
Salunkhe AH, Pratinidhi AK, Salunkhe JA, Kakade SV, Mohite VR, Patange RP. Antenatal Risk Scoring Scale for Predication of Low Birth Weight and its validity. Indian J Community Med. 2019;44(2):97–101.
Rottenstreich A, Regev N, Levin G, Ezra Y, Yagel S, Sompolinsky Y, Mankuta D, Kalish Y, Elchalal U. Factors associated with postcesarean blood transfusion: a case control study. J Matern Fetal Neonatal Med. 2022;35(3):495–502.
Ronkainen J, Lowry E, Heiskala A, Uusitalo I, Koivunen P, Kajantie E, Vaarasmaki M, Jarvelin MR, Sebert S. Maternal hemoglobin associates with preterm delivery and small for gestational age in two finnish birth cohorts. Eur J Obstet Gynecol Reprod Biol. 2019;238:44–8.
Rayis DA, Musa IR, Al-Shafei AI, Moheldein AH, El-Gendy OA, Adam I. High haemoglobin levels in early pregnancy and gestational diabetes mellitus among sudanese women. J Obstet Gynaecol. 2021;41(3):385–9.
Ray JG, Davidson A, Berger H, Dayan N, Park AL. Haemoglobin levels in early pregnancy and severe maternal morbidity: population-based cohort study. BJOG. 2020;127(9):1154–64.
Randall DA, Patterson JA, Gallimore F, Morris JM, Simpson JM, McGee TM, Ford JB. Obstetric transfusion steering G: haemoglobin trajectories during pregnancy and associated outcomes using pooled maternity and hospitalization data from two tertiary hospitals. Vox Sang. 2019;114(8):842–52.
Randall DA, Patterson JA, Gallimore F, Morris JM, McGee TM, Ford JB, Obstetric Transfusion Steering G. The association between haemoglobin levels in the first 20 weeks of pregnancy and pregnancy outcomes. PLoS ONE. 2019;14(11):e0225123.
Parks S, Hoffman MK, Goudar SS, Patel A, Saleem S, Ali SA, Goldenberg RL, Hibberd PL, Moore J, Wallace D et al. Maternal anaemia and maternal, fetal, and neonatal outcomes in a prospective cohort study in India and Pakistan. BJOG. 2019; 126(6):737–43.
Oaks BM, Jorgensen JM, Baldiviez LM, Adu-Afarwuah S, Maleta K, Okronipa H, Sadalaki J, Lartey A, Ashorn P, Ashorn U, et al. Prenatal Iron Deficiency and Replete Iron Status are Associated with adverse birth outcomes, but Associations Differ in Ghana and Malawi. J Nutr. 2019;149(3):513–21.
Nsereko E, Uwase A, Mukabutera A, Muvunyi CM, Rulisa S, Ntirushwa D, Moreland P, Corwin EJ, Santos N, Nzayirambaho M, et al. Maternal genitourinary infections and poor nutritional status increase risk of preterm birth in Gasabo District, Rwanda: a prospective, longitudinal, cohort study. BMC Pregnancy Childbirth. 2020;20(1):345.
Mekuriyaw AM, Mihret MS, Yismaw AE. Determinants of Preterm Birth among Women Who Gave Birth in Amhara Region Referral Hospitals, Northern Ethiopia, 2018: Institutional Based Case Control Study. Int J Pediatr. 2020;2020:1854073.
Mekie M, Taklual W. Magnitude of low birth weight and maternal risk factors among women who delivered in Debre Tabor Hospital, Amhara Region, Ethiopia: a facility based cross-sectional study. Ital J Pediatr. 2019;45(1):86.
Maeda Y, Ogawa K, Morisaki N, Tachibana Y, Horikawa R, Sago H. Association between perinatal anemia and postpartum depression: a prospective cohort study of japanese women. Int J Gynaecol Obstet. 2020;148(1):48–52.
Locks LM, Patel A, Katz E, Simmons E, Hibberd P. Seasonal trends and maternal characteristics as predictors of maternal undernutrition and low birthweight in Eastern Maharashtra, India. Matern Child Nutr. 2021;17(2):e13087.
Lake EA, Olana Fite R. Low Birth Weight and its Associated factors among newborns delivered at Wolaita Sodo University Teaching and Referral Hospital, Southern Ethiopia, 2018. Int J Pediatr. 2019;2019:4628301.
Kumari S, Garg N, Kumar A, Guru PKI, Ansari S, Anwar S, Singh KP, Kumari P, Mishra PK, Gupta BK, et al. Maternal and severe anaemia in delivering women is associated with risk of preterm and low birth weight: a cross sectional study from Jharkhand, India. One Health. 2019;8:100098.
Kim HY, Kim J, Noh E, Ahn KH, Cho GJ, Hong SC, Oh MJ, Kim HJ. Prepregnancy hemoglobin levels and gestational diabetes mellitus in pregnancy. Diabetes Res Clin Pract. 2021;171:108608.
Kelkay B, Omer A, Teferi Y, Moges Y. Factors Associated with Singleton Preterm Birth in Shire Suhul General Hospital, Northern Ethiopia, 2018. J Pregnancy. 2019;2019:4629101.
Kebede BA, Abdo RA, Anshebo AA, Gebremariam BM. Prevalence and predictors of primary postpartum hemorrhage: an implication for designing effective intervention at selected hospitals, Southern Ethiopia. PLoS ONE. 2019;14(10):e0224579.
Hussein H, Shamsipour M, Yunesian M, Hasanvand MS, Fotouhi A. Association of adverse birth outcomes with exposure to fuel type use: a prospective cohort study in the northern region of Ghana. Heliyon. 2020;6(6):e04169.
Gurung A, Wrammert J, Sunny AK, Gurung R, Rana N, Basaula YN, Paudel P, Pokhrel A, Kc A. Incidence, risk factors and consequences of preterm birth - findings from a multi-centric observational study for 14 months in Nepal. Arch Public Health. 2020;78:64.
Guignard J, Deneux-Tharaux C, Seco A, Beucher G, Kayem G, Bonnet MP. group E: Gestational anaemia and severe acute maternal morbidity: a population-based study. Anaesthesia. 2021;76(1):61?71.
Girma S, Fikadu T, Agdew E, Haftu D, Gedamu G, Dewana Z, Getachew B. Factors associated with low birthweight among newborns delivered at public health facilities of Nekemte town, West Ethiopia: a case control study. BMC Pregnancy Childbirth. 2019;19(1):220.
Finkelstein JL, Kurpad AV, Bose B, Thomas T, Srinivasan K, Duggan C. Anaemia and iron deficiency in pregnancy and adverse perinatal outcomes in Southern India. Eur J Clin Nutr. 2020;74(1):112–25.
Finkelstein JL, Herman HS, Plenty A, Mehta S, Natureeba P, Clark TD, Kamya MR, Ruel T, Charlebois ED, Cohan D, et al. Anemia and micronutrient status during pregnancy, and their Associations with Obstetric and Infant Outcomes among HIV-Infected Ugandan Women receiving antiretroviral therapy. Curr Dev Nutr. 2020;4(5):nzaa075.
Figueiredo A, Gomes-Filho IS, Batista JET, Orrico GS, Porto ECL, Cruz Pimenta RM, Dos Santos Conceicao S, Brito SM, Ramos MSX, Sena MCF, et al. Maternal anemia and birth weight: a prospective cohort study. PLoS ONE. 2019;14(3):e0212817.
Egbe TO, Ewane EN, Tendongfor N. Stillbirth rates and associated risk factors at the Buea and Limbe regional hospitals, Cameroon: a case-control study. BMC Pregnancy Childbirth. 2020;20(1):75.
Dessu S, Dawit Z. Perinatal mortality and Associated factors among Antenatal Care attended pregnant mothers at Public Hospitals in Gamo Zone, Southern Ethiopia. Front Pediatr. 2020;8:586747.
Chumak EL, Grjibovski AM. Anemia in pregnancy and its association with pregnancy outcomes in the Arctic Russian town of Monchegorsk, 1973–2002. Int J Circumpolar Health. 2010;69(3):265–77.
Chu FC, Shaw SW, Lo LM, Hsieh TT, Hung TH. Association between maternal anemia at admission for delivery and adverse perinatal outcomes. J Chin Med Assoc. 2020;83(4):402–7.
Chaudhary N, Yadav SN, Kalra SK, Pathak S, Gupta BK, Shrestha S, Patel M, Satia I, Sadhra S, Bolton CE, et al. Prognostic factors associated with small for gestational age babies in a tertiary care hospital of Western Nepal: a cross-sectional study. Health Sci Rep. 2021;4(1):e250.
Beckert RH, Baer RJ, Anderson JG, Jelliffe-Pawlowski LL, Rogers EE. Maternal anemia and pregnancy outcomes: a population-based study. J Perinatol. 2019;39(7):911–9.
Ardic C, Usta O, Omar E, Yildiz C, Memis E, Zeren Ozturk G. Relationship between anaemia during pregnancy and preterm delivery. J Obstet Gynaecol. 2019;39(7):903–6.
Ali SA, Tikmani SS, Saleem S, Patel AB, Hibberd PL, Goudar SS, Dhaded S, Derman RJ, Moore JL, McClure EM, et al. Hemoglobin concentrations and adverse birth outcomes in south asian pregnant women: findings from a prospective maternal and neonatal Health Registry. Reprod Health. 2020;17(Suppl 2):154.
Alemu B, Gashu D. Association of maternal anthropometry, hemoglobin and serum zinc concentration during pregnancy with birth weight. Early Hum Dev. 2020;142:104949.
Ajepe AA, Okunade KS, Sekumade AI, Daramola ES, Beke MO, Ijasan O, Olowoselu OF, Afolabi BB. Prevalence and foetomaternal effects of iron deficiency anaemia among pregnant women in Lagos, Nigeria. PLoS ONE. 2020;15(1):e0227965.
Ahmed S, Hassen K, Wakayo T. A health facility based case-control study on determinants of low birth weight in Dassie town, Northeast Ethiopia: the role of nutritional factors. Nutr J. 2018;17(1):103.
Adler L, Tsamir J, Katz R, Koren G, Yehoshua I. Associations of sociodemographic and clinical factors with perinatal depression among israeli women: a cross-sectional study. BMC Psychiatry. 2019;19(1):331.
Adam Z, Ameme DK, Nortey P, Afari EA, Kenu E. Determinants of low birth weight in neonates born in three hospitals in Brong Ahafo region, Ghana, 2016- an unmatched case-control study. BMC Pregnancy Childbirth. 2019;19(1):174.
Abera Z, Ejara D, Gebremedhin S. Nutritional and non-nutritional factors associated with low birth weight in Sawula Town, Gamo Gofa Zone, Southern Ethiopia. BMC Res Notes. 2019;12(1):540.
Abadiga M, Wakuma B, Oluma A, Fekadu G, Hiko N, Mosisa G. Determinants of preterm birth among women delivered in public hospitals of western Ethiopia, 2020: unmatched case-control study. PLoS ONE. 2021;16(1):e0245825.
Zhou LM, Yang WW, Hua JZ, Deng CQ, Tao X, Stoltzfus RJ. Relation of hemoglobin measured at different times in pregnancy to preterm birth and low birth weight in Shanghai, China. Am J Epidemiol. 1998;148(10):998–1006.
Zhang Y, Li Z, Li H, Jin L, Zhang Y, Zhang L, Liu J, Ye R, Liu J, Ren A. Maternal haemoglobin concentration and risk of preterm birth in a chinese population. J Obstet Gynaecol. 2018;38(1):32–7.
Zhang X, Xu Q, Yang Y, Wang L, Liu F, Li Q, Ji M, He Y, Wang Y, Zhang Y, et al. Preconception hb concentration and risk of preterm birth in over 2.7 million chinese women aged 20–49 years: a population-based cohort study. Br J Nutr. 2018;120(5):508–16.
Zhang J, Cai WW, Lee DJ. Pregnancy-induced hypertension and early neonatal death: a case-control study. Am J Perinatol. 1993;10(5):401–3.
Zhang J, Cai WW. Risk factors associated with antepartum fetal death. Early Hum Dev. 1992;28(3):193–200.
Yi SW, Han YJ, Ohrr H. Anemia before pregnancy and risk of preterm birth, low birth weight and small-for-gestational-age birth in korean women. Eur J Clin Nutr. 2013;67(4):337–42.
Yatich NJ, Funkhouser E, Ehiri JE, Agbenyega T, Stiles JK, Rayner JC, Turpin A, Ellis WO, Jiang Y, Williams JH et al. Malaria, intestinal helminths and other risk factors for stillbirth in Ghana. Infect Dis Obstet Gynecol. 2010;2010:350763.
Xiong X, Buekens P, Fraser WD, Guo Z. Anemia during pregnancy in a chinese population. Int J Gynaecol Obstet. 2003;83(2):159–64.
Wang C, Lin L, Su R, Zhu W, Wei Y, Yan J, Feng H, Li B, Li S, Yang H. Hemoglobin levels during the first trimester of pregnancy are associated with the risk of gestational diabetes mellitus, pre-eclampsia and preterm birth in chinese women: a retrospective study. BMC Pregnancy Childbirth. 2018;18(1):263.
Walker SP, Ewan-Whyte C, Chang SM, Powell CA, Fletcher H, McDonald D, Grantham-McGregor SM. Factors associated with size and proportionality at birth in term jamaican infants. J Health Popul Nutr. 2003;21(2):117–26.
Verhoeff FH, Brabin BJ, van Buuren S, Chimsuku L, Kazembe P, Wit JM, Broadhead RL. An analysis of intra-uterine growth retardation in rural Malawi. Eur J Clin Nutr. 2001;55(8):682–9.
Unger HW, Ome-Kaius M, Karl S, Singirok D, Siba P, Walker J, Wangnapi RA, Mueller I, Rogerson SJ. Factors associated with ultrasound-aided detection of suboptimal fetal growth in a malaria-endemic area in Papua New Guinea. BMC Pregnancy Childbirth. 2015;15:83.
Tzur T, Weintraub AY, Sergienko R, Sheiner E. Can anemia in the first trimester predict obstetrical complications later in pregnancy? J Matern Fetal Neonatal Med. 2012;25(11):2454–7.
Tsu VD. Postpartum haemorrhage in Zimbabwe: a risk factor analysis. Br J Obstet Gynaecol. 1993;100(4):327–33.
Thakur N, Saili A, Kumar A, Kumar V. Predictors of mortality of extremely low birthweight babies in a tertiary care centre of a developing country. Postgrad Med J. 2013;89(1058):679–84.
Tandu-Umba B, Mbangama AM. Association of maternal anemia with other risk factors in occurrence of great obstetrical syndromes at university clinics, Kinshasa, DR Congo. BMC Pregnancy Childbirth. 2015;15:183.
Stephansson O, Dickman PW, Johansson A, Cnattingius S. Maternal hemoglobin concentration during pregnancy and risk of stillbirth. JAMA. 2000;284(20):2611–7.
Steer P, Alam MA, Wadsworth J, Welch A. Relation between maternal haemoglobin concentration and birth weight in different ethnic groups. BMJ. 1995;310(6978):489–91.
Smithers LG, Gialamas A, Scheil W, Brinkman S, Lynch JW. Anaemia of pregnancy, perinatal outcomes and children’s developmental vulnerability: a whole-of-population study. Paediatr Perinat Epidemiol. 2014;28(5):381–90.
Shehata N, Chasse M, Colas JA, Murphy M, Forster AJ, Malinowski AK, Ducharme R, Fergusson DA, Tinmouth A, Wilson K. Risks and trends of red blood cell transfusion in obstetric patients: a retrospective study of 45,213 deliveries using administrative data. Transfusion. 2017;57(9):2197–205.
Sharma SR, Giri S, Timalsina U, Bhandari SS, Basyal B, Wagle K, Shrestha L. Low birth weight at term and its determinants in a tertiary hospital of Nepal: a case-control study. PLoS ONE. 2015;10(4):e0123962.
Scholl TO, Hediger ML, Fischer RL, Shearer JW. Anemia vs iron deficiency: increased risk of preterm delivery in a prospective study. Am J Clin Nutr. 1992;55(5):985–8.
Scholl TO, Hediger ML. Anemia and iron-deficiency anemia: compilation of data on pregnancy outcome.Am J Clin Nutr1994, 59(2 Suppl):492S-500S discussion 500S.
Schmiegelow C, Minja D, Oesterholt M, Pehrson C, Suhrs HE, Bostrom S, Lemnge M, Magistrado P, Rasch V, Lusingu J, et al. Factors associated with and causes of perinatal mortality in northeastern Tanzania. Acta Obstet Gynecol Scand. 2012;91(9):1061–8.
Scanlon KS, Yip R, Schieve LA, Cogswell ME. High and low hemoglobin levels during pregnancy: differential risks for preterm birth and small for gestational age. Obstet Gynecol. 2000;96(5 Pt 1):741–8.
Saeed OA, Ahmed HA, Ibrahim AM, Mahmood EA, Abdu-Allah TO. Risk factors of low birth weight at three hospitals in Khartoum State, Sudan. Sudan J Paediatr. 2014;14(2):22–8.
Rukuni R, Bhattacharya S, Murphy MF, Roberts D, Stanworth SJ, Knight M. Maternal and neonatal outcomes of antenatal anemia in a scottish population: a retrospective cohort study. Acta Obstet Gynecol Scand. 2016;95(5):555–64.
Ronnenberg AG, Wood RJ, Wang X, Xing H, Chen C, Chen D, Guang W, Huang A, Wang L, Xu X. Preconception hemoglobin and ferritin concentrations are associated with pregnancy outcome in a prospective cohort of chinese women. J Nutr. 2004;134(10):2586–91.
Ribot B, Isern R, Hernandez-Martinez C, Canals J, Aranda N, Arija V. [Effects of tobacco habit, second-hand smoking and smoking cessation during pregnancy on newborn’s health]. Med Clin (Barc). 2014;143(2):57–63.
Ren A, Wang J, Ye RW, Li S, Liu JM, Li Z. Low first-trimester hemoglobin and low birth weight, preterm birth and small for gestational age newborns. Int J Gynaecol Obstet. 2007;98(2):124–8.
Raisanen S, Kancherla V, Gissler M, Kramer MR, Heinonen S. Adverse perinatal outcomes associated with moderate or severe maternal anaemia based on parity in Finland during 2006-10. Paediatr Perinat Epidemiol. 2014;28(5):372–80.
Raisanen S, Gissler M, Saari J, Kramer M, Heinonen S. Contribution of risk factors to extremely, very and moderately preterm births - register-based analysis of 1,390,742 singleton births. PLoS ONE. 2013;8(4):e60660.
Poespoprodjo JR, Fobia W, Kenangalem E, Lampah DA, Warikar N, Seal A, McGready R, Sugiarto P, Tjitra E, Anstey NM, et al. Adverse pregnancy outcomes in an area where multidrug-resistant plasmodium vivax and Plasmodium falciparum infections are endemic. Clin Infect Dis. 2008;46(9):1374–81.
Phaloprakarn C, Tangjitgamol S. Impact of high maternal hemoglobin at first antenatal visit on pregnancy outcomes: a cohort study. J Perinat Med. 2008;36(2):115–9.
Patel A, Prakash AA, Das PK, Gupta S, Pusdekar YV, Hibberd PL. Maternal anemia and underweight as determinants of pregnancy outcomes: cohort study in eastern rural Maharashtra, India. BMJ Open. 2018;8(8):e021623.
Ota E, Ganchimeg T, Morisaki N, Vogel JP, Pileggi C, Ortiz-Panozo E, Souza JP, Mori R. Risk factors and adverse perinatal outcomes among term and preterm infants born small-for-gestational-age: secondary analyses of the WHO Multi-Country Survey on maternal and Newborn Health. PLoS ONE. 2014;9(8):e105155.
Obadi MA, Taher R, Qayad M, Khader YS. Risk factors of stillbirth in Yemen. J neonatal-perinatal Med. 2018;11(2):131–6.
Nyflot LT, Sandven I, Stray-Pedersen B, Pettersen S, Al-Zirqi I, Rosenberg M, Jacobsen AF, Vangen S. Risk factors for severe postpartum hemorrhage: a case-control study. BMC Pregnancy Childbirth. 2017;17(1):17.
Nair M, Churchill D, Robinson S, Nelson-Piercy C, Stanworth SJ, Knight M. Association between maternal haemoglobin and stillbirth: a cohort study among a multi-ethnic population in England. Br J Haematol. 2017;179(5):829–37.
Mumbare SS, Maindarkar G, Darade R, Yenge S, Tolani MK, Patole K. Maternal risk factors associated with term low birth weight neonates: a matched-pair case control study. Indian Pediatr. 2012;49(1):25–8.
Msuya SE, Hussein TH, Uriyo J, Sam NE, Stray-Pedersen B. Anaemia among pregnant women in northern Tanzania: prevalence, risk factors and effect on perinatal outcomes. Tanzan J Health Res. 2011;13(1):33–9.
Mola G, Permezel M, Amoa AB, Klufio CA. Anaemia and perinatal outcome in Port Moresby. Aust N Z J Obstet Gynaecol. 1999;39(1):31–4.
Mohamed MA, Ahmad T, Macri C, Aly H. Racial disparities in maternal hemoglobin concentrations and pregnancy outcomes. J Perinat Med. 2012;40(2):141–9.
Meis PJ, Michielutte R, Peters TJ, Wells HB, Sands RE, Coles EC, Johns KA. Factors associated with preterm birth in Cardiff, Wales. I. Univariable and multivariable analysis. Am J Obstet Gynecol. 1995;173(2):590–6.
Masukume G, Khashan AS, Kenny LC, Baker PN, Nelson G. Risk factors and birth outcomes of anaemia in early pregnancy in a nulliparous cohort. PLoS ONE. 2015;10(4):e0122729.
Marti A, Pena-Marti G, Munoz S, Lanas F, Comunian G. Association between prematurity and maternal anemia in venezuelan pregnant women during third trimester at labor. Arch Latinoam Nutr. 2001;51(1):44–8.
Marchant T, Schellenberg JA, Nathan R, Abdulla S, Mukasa O, Mshinda H, Lengeler C. Anaemia in pregnancy and infant mortality in Tanzania. Trop Med Int Health. 2004;9(2):262–6.
Mamun AA, Padmadas SS, Khatun M. Maternal health during pregnancy and perinatal mortality in Bangladesh: evidence from a large-scale community-based clinical trial. Paediatr Perinat Epidemiol. 2006;20(6):482–90.
Malhotra M, Sharma JB, Batra S, Sharma S, Murthy NS, Arora R. Maternal and perinatal outcome in varying degrees of anemia. Int J Gynaecol Obstet. 2002;79(2):93–100.
Lone FW, Qureshi RN, Emanuel F. Maternal anaemia and its impact on perinatal outcome. Trop Med Int Health. 2004;9(4):486–90.
Levy A, Fraser D, Katz M, Mazor M, Sheiner E. Maternal anemia during pregnancy is an independent risk factor for low birthweight and preterm delivery. Eur J Obstet Gynecol Reprod Biol. 2005;122(2):182–6.
Lao TT, Chan LY, Tam KF, Ho LF. Maternal hemoglobin and risk of gestational diabetes mellitus in chinese women. Obstet Gynecol. 2002;99(5 Pt 1):807–12.
Koura GK, Ouedraogo S, Le Port A, Watier L, Cottrell G, Guerra J, Choudat I, Rachas A, Bouscaillou J, Massougbodji A, et al. Anaemia during pregnancy: impact on birth outcome and infant haemoglobin level during the first 18 months of life. Trop Med Int Health. 2012;17(3):283–91.
Knottnerus JA, Delgado LR, Knipschild PG, Essed GG, Smits F. Haematologic parameters and pregnancy outcome. A prospective cohort study in the third trimester. J Clin Epidemiol. 1990;43(5):461–6.
Khattar D, Awasthi S, Das V. Residential environmental tobacco smoke exposure during pregnancy and low birth weight of neonates: case control study in a public hospital in Lucknow, India. Indian Pediatr. 2013;50(1):134–8.
Khan NS, Ashraf RN, Noor S, Mahmood ur R, Mashhadi SF, Rashid Z, Sajjad F, Nazar AF, Nazar HS, Syed R. ASSOCIATION OF MATERNAL PERIODONTITIS WITH LOW BIRTH WEIGHT IN NEWBORNS IN A TERTIARY CARE HOSPITAL. J Ayub Med Coll Abbottabad. 2016;28(1):120–5.
Kattula D, Sarkar R, Sivarathinaswamy P, Velusamy V, Venugopal S, Naumova EN, Muliyil J, Ward H, Kang G. The first 1000 days of life: prenatal and postnatal risk factors for morbidity and growth in a birth cohort in southern India. BMJ Open. 2014;4(7):e005404.
Kalanda BF, Verhoeff FH, Chimsuku L, Harper G, Brabin BJ. Adverse birth outcomes in a malarious area. Epidemiol Infect. 2006;134(3):659–66.
Jaleel R, Khan A. Post-partum haemorrhage–a risk factor analysis. Mymensingh Med J. 2010;19(2):282–9.
Hwang HS, Kim YH, Kwon JY, Park YW. Uterine and umbilical artery Doppler velocimetry as a predictor for adverse pregnancy outcomes in pregnant women with anemia. J Perinat Med. 2010;38(5):467–71.
Hinderaker SG, Olsen BE, Bergsjo PB, Gasheka P, Lie RT, Kvale G. Perinatal mortality in northern rural Tanzania. J Health Popul Nutr. 2003;21(1):8–17.
Hamalainen H, Hakkarainen K, Heinonen S. Anaemia in the first but not in the second or third trimester is a risk factor for low birth weight. Clin Nutr. 2003;22(3):271–5.
Gonzales GF, Tapia V, Gasco M, Carrillo CE, Fort AL. Association of hemoglobin values at booking with adverse maternal outcomes among peruvian populations living at different altitudes. Int J Gynaecol Obstet. 2012;117(2):134–9.
Gonzales GF, Tapia V, Gasco M. Correcting haemoglobin cut-offs to define anaemia in high-altitude pregnant women in Peru reduces adverse perinatal outcomes. Arch Gynecol Obstet. 2014;290(1):65–74.
Gonzales GF, Steenland K, Tapia V. Maternal hemoglobin level and fetal outcome at low and high altitudes. Am J Physiol Regul Integr Comp Physiol. 2009;297(5):R1477–1485.
Getiye Y, Fantahun M. Factors associated with perinatal mortality among public health deliveries in Addis Ababa, Ethiopia, an unmatched case control study. BMC Pregnancy Childbirth. 2017;17(1):245.
Geelhoed D, Agadzi F, Visser L, Ablordeppey E, Asare K, O’Rourke P, Van Leeuwen JS, Van Roosmalen J. Maternal and fetal outcome after severe anemia in pregnancy in rural Ghana. Acta Obstet Gynecol Scand. 2006;85(1):49–55.
Ganesh Kumar S, Harsha Kumar HN, Jayaram S, Kotian MS. Determinants of low birth weight: a case control study in a district hospital in Karnataka. Indian J Pediatr. 2010;77(1):87–9.
Gaillard R, Eilers PH, Yassine S, Hofman A, Steegers EA, Jaddoe VW. Risk factors and consequences of maternal anaemia and elevated haemoglobin levels during pregnancy: a population-based prospective cohort study. Paediatr Perinat Epidemiol. 2014;28(3):213–26.
Ferdous J, Ahmed A, Dasgupta SK, Jahan M, Huda FA, Ronsmans C, Koblinsky M, Chowdhury ME. Occurrence and determinants of postpartum maternal morbidities and disabilities among women in Matlab, Bangladesh. J Health Popul Nutr. 2012;30(2):143–58.
Eng C, Karki S, Trivedi AN. Risk factors of stillbirths in Victoria (Australia): a case-control study. J Obstet Gynaecol. 2016;36(6):754–7.
Elhassan EM, Abbaker AO, Haggaz AD, Abubaker MS, Adam I. Anaemia and low birth weight in Medani, Hospital Sudan. BMC Res Notes. 2010;3:181.
Ehrenthal DB, Chichester ML, Cole OS, Jiang X. Maternal risk factors for peripartum transfusion. J Womens Health (Larchmt). 2012;21(7):792–7.
Drukker L, Hants Y, Farkash R, Ruchlemer R, Samueloff A, Grisaru-Granovsky S. Iron deficiency anemia at admission for labor and delivery is associated with an increased risk for cesarean section and adverse maternal and neonatal outcomes. Transfusion. 2015;55(12):2799–806.
Domple VK, Doibale MK, Nair A, Rajput PS. Assessment of maternal risk factors associated with low birth weight neonates at a tertiary hospital, Nanded, Maharashtra. Niger Med J. 2016;57(1):37–43.
Delpisheh A, Brabin L, Drummond S, Brabin BJ. Prenatal smoking exposure and asymmetric fetal growth restriction. Ann Hum Biol. 2008;35(6):573–83.
Cung TG, Paus AS, Aghbar A, Kiserud T, Hinderaker SG. Stillbirths at a hospital in Nablus, 2010: a cohort study. Glob Health Action. 2014;7:25222.
Chumak EL, Grijbovski AM. Association between different levels of hemoglobin in pregnancy and pregnancy outcomes: a registry-based study in Northwest Russia. Int J Circumpolar Health. 2016;70(5):457–9.
Chen JH, Guo XF, Liu S, Long JH, Zhang GQ, Huang MC, Qiu XQ. [Impact and changes of maternal hemoglobin on birth weight in pregnant women of Zhuang Nationality, in Guangxi]. Zhonghua Liu Xing Bing Xue Za Zhi. 2017;38(2):154–7.
Chen C, Grewal J, Betran AP, Vogel JP, Souza JP, Zhang J. Severe anemia, sickle cell disease, and thalassemia as risk factors for hypertensive disorders in pregnancy in developing countries. Pregnancy Hypertens. 2018;13:141–7.
Butwick AJ, Ramachandran B, Hegde P, Riley ET, El-Sayed YY, Nelson LM. Risk factors for severe Postpartum Hemorrhage after Cesarean Delivery: case-control studies. Anesth Analg; 2017.
Borah M, Agarwalla R. Maternal and socio-demographic determinants of low birth weight (LBW): a community-based study in a rural block of Assam. J Postgrad Med. 2016;62(3):178–81.
Bodeau-Livinec F, Briand V, Berger J, Xiong X, Massougbodji A, Day KP, Cot M. Maternal anemia in Benin: prevalence, risk factors, and association with low birth weight. Am J Trop Med Hyg. 2011;85(3):414–20.
Bilano VL, Ota E, Ganchimeg T, Mori R, Souza JP. Risk factors of pre-eclampsia/eclampsia and its adverse outcomes in low- and middle-income countries: a WHO secondary analysis. PLoS ONE. 2014;9(3):e91198.
Bian Y, Zhang Z, Liu Q, Wu D, Wang S. Maternal risk factors for low birth weight for term births in a developed region in China: a hospital-based study of 55,633 pregnancies. J Biomed Res. 2013;27(1):14–22.
Banhidy F, Acs N, Puho EH, Czeizel AE. Iron deficiency anemia: pregnancy outcomes with or without iron supplementation. Nutrition. 2011;27(1):65–72.
Baig SA, Khan N, Baqai T, Fatima A, Karim SA, Aziz S. Preterm birth and its associated risk factors. A study at tertiary care hospitals of Karachi, Pakistan. J Pak Med Assoc. 2013;63(3):414–8.
Bader E, Alhaj AM, Hussan AA, Adam I. Malaria and stillbirth in Omdurman Maternity Hospital, Sudan. Int J Gynaecol Obstet. 2010;109(2):144–6.
Alwan NA, Cade JE, McArdle HJ, Greenwood DC, Hayes HE, Simpson NA. Maternal iron status in early pregnancy and birth outcomes: insights from the Baby’s vascular health and Iron in pregnancy study. Br J Nutr. 2015;113(12):1985–92.
Ali AA, Rayis DA, Abdallah TM, Elbashir MI, Adam I. Severe anaemia is associated with a higher risk for preeclampsia and poor perinatal outcomes in Kassala hospital, eastern Sudan. BMC Res Notes. 2011;4:311.
Adams MM, Sarno AP, Harlass FE, Rawlings JS, Read JA. Risk factors for preterm delivery in a healthy cohort. Epidemiology. 1995;6(5):525–32.
Adam I, Haggaz AD, Mirghani OA, Elhassan EM. Placenta previa and pre-eclampsia: analyses of 1645 cases at medani maternity hospital, Sudan. Front Physiol. 2013;4:32.
Abeysena C, de Jayawardana P. Maternal haemoglobin level at booking visit and its effect on adverse pregnancy outcome. Aust N Z J Obstet Gynaecol. 2010;50(5):423–7.
Babu GR, Murthy GVS, Singh N, Nath A, Rathnaiah M, Saldanha N, Deepa R, Kinra S. Sociodemographic and medical risk factors Associated with Antepartum Depression. Front Public Health. 2018;6:127.
Jessani S, Saleem S, Hoffman MK, Goudar SS, Derman RJ, Moore JL, Garces A, Figueroa L, Krebs NF, Okitawutshu J, et al. Association of haemoglobin levels in the first trimester and at 26–30 weeks with fetal and neonatal outcomes: a secondary analysis of the Global Network for Women’s and Children’s Health’s ASPIRIN Trial. BJOG. 2021;128(9):1487–96.
Woldetensay YK, Belachew T, Biesalski HK, Ghosh S, Lacruz ME, Scherbaum V, Kantelhardt EJ. The role of nutrition, intimate partner violence and social support in prenatal depressive symptoms in rural Ethiopia: community based birth cohort study. BMC Pregnancy Childbirth. 2018;18(1):374.
Goshtasebi A, Alizadeh M, Gandevani SB. Association between maternal anaemia and postpartum depression in an urban sample of pregnant women in Iran. J Health Popul Nutr. 2013;31(3):398–402.
Kozuki N, Lee AC, Katz J, Child Health Epidemiology Reference G. Moderate to severe, but not mild, maternal anemia is associated with increased risk of small-for-gestational-age outcomes. J Nutr. 2012;142(2):358–62.
Rahmati S, Delpishe A, Azami M, Hafezi Ahmadi MR, Sayehmiri K. Maternal Anemia during pregnancy and infant low birth weight: a systematic review and Meta-analysis. Int J Reprod Biomed (Yazd). 2017;15(3):125–34.
Sukrat B, Wilasrusmee C, Siribumrungwong B, McEvoy M, Okascharoen C, Attia J, Thakkinstian A. Hemoglobin concentration and pregnancy outcomes: a systematic review and meta-analysis. Biomed Res Int. 2013;2013:769057.
Mireku MO, Davidson LL, Koura GK, Ouedraogo S, Boivin MJ, Xiong X, Accrombessi MM, Massougbodji A, Cot M, Bodeau-Livinec F. Prenatal hemoglobin levels and early cognitive and motor functions of one-year-old children. Pediatrics. 2015;136(1):e76–83.
Haider BA, Olofin I, Wang M, Spiegelman D, Ezzati M, Fawzi WW. Nutrition Impact Model Study G: anaemia, prenatal iron use, and risk of adverse pregnancy outcomes: systematic review and meta-analysis. BMJ. 2013;346:f3443.
Rahman MM, Abe SK, Rahman MS, Kanda M, Narita S, Bilano V, Ota E, Gilmour S, Shibuya K. Maternal anemia and risk of adverse birth and health outcomes in low- and middle-income countries: systematic review and meta-analysis. Am J Clin Nutr. 2016;103(2):495–504.
Figueiredo A, Gomes-Filho IS, Silva RB, Pereira PPS, Mata F, Lyrio AO, Souza ES, Cruz SS, Pereira MG. Maternal Anemia and Low Birth Weight: A Systematic Review and Meta-Analysis.Nutrients2018, 10(5).
Vricella LK. Emerging understanding and measurement of plasma volume expansion in pregnancy. Am J Clin Nutr. 2017;106(Suppl 6):1620s–5.
Di Renzo GC, Spano F, Giardina I, Brillo E, Clerici G, Roura LC. Iron Deficiency Anemia in pregnancy. Women’s Health. 2015;11(6):891–900.
Wawer AA, Hodyl NA, Fairweather-Tait S, Froessler B. Are Pregnant Women Who Are Living with Overweight or Obesity at Greater Risk of Developing Iron Deficiency/Anaemia? Nutrients 2021, 13(5).
Bothwell TH. Iron requirements in pregnancy and strategies to meet them. Am J Clin Nutr. 2000;72(1 Suppl):257S–64.
Zimmermann MB, Hurrell RF. Nutritional iron deficiency. Lancet. 2007;370(9586):511–20.
Capanzana MV, MA LM, Smith G, Angeles-Agdeppa I, Perlas L, Los Reyes F, Amarra MS. Thalassemia and other hemoglobinopathies among anemic individuals in Metro Manila, Philippines and their intake of iron supplements. Asia Pac J Clin Nutr. 2018;27(3):519–26.
Merrill RD, Shamim AA, Ali H, Labrique AB, Schulze K, Christian P, West KP Jr. High prevalence of anemia with lack of iron deficiency among women in rural Bangladesh: a role for thalassemia and iron in groundwater. Asia Pac J Clin Nutr. 2012;21(3):416–24.
Guimaraes JS, Cominal JG, Silva-Pinto AC, Olbina G, Ginzburg YZ, Nandi V, Westerman M, Rivella S, de Souza AM. Altered erythropoiesis and iron metabolism in carriers of thalassemia. Eur J Haematol. 2015;94(6):511–8.
Salas SP, Marshall G, Gutierrez BL, Rosso P. Time course of maternal plasma volume and hormonal changes in women with preeclampsia or fetal growth restriction. Hypertension. 2006;47(2):203–8.
Burton GJ, Redman CW, Roberts JM, Moffett A. Pre-eclampsia: pathophysiology and clinical implications. BMJ. 2019;366:l2381.
Roberts JM, Lain KY. Recent insights into the pathogenesis of pre-eclampsia. Placenta. 2002;23(5):359–72.
Melchiorre K, Sharma R, Khalil A, Thilaganathan B. Maternal Cardiovascular function in normal pregnancy: evidence of maladaptation to chronic volume overload. Hypertension. 2016;67(4):754–62.
Thilaganathan B. Association of higher maternal blood pressure with Lower Infant Birthweight: placental cause or Cardiovascular Effect? Hypertension. 2016;67(3):499–500.
Casanueva E, Viteri FE. Iron and oxidative stress in pregnancy. J Nutr. 2003;133(5 Suppl 2):1700S–8.
Kehrer JP. The Haber-Weiss reaction and mechanisms of toxicity. Toxicology. 2000;149(1):43–50.
Fernandez-Real JM, Lopez-Bermejo A, Ricart W. Cross-talk between iron metabolism and diabetes. Diabetes. 2002;51(8):2348–54.
Kataria Y, Wu Y, Horskjaer PH, Mandrup-Poulsen T, Ellervik C. Iron Status and Gestational Diabetes-A Meta-Analysis.Nutrients2018, 10(5).
Steer PJ. Results of pre-eclampsia screening vary by race; cut-offs versus continuums. BJOG. 2023;130(1):88.
Clark SL, Saade GA, Tolcher MC, Belfort MA, Rouse DW, Barton JR, Silver RM, Sibai BM. Gestational hypertension and “severe” disease: time for a change.Am J Obstet Gynecol2022.
Viera AJ. Odds ratios and risk ratios: what’s the difference and why does it matter? South Med J. 2008;101(7):730–4.
Acknowledgements
We would like to thank the Emory University Library staff (Shenita Peterson) for their assistance with search strategy and data extraction. Original review was commissioned and financially supported by the Evidence and Programme Guidance Unit, Department of Nutrition for Health and Development of the World Health Organization (WHO), Geneva, Switzerland.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
MFY, BMO, ST, PR, RM, KGD, ASW were involved in study design and protocol development. PR, ST, MFY, BMO, ASW led data abstraction and analysis. MFY, PR, ST, BMO, ASW contributed to writing manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
No authors have conflict of interests to report.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Young, M.F., Oaks, B.M., Rogers, H.P. et al. Maternal low and high hemoglobin concentrations and associations with adverse maternal and infant health outcomes: an updated global systematic review and meta-analysis. BMC Pregnancy Childbirth 23, 264 (2023). https://doi.org/10.1186/s12884-023-05489-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12884-023-05489-6