Abstract
Background
Third trimester fetal anthropometric parameters are known to predict neonatal complications. A better understanding of predictors of adverse fetal parameters might help to personalize the use and frequency of fetal ultrasound. The objectives of this study were:
(a) to evaluate the utility of maternal sociodemographic, anthropometric and metabolic predictors to predict 3rd trimester fetal anthropometric parameters in women with gestational diabetes mellitus (GDM),
(b) to assess whether the impact of these maternal predictors is fetal sex-dependent, and
(c) to provide a risk stratification for markers of fetal overgrowth (fetal weight centile (FWC) and fetal abdominal circumference centile (FACC) depending on prepregnancy BMI and gestational weight gain (GWG) until the 1st GDM visit.
Methods
This prospective study included 189 women with GDM. Maternal predictors were age, ethnicity, prepregnancy BMI, GWG and excessive weight gain until the 1st GDM visit, fasting, 1-hour and 2-hour blood glucose oral glucose tolerance test values, HbA1c at the 1st visit and medical treatment requirement. Fetal outcomes included FWC, FWC >90% and <10%, FACC, FACC >90% and <10%, at 29 0/7 to 35 6/7 weeks of gestational age. We performed univariate and multivariate regression analyses and probability analyses.
Results
In multivariate analyses, prepregnancy BMI was associated with FWC, FWC > 90% and FACC. GWG until the 1st GDM visit was associated with FWC, FACC and FACC > 90% (all p ≤ 0.045). Other maternal parameters were not significantly associated with fetal anthropometry in multivariate analyses (all p ≥ 0.054). In female fetuses, only GWG was associated with FACC (p= 0.044). However, in male fetuses, prepregnancy BMI was associated with FWC, FWC > 90% and FACC and GWG with FWC in multivariate analyses (all p ≤ 0.030). In women with a prepregnancy BMI of ≥ 25 kg/m2 and a GWG until the 1st GDM visit ≥ 10.3 kg (mean GWG), the risk for FWC > 90% and FACC > 90% was 5.3 and 4 times higher than in their counterparts.
Conclusions
A personalized fetal ultrasound surveillance guided by fetal sex, prepregnancy BMI and GWG may be beneficial in reducing adverse fetal and neonatal outcomes.
Similar content being viewed by others
Background
Gestational diabetes mellitus (GDM), defined as any degree of glucose intolerance with onset or first recognition during pregnancy, exposes both mothers and their offspring to short and long-term adverse outcomes [1,2,3,4,5,6]. Among these, large for gestational and macrosomia age are one of the most frequent adverse neonatal outcomes and they also have a more long-term impact [7, 8]. Maternal prepregnancy BMI and gestational weight gain are associated with adverse neonatal outcomes such as LGA [9,10,11,12,13] . Weight gain in excess of the weight gain recommended by the Institute of Medicine [14] significantly increases the risk for LGA, as well as other neonatal complications, including hypoglycemia, polycythemia, low 5-minute Apgar score, meconium aspiration syndrome [15, 16].
A recent analysis of our group has shown that 3rd trimester fetal anthropometric parameters, such as fetal weight centile (FWC), FWC > 90%, fetal abdominal circumference centile (FACC), and FACC > 90% can predict neonatal complications, including small and large for gestational age (SGA, LGA), prematurity, and emergency cesarean section [17].
Only a few studies have investigated the impact of maternal anthropometric and metabolic parameters on fetal anthropometry in late pregnancy; these involved predominantly healthy pregnancies with low GDM incidence. A population-based study by Galjaard et al. [18] showed a positive correlation between gestational weight gain (GWG) and estimated fetal weight (EFW) apparent from the end of the 2nd trimester. Moreover, prepregnancy BMI has also been associated with EFW especially from midpregnancy onward, in predominantly healthy populations [18,19,20]. In a Korean study population with low GDM incidence (5.1%), maternal age and HbA1c at 24-28 gestational weeks were associated with fetal abdominal overgrowth ratios [21]. In a small cross-sectional study including 19 women with normal glucose tolerance and 12 women with gestational diabetes, estimated fetal weight in late gestation (32-36.6 gestational weeks) was correlated with hepatic glucose production and insulin sensitivity glucose infusion rate at the same timepoint [22].
Male sex has been found to be an independent factor for adverse pregnancy outcomes in predominantly healthy as well as GDM populations [23, 24]. Furthermore, the impact of GDM on fetal abdominal circumference could also be more pronounced in male fetuses, which could mean that this sexual dimorphism starts in utero [25]. Thus, Macaulay et al [25] found a positive correlation between GDM diagnosis and fetal abdominal circumference in the whole population, but when stratified by sex, this was only observed in the male fetuses.
Data on the association between maternal characteristics and 3rd trimester fetal anthropometry in pregnancies with GDM are still lacking. Moreover, it is unknown whether these associations follow a sex dimorphism. A personalized follow-up based on maternal characteristics, fetal sex and 3rd trimester anthropometry could possibly lead to a reduction of neonatal complications and long-term adverse outcomes in the offspring.
The objectives of this study were: (a) to evaluate the utility of maternal sociodemographic, anthropometric and metabolic parameters for the prediction of 3rd trimester fetal anthropometric parameters known to be associated with adverse neonatal outcomes in women with GDM, (b) to assess whether the impact of these maternal parameters is fetal sex-dependent and (c) to provide a risk stratification for FWC > 90% and FACC > 90% depending on the prepregnancy BMI and GWG until the 1st GDM visit.
Methods
This prospective observational study included pregnant women with GDM followed in the Diabetes and Pregnancy Unit in the Centre Hospitalier Universitaire Vaudois (CHUV), Lausanne, Switzerland, between April 2012 and October 2017. Detailed information on the materials and methods have been included in previous publications of our group [12, 17, 26,27,28,29] Briefly, all women with GDM who had signed an informed consent were included in this study. Exclusion criteria for the current analysis were: multiple gestation, pregestational diabetes or diabetes diagnosed before 13 weeks of gestation, missing fetal sex and missing fetal ultrasound data between 29 0/7 and 35 6/7 gestational weeks.
GDM was diagnosed according to the International Association of the Diabetes and Pregnancy Study Groups criteria [30], with a 75-g oral glucose tolerance test (oGTT) at 24–28 gestational weeks. The treatment was based on the latest guidelines of the American Diabetes Association [31] and of the Endocrine Society [32]. The patients were followed by a multidisciplinary team specialised in GDM, composed by medical doctors, nurses, and dieticians. At their first clinical appointment, patients received information on GDM, and were instructed on lifestyle adaptations and how to perform a capillary blood glucose test. There were seen a week later by a dietician, who provided them with advice to optimal glycemic control, optimal gestational weight gain and a balanced diet. Women were encouraged to increase physical activity and were offered the possibility to receive physical activity counselling by a physiotherapist, and to participate in GDM physical activity groups.
According to international and local guidelines (Vaud Cantonal Diabetes Program women were asked to check their capillary glucose values 4x/day. If, despite lifestyle changes, glucose values remained above targets, metformin or insulin treatment was introduced, according to guidelines [30,31,32,33].
Maternal predictors and fetal anthropometric outcomes
Maternal predictors included age, ethnicity, prepregnancy body mass index (BMI), GWG until the 1st GDM visit, excessive weight gain (EWG) up to the 1st GDM visit, fasting, 1-h and 2-h blood glucose values during the 75g oGTT at 24–28 weeks of GA, HbA1c at 1st GDM visit, and glucose lowering medical treatment requirement (metformin and/or insulin). Maternal ethnicity was classified, according to official criteria, in Low (Europe, North America) and High Risk (Asia, Central and South America, Africa, Oceania) ethnic groups [34]. Prepregnancy BMI was calculated based on pre-pregnancy weight (retrieved from medical charts or self-reported), and on height measured at the first visit at the GDM clinic, using the formula weight (kg)/(height(m))2. GWG was determined as the difference between the last weight measured before delivery and prepregnancy weight. GWG until the 1st GDM visit was determined as the difference between the weight measured at the 1st GDM visit and the prepregnancy weight. EWG at the 1st GDM visit was defined as gestational weight gain exceeding the thresholds established by the Institute of Medicine (IOM) Guidelines 2009 for the respective maternal pre-pregnancy BMI category [14]. HbA1c at the 1st GDM visit was measured using a chemical photometric method (conjugation with boronate; Afinion®). Maternal treatment was classified into 2 categories (no treatment, treatment with metformin and/or insulin; where almost all women were treated with insulin).
Fetal anthropometric outcomes consisted of FWC (ranging from 0–100%), FWC > 90%, FWC < 10%, FACC (ranging from 0–100%), FACC > 90% and FACC < 10%. Fetal ultrasounds (one per patient) were performed between 29 0/7 and 35 6/7 weeks of gestation by experienced obstetricians at the CHUV. Estimated FW was calculated using the Hadlock formula [35] and fetal centiles using the Intergrowth 21st fetal size application tool [36].
All maternal and fetal data were retrieved from the CHUV’s patient electronic medical chart.
Statistical analysis
All data were analysed using Stata/SE 16.0 (StataCorp LLC, TX, USA). The normality of continuous variables was assessed using QQ plots. Continuous variables were normally distributed and described as means and standard deviations (SDs). Binary outcomes were described as N (percentages) (Table 1). These calculations were performed for the total population, as well as for the population stratified by fetal sex, as defined at birth. Comparisons between the female and male subpopulations were initially made using the unpaired t-test for continuous variables and the Fisher’s exact test for binary variables (Table 1). Linear and logistic univariate regression analyses were performed for all women, as well as stratified by fetal sex. In these analyses, fetal outcome was the dependent variable, and analyses were adjusted for fetal gestational age and sex where appropriate (Table 2, Supplementary Tables, Additional files 1 and 2). In female fetuses, for the rare outcome FACC < 10%, two analyses (for ethnicity and excessive weight gain until the 1st GDM visit) was not possible due to the small sample size.
Maternal predictors with a p-value < 0.05 in univariate analysis were included in a stepwise multiple regression analysis model. These analyses were also adjusted for fetal gestational age and gender where appropriate. These analyses were performed in order to identify the most important maternal predictors of fetal anthropometric parameters associated with adverse neonatal outcomes in previous studies (Tables 3 and 4). We tested for collinearity and collinearity index was less than 0.6 for all predictors.
Probability analyses according to logistic regression models were used to evaluate the risk of FWC > 90% and FACC > 90% based on two maternal parameters that are easily available at the 1st GDM booking and turned out to be very predictive: prepregnancy BMI and GWG until the 1st GDM visit > 10.35 kg, which corresponds to the median value at this timepoint (Table 5). We used GWG as it was the most relevant predictor for fetal outcomes, in addition to prepregnancy BMI. However, we also tested the same analyses using the presence or not of EWG based on the IOM criteria instead of the median GWG until the 1st GDM visit (Additional file 3, Table A3).
For all analyses, beta-coefficients (for continuous outcomes such as FWC and FACC) and adjusted odds ratios (aORs-for binary outcome, including FWC > 90%, FACC > 90% and <10%) are reported along with their 95% confidence intervals (CIs), and statistical significance was set at 0.05.
Results
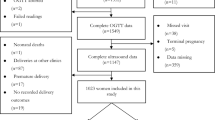
The initial population included 831 adult women with gestational diabetes, of whom 9 were excluded because they participated in an intervention trial and 111 because they refused to participate in the study. Of the 711 women who agreed to participate and signed informed consent, the following were excluded: 142 due to multiple gestation and/or missing sex at birth, 5 because the diagnosis of GDM was done before 13 weeks of gestation, raising the suspicion of pre-existent diabetes and 375 because of missing fetal ultrasound data between 29 0/7 and 35 6/7 gestational weeks (as there were done in outside private practices). Thus, 189 women were included in the final analysis.
Maternal sociodemographic, anthropometric and metabolic characteristics and fetal anthropometric parameters
Detailed information on the maternal characteristics and fetal anthropometric parameters of the total population, as well as the population stratified by fetal sex, are displayed in Table 1. No significant differences in the maternal characteristics and fetal anthropometry were found when stratifying the population by fetal sex.
Associations between maternal predictors and fetal anthropometric parameters
Total population
The results of the univariate analyses are shown in Table 2: Prepregnancy BMI showed a significant association with FWC, FWC > 90% and FACC, and 1-hour-oGTT glucose with FWC > 90%, FACC and FACC > 90%. GWG until the 1st visit at the GDM clinic predicted FWC, FWC > 90%, FACC and FACC > 90%. Excessive weight gain until the 1st visit was associated with FWC, and FACC. Maternal medical treatment requirement (metformin and/or insulin) was associated with FWC and FACC (all p ≤ 0.047). Maternal age, ethnicity, fasting-oGTT glucose, 2-hour-oGTT glucose and HbA1c at the first and last GDM visit did not show any association with fetal outcomes.
The results of the multivariate analyses are shown in Table 3: GWG until the 1st visit at the GDM clinic predicted FWC, FACC and FACC > 90% and prepregnancy BMI showed a significant association with FWC, FWC > 90% and FACC (all p ≤ 0.045). A marginal association was found between maternal medical treatment requirement and FWC (p= 0.057) as well as 1-hour-oGTT glucose and FWC > 90% (p= 0.054).
Population stratified by fetal gender
In female fetuses, only GWG until the 1st GDM visit showed a signification association with FACC (both in the univariate and thus also in the multivariate analyses; p= 0.040, see Additional file 1, Table A1). Otherwise, no other association was found between maternal predictors and female fetal anthropometric parameters.
In male fetuses, however, prepregnancy BMI, GWG until the 1st GDM visit and maternal medical treatment requirement showed a significant association with one or more fetal anthropometric parameters. More precisely, prepregnancy BMI predicted FWC, FWC > 90% and FACC (all p ≤ 0.009 see Additional file 2, Table A2). GWG until the 1st GDM visit showed a significant association with FWC and a marginally significant association with FACC > 90% and the need for maternal treatment a significant association with FACC (all p ≤ 0.050 see see Additional file 2, Table A2). In multivariate analyses for the US outcomes of males fetuses, prepregnancy BMI was associated with FWC, FWC > 90% and FACC and GWG until the 1st GDM visit with FWC (all p ≤ 0.030 see Table 4).
Risk stratification
Probability analyses using logistic regression models were performed to provide a risk stratification for FWC > 90%, and FACC > 90%, according to the prepregnancy BMI (< 25 vs ≥ 25 kg/m2) and GWG until the 1st GDM visit (< 10.3 vs > 10.3 kg corresponding to the median GWG until his timepoint). In the lowest risk group (BMI < 25 kg/m2 and GWG until the 1st GDM visit < 10.3 kg), the probability for FWC > 90% was 6% and for FACC > 90% was 12%, whereas this risk was 5.3 times higher (32%) for FWC > 90% and 4.0 times higher (48%) for FACC > 90% in the highest risk group (BMI ≥ 25 kg/m2 and GWG until the 1st GDM visit ≥ 10.3kg) (Table 5). Results were similar when using EWG instead of GWG (see Additional file 3, Table A3).
Discussion
This prospective cohort of 189 singleton women with GDM showed that two maternal anthropometric parameters, prepregnancy BMI and GWG until the 1st visit at the GDM clinic were the only independent predictors of 3rd trimester fetal anthropometry Thus, these two parameters could serve to determine the frequency of US monitoring in this metabolically high-risk population of women with GDM. When stratifying the population by fetal sex, prepregnancy BMI and/or GWG until the 1st GDM visit predicted FWC, FWC > 90%, and FACC, in multivariate analyses in male fetuses. In female fetuses, however, only GWG until the 1st GDM visit was predictive and predicted only FACC. A risk stratification model showed that in overweight or obese women with a GWG until the 1st GDM visit above the median (≥10.3 kg at 28.2 weeks of GA), the risk for FWC > 90% and FACC > 90% was 4-5 times higher than in normal-weight women with a GWG below the median. For the total population, prepregnancy BMI predicted FWC, FWC > 90% and FACC and GWG until the 1st visit at the GDM clinic predicted FWC, FACC and FACC > 90% in multivariate analyses. In addition, in univariate analysis, 1-h glucose values after oGTT and the need for medical treatment also had an impact on fetal anthropometry, but not the other tested sociodemographic or metabolic predictors. The impact of various maternal parameters on 3rd trimester fetal anthropometry in pregnancies complicated with GDM has been previously poorly studied; most of the existing data have been obtained from healthy pregnancies with low GDM or unspecified GDM incidence, while for pregnancies with GDM, data are based on neonatal, but not fetal anthropometry [12, 18, 21, 37].
In population-based mostly healthy pregnancies, GWG has been positively associated with estimated FW and FAC [18, 38]. Similarly, prepregnancy BMI has been significantly associated with estimated FW from midprepregnancy onwards [20]. Moreover, a cohort study with 11.2% of women with GDM, described a positive association between GDM status and FAC [25] . However, in this same study, maternal BMI and weight change per week had a direct effect on FAC that was independent of GDM status. A prospective cohort study in a population with 4.2% GDM incidence, demonstrated that obese women with GDM had an increased risk of FACC >90% [39].
In a study investigating fetal growth in pregnancies with Type 1, Type 2 diabetes, and GDM vs controls, the diabetes category influenced the AC growth trajectory, but ethnicity, maternal prepregnancy weight and BMI did not [40]. Moreover, in a small study of 31 women including also women with GDM (present in 39% of the population), insulin resistance, but not maternal age or weight gain was correlated to estimated fetal weight. Differences between these studies and ours are that we used a larger and homogenous cohort of women with GDM. Thus, the impact of BMI and weight gain in the cited studies might have been diluted by differences in glucose control. Furthermore, in contrast to the first study, we used fetal anthropometry at a given time point and not the trajectory [22].
In our study, excessive weight gain until the 1st GDM visit showed a significant association with FWC and FACC in univariate but not in multivariate analyses. That could mean that the GWG and not EWG according to the IOM 2009 criteria, is the most relevant parameter and could question the relevance of the IOM cut-offs for this population [41]. This finding is in accordance with a previous study of our group which found that in the presence of GWG, EWG was not associated with adverse neonatal outcomes, suggesting again the superiority of absolute GWG to predict these outcomes [12]. To the best of our knowledge, the effect of EWG on fetal anthropometry has not been studied previously.
Male offspring are known to have higher perinatal risks, such as preterm birth, cord prolapse, cesarean section, lower Apgar score at 1 minute, and higher fetal anthropometry measures [23, 24]. In a general healthy Caucasian population, higher abdominal and head circumference, were documented in male vs female fetuses throughout gestation [42]. We therefore stratified our analyses by sex: when doing this, the association between maternal parameters and fetal anthropometry was predominantly observed among male fetuses. To the best of our knowledge, this is the first study investigating the presence of a sexual dimorphism in the effect of maternal predictors on 3rd trimester anthropometry in pregnancies with GDM. Most of the existing studies in populations with GDM have investigated the effect of the fetal sex on pregnancy outcomes, as well as metabolic complications in neonates or later on in life. Fetal growth in populations with GDM has previously been studied without taking fetal sex into account. In the general population, fetal growth is also monitored without consideration for fetal sex, even though some sex-specific growth charts exist [42, 43]. Regarding neonatal complications, previous population-based studies, have shown that male sex was an independent risk factor for adverse perinatal outcomes, including preterm birth, lower Apgar scores, macrosomia, [23, 44,45,46] as well as higher birthweight, and lower arterial pH [42, 46]. A higher risk for pregnancy complications has been also found for women carrying male fetuses, with higher incidence of gestational diabetes, caesarean section, cord prolapse and nuchal cords [23]. A recent study in a population with GDM found higher birthweight, and fat mass at birth among male vs female neonates, and a more frequent need for insulin treatment in their mothers [24]. Hu et al., [46] found a higher risk for neonatal infection, acute respiratory disorders and abnormal neonatal central nervous system development in male vs female fetuses in pregnancies with GDM. Lastly, a higher BMI and risk for obesity during childhood was observed among male but not female offspring from GDM exposed pregnancies [47].
Finally, we performed probability analyses using logistic regression models in order to provide a risk stratification for two markers of 3rd trimester fetal overgrowth, according to the prepregnancy BMI and the GWG until the 1st GDM visit. FWC > 90%, and FACC > 90% have been found to be powerful predictors of adverse neonatal outcomes [17], and prepregnancy BMI and GWG until the 1st GDM visit are easily available at the 1st visit. In the highest risk group (prepregnancy BMI of ≥ 25 kg/m2 and GWG until the 1st GDM visit ≥ 10.3 kg), the risk for FWC > 90% and FACC > 90% was 4-5 times higher compared to the lowest risk group. Interestingly, although the proposed ideal weight gain for normal-weight women for the entire pregnancy is 11.3-16 kg [48], gaining more than 10.3 kg up to the first GDM visit in this group was also associated with 2.5-3 times increase in fetal overgrowth. Of note, none of the fetal US showed a FWC < 10% and for FACC < 10% this was only observed in 6.4% of the total population. Using EWG instead of GWG for the risk stratification provided similar results. To the best of our knowledge, this is the first study providing a risk stratification model for 3rd trimester fetal overgrowth, according to maternal anthropometric parameters.
Based on our results, aiming to reduce GWG starting early in pregnancy seems crucial, as more pronounced weight gain in this time period may be associated with the risk of fetal overgrowth but also offspring complications [12]. Similarly, achieving weight loss in overweight and obese women before pregnancy may help reduce fetal overgrowth and neonatal complications. In the long-term, a personalized follow-up may be offered to women with GDM based on their prepregnancy BMI, their gestational weight gain, as well as anthropometric fetal parameters and fetal gender.
The strengths of our study encompass its original findings in the multivariate and stratified analyses as well as its prospective nature, as it contains thorough information on maternal and fetal parameters of interest. Some limitations may nevertheless be mentioned. For the rare outcome FACC < 10%, two analyses were not possible due to the small sample size. Moreover, we included only 3rd and not 2nd trimester fetal anthropometric data due to the limited number of patients followed at our hospital before the diagnosis of GDM. In order to ensure data quality, all fetal anthropometric data included in the analyses were exclusively obtained from ultrasounds performed in our CHUV tertiary hospital, by equally experienced gynecologists, using the same methodology. Lastly, we included in our analyses 189 patients of the initial population of 831 women. Although metabolic parameters were similar in our selected cohort compared to the initial population of 711 women who consented, we cannot be completely sure that this is a representative sample.
Conclusions
This study showed that in women with GDM, prepregnancy BMI and GWG until the 1st visit at the GDM clinic are the only significant predictors of 3rd trimester fetal anthropometric parameters. The influence of these maternal parameters presents a sex dimorphism, affecting predominantly male fetuses. Compared to normal-weight women with a GWG less than 10.3 kg up to 28.2 weeks of GA, fetal overgrowth is 2.5-3 times higher in those who gain more than 10.3 kg in this time period and even 4-5 times higher in overweight or obese women with a GWG of 10.3 kg or more. A personalized follow-up guided by the fetal sex and anthropometry as well as maternal metabolic control may be useful in women with high GWG until the end of the second trimester and/or high prepregnancy BMI.
Availability of data and materials
The datasets generated and/or analysed during the current study are not publicly available, as many of them are still being cleaned and worked on but are available from the corresponding author on reasonable request.
Abbreviations
- BMI:
-
Body mass index
- CHUV:
-
Centre Hospitalier Universitaire Vaudois
- CI:
-
Confidence interval
- EFW:
-
Estimated fetal weight
- EGW:
-
Excessive weight gain
- FACC:
-
Fetal abdominal circumference centile
- FWC:
-
Fetal weight centile
- GDM:
-
Gestational diabetes mellitus
- GWG:
-
Gestational weight gain
- HbA1c:
-
Glycated hemoglobin
- IOM:
-
Institute of medicine
- LGA:
-
Large for gestational age
- oGTT:
-
Oral glucose tolerance test
- OR:
-
Odds ratio
- SGA:
-
Small for gestational age; US: ultrasound
References
RyserRuetschi J, Jornayvaz FR, Rivest R, Huhn EA, Irion O, Boulvain M. Fasting glycaemia to simplify screening for gestational diabetes. BJOG. 2016;123(13):2219–22.
Mitanchez D, Yzydorczyk C, Siddeek B, Boubred F, Benahmed M, Simeoni U. The offspring of the diabetic mother–short- and long-term implications. Best Pract Res Clin Obstet Gynaecol. 2015;29(2):256–69.
Management of Diabetes in Pregnancy. <em>Standards of Medical Care in Diabetes—2020</em>. Diabetes Care. 2020;43(Supplement 1):S183–92.
Zhu Y, Zhang C. Prevalence of Gestational Diabetes and Risk of Progression to Type 2 Diabetes: a Global Perspective. Curr Diab Rep. 2016;16(1):7.
Nijs H, Benhalima K. Gestational diabetes mellitus and the long-term risk for glucose intolerance and overweight in the offspring: a narrative review. J Clin Med. 2020;9(2):599.
Minschart C, Amuli K, Delameillieure A, Calewaert P, Mathieu C, Benhalima K. Multidisciplinary group education for gestational diabetes mellitus: a prospective observational cohort study. J Clin Med. 2020;9(2):509.
Yessoufou A, Moutairou K. Maternal diabetes in pregnancy: early and long-term outcomes on the offspring and the concept of “metabolic memory.” Exp Diabetes Res. 2011;2011: 218598.
Scifres CM. Short- and long-term outcomes associated with large for gestational age birth weight. Obstet Gynecol Clin North Am. 2021;48(2):325–37.
Catalano PM, McIntyre HD, Cruickshank JK, McCance DR, Dyer AR, Metzger BE, et al. The hyperglycemia and adverse pregnancy outcome study: associations of GDM and obesity with pregnancy outcomes. Diabetes Care. 2012;35(4):780–6.
Aune D, Saugstad OD, Henriksen T, Tonstad S. Maternal body mass index and the risk of fetal death, stillbirth, and infant death: a systematic review and meta-analysis. JAMA. 2014;311(15):1536–46.
Bianchi C, de Gennaro G, Romano M, Aragona M, Battini L, Del Prato S, et al. Pre-pregnancy obesity, gestational diabetes or gestational weight gain: Which is the strongest predictor of pregnancy outcomes? Diabetes Res Clin Pract. 2018;144:286–93.
Antoniou MC, Gilbert L, Gross J, Rossel JB, Fischer Fumeaux CJ, Vial Y, et al. Potentially modifiable predictors of adverse neonatal and maternal outcomes in pregnancies with gestational diabetes mellitus: can they help for future risk stratification and risk-adapted patient care? BMC Pregnancy Childbirth. 2019;19(1):469.
Barnes RA, Edghill N, Mackenzie J, Holters G, Ross GP, Jalaludin BB, et al. Predictors of large and small for gestational age birthweight in offspring of women with gestational diabetes mellitus. Diabet Med. 2013;30(9):1040–6.
Rasmussen KM, Catalano PM, Yaktine AL. New guidelines for weight gain during pregnancy: what obstetrician/gynecologists should know. Curr Opin Obstet Gynecol. 2009;21(6):521–6.
Stotland NE, Cheng YW, Hopkins LM, Caughey AB. Gestational weight gain and adverse neonatal outcome among term infants. Obstet Gynecol. 2006;108(3 Pt 1):635–43.
Barnes RA, Wong T, Ross GP, Griffiths MM, Smart CE, Collins CE, et al. Excessive weight gain before and during gestational diabetes mellitus management: what is the impact? Diabetes Care. 2020;43(1):74–81.
Antoniou MC, Gilbert L, Gross J, Rossel JB, Fumeaux CJF, Vial Y, et al. Main fetal predictors of adverse neonatal outcomes in pregnancies with gestational diabetes mellitus. J Clin Med. 2020;9(8):2409.
Galjaard S, Pexsters A, Devlieger R, Guelinckx I, Abdallah Y, Lewis C, et al. The influence of weight gain patterns in pregnancy on fetal growth using cluster analysis in an obese and nonobese population. Obesity. 2013;21(7):1416–22.
Zhang C, Hediger ML, Albert PS, Grewal J, Sciscione A, Grobman WA, et al. Association of maternal obesity with longitudinal ultrasonographic measures of fetal growth: findings from the NICHD fetal growth studies-singletons. JAMA Pediatr. 2018;172(1):24–31.
Ay L, Kruithof CJ, Bakker R, Steegers EA, Witteman JC, Moll HA, et al. Maternal anthropometrics are associated with fetal size in different periods of pregnancy and at birth. The Generation R Study BJOG. 2009;116(7):953–63.
Kim W, Park SK, Kim YL. Gestational diabetes mellitus diagnosed at 24 to 28 weeks of gestation in older and obese Women: Is it too late? PLoS ONE. 2019;14(12):e0225955.
Rohl J, Huston-Presley L, Amini S, Stepanchak B, Catalano P. Factors associated with fetal growth and body composition as measured by ultrasound. Am J Obstet Gynecol. 2001;185(6):1416–20.
Di Renzo GC, Rosati A, Sarti RD, Cruciani L, Cutuli AM. Does fetal sex affect pregnancy outcome? Gend Med. 2007;4(1):19–30.
Giannubilo SR, Pasculli A, Ballatori C, Biagini A, Ciavattini A. Fetal sex, need for insulin, and perinatal outcomes in gestational diabetes mellitus: an observational cohort study. Clin Ther. 2018;40(4):587–92.
Macaulay S, Munthali RJ, Dunger DB, Norris SA. The effects of gestational diabetes mellitus on fetal growth and neonatal birth measures in an African cohort. Diabet Med. 2018;35(10):1425–33.
Quansah DY, Gilbert L, Gross J, Horsch A, Puder JJ. Intuitive eating is associated with improved health indicators at 1-year postpartum in women with gestational diabetes mellitus. J Health Psychol. 2021;26(8):1168–84. https://doi.org/10.1177/1359105319869814.
Quansah DY, Gross J, Gilbert L, Arhab A, Horsch A, Puder JJ. Predictors and consequences of weight retention in the early and late postpartum period in women with gestational diabetes. Diabetes Res Clin Pract. 2020;165:108238.
Quansah DY, Gross J, Gilbert L, Helbling C, Horsch A, Puder JJ. Intuitive eating is associated with weight and glucose control during pregnancy and in the early postpartum period in women with gestational diabetes mellitus (GDM): a clinical cohort study. Eat Behav. 2019;34:101304.
Gilbert L, Nikolaou A, Quansah DY, Rossel JB, Horsch A, Puder JJ. Mental health and its associations with glucose-lowering medication in women with gestational diabetes mellitus a prospective clinical cohort study. Psychoneuroendocrinology. 2021;124:105095.
International Association of Diabetes and Pregnancy Study Groups Consensus Panel, Metzger BE, Gabbe SG, Persson B, Buchanan TA, Catalano PA, Damm P, Dyer AR, Leiva Ad, Hod M, Kitzmiler JL, Lowe LP, McIntyre HD, Oats JJ, Omori Y, Schmidt MI. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33(3):676–82. https://doi.org/10.2337/dc09-1848.
Management of Diabetes in Pregnancy. Standards of Medical Care in Diabetes-2021. Diabetes Care. 2021;44(Suppl 1):S200–10.
Blumer I, Hadar E, Hadden DR, Jovanovič L, Mestman JH, Murad MH, et al. Diabetes and pregnancy: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2013;98(11):4227–49.
Rosselet P, Puder J, Vial Y, Hagon-Traub I, Burnand B. Diagnostic et prise en charge du diabète gestationnel - Prise en charge multidisciplinaire du diabète : recommandations pour la pratique clinique. Rev Med Suisse. 2017;13(568):1305.
Introduction: Standards of Medical Care in Diabetes—2021. Diabetes Care. 2021;44 (Supplement_1): S1–S2. https://doi.org/10.2337/dc21-Sint.
Hadlock FP, Harrist RB, Sharman RS, Deter RL, Park SK. Estimation of fetal weight with the use of head, body, and femur measurements–a prospective study. Am J Obstet Gynecol. 1985;151(3):333–7.
Papageorghiou AT, Ohuma EO, Altman DG, Todros T, Cheikh Ismail L, Lambert A, et al. International standards for fetal growth based on serial ultrasound measurements: the Fetal Growth Longitudinal Study of the Intergrowth-21st Project. Lancet. 2014;384(9946):869–79.
Ott R, Stupin JH, Loui A, Eilers E, Melchior K, Rancourt RC, et al. Maternal overweight is not an independent risk factor for increased birth weight, leptin and insulin in newborns of gestational diabetic women: observations from the prospective ‘EaCH’ cohort study. BMC Pregnancy Childbirth. 2018;18(1):250.
Hure AJ, Collins CE, Giles WB, Paul JW, Smith R. Greater maternal weight gain during pregnancy predicts a large but lean fetal phenotype: a prospective cohort study. Matern Child Health J. 2012;16(7):1374–84.
Sovio U, Murphy HR, Smith GCS. Accelerated fetal growth prior to diagnosis of gestational diabetes mellitus: a prospective cohort study of nulliparous women. Diabetes Care. 2016;39(6):982–7.
Hammoud NM, Visser GH, Peters SA, Graatsma EM, Pistorius L, de Valk HW. Fetal growth profiles of macrosomic and non-macrosomic infants of women with pregestational or gestational diabetes. Ultrasound Obstet Gynecol. 2013;41(4):390–7.
Voerman E, Santos S, Inskip H, Amiano P, Barros H, Charles MA, et al. Association of gestational weight gain with adverse maternal and infant outcomes. JAMA. 2019;321(17):1702–15.
Galjaard S, Ameye L, Lees CC, Pexsters A, Bourne T, Timmerman D, et al. Sex differences in fetal growth and immediate birth outcomes in a low-risk Caucasian population. Biol Sex Differ. 2019;10(1):48.
Schwärzler P, Bland JM, Holden D, Campbell S, Ville Y. Sex-specific antenatal reference growth charts for uncomplicated singleton pregnancies at 15–40 weeks of gestation. Ultrasound Obstet Gynecol. 2004;23(1):23–9.
Sheiner E, Levy A, Katz M, Hershkovitz R, Leron E, Mazor M. Gender does matter in perinatal medicine. Fetal Diagn Ther. 2004;19(4):366–9.
Nagy E, Orvos H, Bakki J, Pal A. Sex-differences in Apgar scores for full-term neonates. Acta Paediatrica. 2009;98(5):898–900.
Hu J, Ge Z, Xu Q, Shen S, Wang Y, Zhu D, et al. Influence of fetal sex on perinatal outcomes in women with gestational diabetes mellitus. Diabetes Metab Res Rev. 2020;36(3): e3245.
Li S, Zhu Y, Yeung E, Chavarro JE, Yuan C, Field AE, et al. Offspring risk of obesity in childhood, adolescence and adulthood in relation to gestational diabetes mellitus: a sex-specific association. Int J Epidemiol. 2017;46(5):1533–41.
Rasmussen KM, Abrams B, Bodnar LM, Butte NF, Catalano PM, Maria S-R. Recommendations for weight gain during pregnancy in the context of the obesity epidemic. Obstet Gynecol. 2010;116(5):1191–5.
Acknowledgments
None.
Funding
This study was sponsored by an unrestricted educational grant from Novo Nordisk. The funding body did not take part in the design of the study, the collection, analysis, interpretation of data or in the writing of the manuscript. This study is a pilot of a project grant by the Swiss National Science Foundation (SNF 32003B_176119).
Author information
Authors and Affiliations
Contributions
M.C.A. participated in the conceptual design of the study, the data collection, analysis and interpretation and wrote the original draft. L.G. participated in the data collection and revised the final draft. J.G. participated in the data collection and revised the final draft. J.B.R participated in the statistical analysis and revised the final draft. C.J.F.F. contributed to the design and conceptualization of the study, the fetal and neonatal aspects and revised the final draft. Y.V. contributed to the design and conceptualization of the study, the obstetrical aspects and revised the final draft. J.J.P. was the origin of the cohort, the coordinator of the study, participated in the conceptual design of the study, the data analysis and interpretation and corrected all drafts. All the authors critically reviewed the article for important intellectual content and approved the final version submitted for publication. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Signed informed consent was obtained from all participating women. The study was conducted in accordance with the guidelines of the declaration of Helsinki, and good clinical practice. The Human Research Ethics Committee of the Canton de Vaud approved the study protocol (326/15).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Antoniou, MC., Gilbert, L., Gross, J. et al. Sex-dependent influence of maternal predictors on fetal anthropometry in pregnancies with gestational diabetes mellitus. BMC Pregnancy Childbirth 22, 460 (2022). https://doi.org/10.1186/s12884-022-04767-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12884-022-04767-z