Abstract
Background
The objective of this study was to evaluate the association between red cell distribution width/platelet ratio (RPR) and 30-day and 1-year mortality in acute ischemic stroke (AIS).
Methods
Data for the retrospective cohort study were collected from the Medical Information Mart for Intensive Care (MIMIC) III database. RPR was divided into two groups: RPR ≤ 0.11 and RPR > 0.11. The study outcomes were 30-day mortality and 1-year mortality from AIS. Cox proportional hazard models were utilized to assess the association between RPR and mortality. Subgroup analyses were applied based on age, tissue-type plasminogen activator (IV-tPA), endovascular treatment, and myocardial infarction.
Results
A total of 1,358 patients were included in the study. Short- and long-term mortality occurred in 375 (27.61%) and 560 (41.24%) AIS patients, respectively. A high RPR was significantly associated with increased 30-day [hazard ratio (HR): 1.45, 95% confidence interval (CI): 1.10 to 1.92, P = 0.009] and 1-year mortality (HR: 1.54, 95%CI: 1.23 to 1.93, P < 0.001) in AIS patients. Meanwhile, RPR was found to be significantly related to 30-day mortality in AIS patients aged < 65 years (HR: 2.19, 95% CI: 1.17 to 4.10, P = 0.014), without IV-tPA use (HR: 1.42, 95% CI: 1.05 to 1.90, P = 0.021), without using endovascular treatment (HR: 1.45, 95% CI: 1.08 to 1.94, P = 0.012), and without myocardial infarction (HR: 1.54, 95% CI: 1.13 to 2.10, P = 0.006). Additionally, RPR was associated with 1-year mortality in AIS patients aged < 65 years (HR: 2.54, 95% CI: 1.56 to 4.14, P < 0.001), aged ≥ 65 years (HR: 1.38, 95% CI: 1.06 to 1.19, P = 0.015), with (HR: 1.46, 95% CI: 1.15 to 1.85, P = 0.002) and without using IV-tPA (HR: 2.30, 95% CI: 1.03 to 5.11, P = 0.041), without using endovascular treatment (HR: 1.56, 95% CI: 1.23 to 1.96, P < 0.001), and without myocardial infarction (HR: 1.68, 95% CI: 1.31 to 2.15, P < 0.001).
Conclusion
Elevated RPR is associated with a high risk of short-term and long-term mortality in AIS.
Similar content being viewed by others
Background
Stroke is one of the major causes of death and long-term disability, of which acute ischemic stroke (AIS) is the most common type, accounting for approximately 80% of all strokes [1, 2]. The 2020 American Heart Association report on Heart Disease and Stroke Statistics estimates that the prevalence of stroke in the United States in 2016 was 2.5%, corresponding to 7 million Americans over 20 years of age who had suffered a stroke, nearly 800,000 stroke events, and nearly 150,000 deaths [3]. AIS is a prominent cause of mortality, brings not only physical and mental suffering to the patients, but also placing a huge burden on families and society [4]. Therefore, it is essential to explore the factors associated with AIS and identify patients with poor prognoses for optimal management.
Strokes are caused by cerebral vascular occlusion or hemorrhage leading to oxygen and nutrient deprivation, which causes local inflammatory immune response [5]. Thereby, biochemical markers reflecting systemic immune inflammatory status may be of great significance for disease monitoring and prognosis in AIS patients. Several studies have explored and identified the overall association between serum inflammatory biomarkers and outcomes in patients with ischemic stroke [6,7,8]. A previous study observed that a higher admission systemic inflammatory response index (SIRI) increased the risk of poor outcome at 3 months in patients with AIS [8]. The red cell distribution width (RDW) is a parameter reflecting the variation and dispersion degree of peripheral blood red blood cell volume [9] and is clinically used to distinguish different types of anemia [10]. In recent years, RDW has attracted attention as an inflammatory marker of cardiovascular and cerebrovascular diseases [11]. A cohort study and systematic review reported that RDW is associated with mortality after AIS [12]. The results of a previous study implied that pre-RDW is a reliable predictor of one-year prognosis and mortality after receiving endovascular therapy in acute anterior circulation stroke patients [13]. Platelets attach to the damaged blood vessel wall, release bioactive substances, and promote the formation of thrombosis [14]. Platelets have been found to be predictors of prognosis in cerebral infarction and cerebral infarction [15]. A previous study showed that platelet aggregation-related thrombosis is a key step in the pathophysiology of AIS [16]. RDW to platelet ratio (RPR) is a new marker of inflammation, which has been found to be related to the prognosis of cerebrovascular diseases associated with inflammation such as cerebral hemorrhage and acute myocardial infarction [17, 18]. However, the association of RPR with AIS outcome has not been reported.
Therefore, the aim of this study was to explore the association between RPR and short-term and long-term mortality in patients with AIS and help to evaluate its predictive value for mortality in order to screen out a powerful marker for risk stratification of AIS in the future.
Methods
Study design and patients
This was a retrospective cohort study. We derived data from the Medical Information Mart for Intensive Care-III (MIMIC-III) database with intensive care unit (ICU) admission diagnoses classified as ICD-9 code: 43,301, 43,311, 43,321, 43,331, 43,381, 43,391, 43,401, 43,411, 43,491. MIMIC-III is a large, free accessible intensive care database that contains the detailed information of more than 40,000 patients admitted to the critical care units in the Beth Israel Deaconess Medical Center (BIDMC) from 2001 to 2012, including demographic data, vital signs, comorbidities, and laboratory tests, which provides reliable data resource for clinicians to conduct epidemiological studies. The included criteria were (1) diagnosed as AIS at ICU admission. Excluded criteria were (1) aged < 18 years old; (2) hospitalized in the ICU for less than 24 h and (3) missing key data, such as RDW, and platelet. Our study data were obtained from a public database and therefore did not require approval from the ethics committee of our hospital.
Data collection
We recorded patients’ information including: (1) baseline characteristics: age (years), gender, ethnicity (Black, White, others, and unknown), insurance status (Medicare, private, and others), therapy use [ventilation use (yes or no), vasopressor use (yes or no), renal replacement therapy (Rrt, yes or no), tissue-type plasminogen activator (IV-tPA, yes or no), endovascular treatment (yes or no), Beta-blockers (yes or no)], comorbidities (diabetes, congestive heart failure, atrial fibrillation, acute myocardial infarction, hypertension, cardiogenic shock, acute kidney injury, and myocardial infarct), weight (kg); (2) vital signs: systolic blood pressure (SBP, mmHg), diastolic blood pressure (DBP, mmHg), heart rate (bpm), respiratory rate (bpm), temperature (°C), SpO2 (%); (3) scoring systems: sequential organ failure assessment (SOFA) score, simplified acute physiology score II (SAPSII), quick sepsis-related organ failure assessment (qSOFA) score, Glasgow Coma Scale (GCS), charlson’s comorbidity index (CCI), international normalized ratio (INR); (4) laboratory parameters: white blood cell (WBC) count (K/uL), platelet (K/uL), hemoglobin (g/dL), RDW (109/L), creatinine (mg/dL), prothrombin time (PT, seconds), blood urea nitrogen (BUN, mg/dL), glucose (mg/dL), sodium (mEq/L), potassium (mEq/L), chloride (mEq/L).
Definitions and outcomes
The RPR is defined using the following equation: RDW/platelet count. According to the cut-off value, RPR was divided into groups: RPR ≤ 0.11 and RPR > 0.11. The outcome of the study was 30-day mortality and 1-year mortality, which were defined as the time from ICU admission to death or the last date of follow-up. The follow-up duration was 30.00 (22.05, 30.00) days for the 30-day mortality outcome and 365.00 (22.05, 365.00) days for the 1-year mortality outcome.
Statistical analysis
The measurement data with normal distribution were described by Mean ± standard deviation (Mean ± SD), and the t-test was used to compare the differences between the two groups. The median and quartile [M (Q1, Q3)] were used to describe the distribution of measurement data that did not obey the normal distribution, and the Wilcoxon rank sum test was used to compare the differences between the two groups. The number of cases and constituent ratio [n (%)] was used to describe the distribution of count data, and the chi-square test was used to compare the differences between groups.
Univariate Cox proportional-hazards models were used to explore the effect of all variables on 30-day and 1-year mortality to screen for confounders. The significant variables in univariate analysis were screened one by one by stepwise regression method for multivariate analysis. Model 1 was an unadjusted model, in Model 2 of 30-day mortality, age, ethnicity, ventilation, respiratory rate, SAPSII, SOFA, and glucose were adjusted for; age, ethnicity, ventilation, respiratory rate, heart rate, SAPSII, SOFA, creatinine, and glucose were adjusted in model 2 for 1-year mortality.
The random forest imputation method was used to impute data for variables with missing rates of less than 20%. Sensitivity analysis was performed by comparing the data before and after the imputation. Subgroup analyses were applied based on age, IV-tPA, endovascular treatment, and myocardial infarction. Kaplan–Meier (KM) survival analysis was used to assess and compare the disease-related survival of patients with different RPR classifications. The hazard ratio (HR) with a 95% confidence interval (CI) was used to assess the results. X-tile Version 3.6.1 software was used to determine the best cut-off value for RPR classification. We also used restricted cubic spline (RCS) transformations to assess nonlinear relationships between RPR and mortality risk in patients with AIS. The significance for all tests was set at an alpha level of 0.05. Statistical analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, NC, USA), Python 3.7.4, and R version 4.2.0 (2022–04-22 ucrt).
Results
Baseline characteristics of included patients
A loss to follow-up rate of 0% was estimated. Based on the inclusion and exclusion criteria, 1,358 patients were included in the study, of which 375 patients (27.61%) had 30-day mortality, and 560 patients (41.24%) ended up with 1-year mortality. The flow chart of data collection is provided in Fig. 1. The mean age of the patients was 68.76 ± 15.60 years. The majority of the included population was White (72.16%). There were significant differences between patients with RPR ≤ 0.11 and patients with RPR > 0.11 in ventilation, vasopressor, Rrt, IV-tPA, SBP, DBP, heart rate, SAPSII, SOFA, qSOFA, CCI, INR, diabetes, atrial fibrillation, WBC, platelet, hemoglobin, RDW, creatinine, PT, BUN, and chloride. Baseline demographic and clinical characteristics and laboratory tests of the included patients are shown in Table 1.
Association of RPR with 30-day and 1-year mortality in patients with AIS
In model 1 analysis, RPR > 0.11 was associated with 30-day mortality (HR: 1.58, 95% CI: 1.22 to 2.04, P < 0.001) and 1-year mortality (HR: 1.85, 95% CI: 1.50 to 2.28, P < 0.001) in patients with AIS. Based on model 2 analysis, RPR > 0.11 was associated with increased risk of 30-day (HR: 1.45, 95% CI: 1.10 to 1.92, P = 0.009) and 1-year mortality (HR: 1.54, 95% CI: 1.23 to 1.93, P < 0.001) in AIS patients. The association of RPR with 30-day and 1-year mortality in patients with AIS is presented in Table 2.
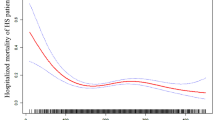
The KM curve shows a higher risk of 30-day and 1-year mortality in patients with an RPR > 0.11. The KM curve for 30-day mortality in AIS is depicted in Fig. 2. The KM curve of 1-year mortality in AIS is shown in Fig. 3. RCS curves show a nonlinear association between RPR and 30-day and 1-year mortality in patients with AIS. The association between RPR and 30-day and 1-year mortality in patients with AIS based on the RCS analysis is shown in Figs. 4 and 5.
Subgroup analyses of the associations of RPR with 30-day and 1-year mortality in patients with AIS
Subgroup analyses based on age, IV-tPA, endovascular treatment, and myocardial infarction were performed to further investigate the association of RPR with 30-day and 1-year mortality in different stratifications. Regarding the outcome of 30-day mortality, RPR was associated with 30-day mortality in AIS patients aged < 65 years (HR: 2.19, 95% CI: 1.17 to 4.10, P = 0.014), in AIS patients without using IV-tPA (HR: 1.42, 95% CI: 1.05 to 1.90, P = 0.021), in AIS patients without using endovascular treatment (HR: 1.45, 95% CI: 1.08 to 1.94, P = 0.012), and in AIS patients without myocardial infarction (HR: 1.54, 95% CI: 1.13 to 2.10, P = 0.006). Regarding the 1-year mortality in patients with AIS, RPR was associated with 1-year mortality in AIS patients with age < 65 years old (HR: 2.54, 95% CI: 1.56 to 4.14, P < 0.001), age ≥ 65 years old (HR: 1.38, 95% CI: 1.06 to 1.19, P = 0.015), in AIS patients with (HR: 1.46, 95% CI: 1.15 to 1.85, P = 0.002) and without using IV-tPA (HR: 2.30, 95% CI: 1.03 to 5.11, P = 0.041), in AIS patients without using endovascular treatment (HR: 1.56, 95% CI: 1.23 to 1.96, P < 0.001), and in AIS patients without myocardial infarction (HR: 1.68, 95% CI: 1.31 to 2.15, P < 0.001). Subgroup analyses of the associations of RPR with 30-day and 1-year mortality in patients with AIS are shown in Fig. 6.
Discussion
In recent years, numerous studies have focused on the role of inflammatory processes in the aetiology and prognosis of AIS [19, 20]. Thus, in this study, we explored the association between RPR and short-term and long-term mortality in patients with AIS. The results of the current study revealed that there was a nonlinear relationship between RPR and mortality in AIS patients, with an upward trend. Elevated RPR was significantly linked to increased 30-day mortality and 1-year mortality in AIS. RPR was also related to 30-day mortality in AIS patients younger than 65 years old, without the use of IV-tPA, without the use of endovascular treatment, and without myocardial infarction. RPR was associated with 1-year mortality in AIS patients aged < 65 years, aged ≥ 65 years, with and without IV-tPA, without endovascular therapy, and without myocardial infarction.
Based on RDW and platelets, RPR has served as a useful systemic inflammatory marker and prognostic indicator of adult inflammatory diseases such as hepatic fibrosis in chronic hepatitis B [21], myocardial infarction [22], and acute pancreatitis [23]. To our knowledge, there are no reports on RPR in mortality in AIS. Our results indicated the association between RPR and short-term and long-term mortality risk in patients with AIS. The roles of RDW and platelet in the motility of AIS may be the underlying mechanisms. An elevated RDW level denotes an aberrant variation of red blood cell size in the peripheral blood [24], which is predisposed to thrombophilia due to increased or ineffective red blood cells production and excessive fragmentation or destruction of red blood cell [25]. RDW has been studied as an inflammatory marker in stroke severity [26]. Previous studies have also revealed that RDW is associated with the occurrence and prognosis of AIS [27,28,29]. A study by Feng et al. revealed higher RDW levels were related to worsening prognosis in AIS patients [30]. Platelets serve as a link between innate and acquired immune responses and contribute to the beginning or exacerbation of the inflammatory process by secreting proinflammatory cytokines and interacting with a variety of immune cells, such as neutrophils, T-lymphocytes, natural killer cells, and macrophages [31]. AIS may cause aberrant platelet function, and activated and hyperresponsive platelets may interact with platelet-binding T lymphocyte cells to create cytokines, interferons, chemokines, and other adhesion molecules that may interfere with AIS recovery [32].
According to the subgroup analysis, the association between high RPR and mortality became non-significant in the subgroups with the IV-tPA use, the endovascular therapy use, and myocardial infarction, however, this association remained significant in the AIS patients without the use of IV-tPA, without the use of endovascular treatment, and without myocardial infarction. We hypothesized that there might be an interaction between RPR and disease severity. Patients with IV-tPA use, endovascular therapy use, and myocardial infarction, to a certain degree, represented patients with inflammation of higher severity. Additionally, treatment of AIS may affect the outcome of AIS. Early administration of IV-tPA and/or endovascular therapy has been found to be associated with improved outcomes in AIS [33]. However, Longstreth et al. indicate that 3-month functional outcomes after an AIS were not significantly associated with IV-tPA use [34]. Similarly, AIS patients with myocardial infarction may differ from AIS without myocardial infarction. Inohara et al. found that among older AIS patients treated with recombinant tissue-type plasminogen activator (rtPA), recent myocardial infarction was associated with an increased risk of in-hospital mortality [35]. Further research is required to confirm the link between RPR and mortality in AIS patients receiving therapy or with myocardial infarction.
In this study, the association between RPR and short- and long-term survival in patients with AIS was confirmed using rigorous statistical methods, which may help in the monitoring and risk stratification management of AIS. Furthermore, in clinical practice, RDW and platelets are commonly used to measure whole blood counts, which can be easily monitored dynamically due to the speed and low cost of the method. There are several limitations to this study that should be considered. Firstly, this study is a retrospective, single-center study design, which inevitably introduces some bias. Second, although the patients included were multiracial, this study used data from ICUs in the United States, and the results may not be generalizable to ICUs in other countries. Third, long-term mortality is difficult to adjust for confounding factors, such as out-of-hospital interventions, and should be interpreted with caution. Fourth, increased inflammatory biomarkers (C-reactive protein and erythrocyte sedimentation rate) have been shown to increase poor outcomes in ischemic stroke. However, C-reactive protein and erythrocyte sedimentation rate data are missing from the database. Further studies are needed to assess the association between RPR and 30-day and 1-year mortality in AIS, with C-reactive protein and erythrocyte sedimentation rate being considered.
Conclusions
In conclusion, higher RPR was correlated with higher 30-day and 1-year mortality in AIS patients, especially in AIS patients without IV-tPA use, without endovascular therapy, and without myocardial infarction.
Availability of data and materials
The datasets generated and/or analyzed during the current study are available in the MIMIC-III database, https://mimic.physionet.org/iii/.
Abbreviations
- AIS:
-
Acute ischemic stroke
- SIRI:
-
Systemic inflammatory response index
- RDW:
-
Red cell distribution width
- RPR:
-
RDW to platelet ratio
- MIMIC-III:
-
Medical Information Mart for Intensive Care-III
- ICU:
-
Intensive care unit
- BIDMC:
-
Beth Israel Deaconess Medical Center
- SBP:
-
Systolic blood pressure
- DBP:
-
Diastolic blood pressure
- SOFA:
-
Sequential organ failure assessment
- SAPSII:
-
Simplified acute physiology score II
- qSOFA:
-
Quick sepsis-related organ failure assessment
- GCS:
-
Glasgow Coma Scale
- CCI:
-
Charlson’s comorbidity index
- INR:
-
International normalized ratio
- WBC:
-
White blood cell
- PT:
-
Prothrombin time
- BUN:
-
Blood urea nitrogen
References
Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, et al. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation. 2020;141(9):e139–596. https://doi.org/10.1161/cir.0000000000000757.
Yang J, Hao J, Lin Y, Guo Y, Liao K, Yang M, et al. Profile and Functional Prediction of Plasma Exosome-Derived CircRNAs From Acute Ischemic Stroke Patients. Front Genetics. 2022; 13: 810974. https://doi.org/10.3389/fgene.2022.810974.
Feske SK. Ischemic Stroke. Am J Med. 2021;134(12):1457–64. https://doi.org/10.1016/j.amjmed.2021.07.027.
Zhang Y, Wang S, Lu F, Zhang M, Kong H, Cheng J, et al. The neuroprotective effect of pretreatment with carbon dots from Crinis Carbonisatus (carbonized human hair) against cerebral ischemia reperfusion injury. J Nanobiotechnol. 2021;19(1):257. https://doi.org/10.1186/s12951-021-00908-2.
Shim R, Wong CH. Ischemia, Immunosuppression and Infection--Tackling the Predicaments of Post-Stroke Complications. Int J Mol Sci. 2016; 17(1). https://doi.org/10.3390/ijms17010064.
Yi HJ, Sung JH, Lee DH. Systemic Inflammation Response Index and Systemic Immune-Inflammation Index Are Associated with Clinical Outcomes in Patients Treated with Mechanical Thrombectomy for Large Artery Occlusion. World Neurosurg. 2021;153:e282–9. https://doi.org/10.1016/j.wneu.2021.06.113.
Świtońska M, Piekuś-Słomka N, Słomka A, Sokal P, Żekanowska E, Lattanzi S. Neutrophil-to-Lymphocyte Ratio and Symptomatic Hemorrhagic Transformation in Ischemic Stroke Patients Undergoing Revascularization. Brain Sci. 2020; 10(11). https://doi.org/10.3390/brainsci10110771.
Lattanzi S, Norata D, Divani AA, Di Napoli M, Broggi S, Rocchi C, et al. Systemic Inflammatory Response Index and Futile Recanalization in Patients with Ischemic Stroke Undergoing Endovascular Treatment. Brain Sci. 2021; 11(9). https://doi.org/10.3390/brainsci11091164.
Liu J, Huang X, Yue S, Wang J, Ye E, Huang J, et al. Association of Red Cell Distribution Width-to-Platelet Ratio and Mortality in Patients with Sepsis. Mediators Inflamm. 2022;2022:4915887. https://doi.org/10.1155/2022/4915887.
Kim HJ, Yoo J, Jung SM, Song JJ, Park YB, Lee SW. Red Blood Cell Distribution Width Can Predict Vasculitis Activity and Poor Prognosis in Granulomatosis with Polyangiitis. Yonsei Med J. 2018;59(2):294–302. https://doi.org/10.3349/ymj.2018.59.2.294.
Hong RH, Zhu J, Li ZZ, Yuan J, Zhao P, Ding J, et al. Red blood cell distribution width is associated with neuronal damage in acute ischemic stroke. Aging. 2020;12(10):9855–67. https://doi.org/10.18632/aging.103250.
Wang L, Wang C, Wu S, Li Y, Guo W, Liu M. Red blood cell distribution width is associated with mortality after acute ischemic stroke: a cohort study and systematic review. Ann Transl Med. 2020;8(4):81. https://doi.org/10.21037/atm.2019.12.142.
Wang Z, Liu Y. Red Cell Distribution Width as a Predictor of One-Year Prognosis and Mortality of Endovascular Therapy for Acute Anterior Circulation Ischemic Stroke. J Stroke Cerebrovasc Dis. 2022; 31(2): 106243. https://doi.org/10.1016/j.jstrokecerebrovasdis.2021.106243.
Gawaz M, Brand K, Dickfeld T, Pogatsa-Murray G, Page S, Bogner C, et al. Platelets induce alterations of chemotactic and adhesive properties of endothelial cells mediated through an interleukin-1-dependent mechanism. Implications for atherogenesis. Atherosclerosis. 2000;148(1):75–85. https://doi.org/10.1016/s0021-9150(99)00241-5.
Yang M, Pan Y, Li Z, Yan H, Zhao X, Liu L, et al. Platelet Count Predicts Adverse Clinical Outcomes After Ischemic Stroke or TIA: Subgroup Analysis of CNSR II. Front Neurol. 2019;10:370. https://doi.org/10.3389/fneur.2019.00370.
Kraft P, Schwarz T, Meijers JC, Stoll G, Kleinschnitz C. Thrombin-activatable fibrinolysis inhibitor (TAFI) deficient mice are susceptible to intracerebral thrombosis and ischemic stroke. PloS one. 2010;5(7):e11658. https://doi.org/10.1371/journal.pone.0011658.
Lehmann F, Schenk LM, Bernstock JD, Bode C, Borger V, Gessler FA, et al. Elevated Red Cell Distribution Width to Platelet Ratio Is Associated With Poor Prognosis in Patients With Spontaneous, Deep-Seated Intracerebral Hemorrhage. Front Neurol. 2021; 12: 751510. https://doi.org/10.3389/fneur.2021.751510.
Tomaniak M, Kołtowski Ł, Jonik S, Kochman J, Rdzanek A, Pietrasik A, et al. Platelet to red cell distribution width ratio for predicting clopidogrel efficacy in patients undergoing percutaneous coronary interventions: insights from the ONSIDE-TEST study. Pol Arch Intern Med. 2019;129(2):117–22. https://doi.org/10.20452/pamw.4441.
Rust R, Grönnert L, Schwab ME. Inflammation after Stroke: A Local Rather Than Systemic Response? Trends Neurosci. 2018;41(12):877–9. https://doi.org/10.1016/j.tins.2018.09.011.
Yang Y, Xie D, Zhang Y. Increased Platelet-to-Lymphocyte Ratio is an Independent Predictor of Hemorrhagic Transformation and In-Hospital Mortality Among Acute Ischemic Stroke with Large-Artery Atherosclerosis Patients. Int J Gen Med. 2021;14:7545–55. https://doi.org/10.2147/ijgm.S329398.
Ferdous A, Ahmed AN, Rahman SA, Hasan T, Mahzabeen L. Role of Red Cell Distribution Width to Platelet Ratio in Predicting Hepatic Fibrosis in Chronic Hepatitis B. Mymensingh Med J. 2018;27(3):550–60.
Pusuroglu H, Cakmak HA, Akgul O, Erturk M, Surgit O, Akkaya E, et al. The prognostic value of admission red cell distribution width-to-platelet ratio in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Rev Port Cardiol. 2015; 34(10): 597–606. https://doi.org/10.1016/j.repc.2015.03.014.
Cetinkaya E, Senol K, Saylam B, Tez M. Red cell distribution width to platelet ratio: new and promising prognostic marker in acute pancreatitis. World J Gastroenterol. 2014;20(39):14450–4. https://doi.org/10.3748/wjg.v20.i39.14450.
Akpinar CK, Gurkaş E, Aykac O, Uysal Z, Ozdemir AO. Elevated Red Blood Cell Distribution Width May Be a Novel Independent Predictor of Poor Functional Outcome in Patients Treated with Mechanical Thrombectomy. Neurointervention. 2021;16(1):34–8. https://doi.org/10.5469/neuroint.2020.00262.
Öztürk ZA, Ünal A, Yiğiter R, Yesil Y, Kuyumcu ME, Neyal M, et al. Is increased red cell distribution width (RDW) indicating the inflammation in Alzheimer’s disease (AD)? Arch Gerontol Geriatr. 2013;56(1):50–4. https://doi.org/10.1016/j.archger.2012.10.002.
Kara H, Degirmenci S, Bayir A, Ak A, Akinci M, Dogru A, et al. Red cell distribution width and neurological scoring systems in acute stroke patients. Neuropsychiatr Dis Treat. 2015;11:733–9. https://doi.org/10.2147/ndt.S81525.
Lappegård J, Ellingsen TS, Skjelbakken T, Mathiesen EB, Njølstad I, Wilsgaard T, et al. Red cell distribution width is associated with future risk of incident stroke The Tromsø Study. Thromb Haemost. 2016;115(1):126–34. https://doi.org/10.1160/th15-03-0234.
Turcato G, Cappellari M, Follador L, Dilda A, Bonora A, Zannoni M, et al. Red Blood Cell Distribution Width Is an Independent Predictor of Outcome in Patients Undergoing Thrombolysis for Ischemic Stroke. Semin Thromb Hemost. 2017;43(1):30–5. https://doi.org/10.1055/s-0036-1592165.
Li B, Liu S, Liu X, Fang J, Zhuang W. Association between red cell distribution width level and risk of stroke: A systematic review and meta-analysis of prospective studies. Medicine. 2020;99(16):e19691. https://doi.org/10.1097/md.0000000000019691.
Feng GH, Li HP, Li QL, Fu Y, Huang RB. Red blood cell distribution width and ischaemic stroke. Stroke Vasc Neurol. 2017;2(3):172–5. https://doi.org/10.1136/svn-2017-000071.
Shen Y, Huang X, Zhang W. Platelet-to-lymphocyte ratio as a prognostic predictor of mortality for sepsis: interaction effect with disease severity-a retrospective study. BMJ open. 2019;9(1):e022896. https://doi.org/10.1136/bmjopen-2018-022896.
Fateh-Moghadam S, Htun P, Tomandl B, Sander D, Stellos K, Geisler T, et al. Hyperresponsiveness of platelets in ischemic stroke. Thromb Haemost. 2007;97(6):974–8.
Goyal M, Menon BK, van Zwam WH, Dippel DW, Mitchell PJ, Demchuk AM, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387(10029):1723–31. https://doi.org/10.1016/s0140-6736(16)00163-x.
Longstreth WT Jr, Katz R, Tirschwell DL, Cushman M, Psaty BM. Intravenous tissue plasminogen activator and stroke in the elderly. Am J Emerg Med. 2010;28(3):359–63. https://doi.org/10.1016/j.ajem.2009.01.025.
Inohara T, Liang L, Kosinski AS, Smith EE, Schwamm LH, Hernandez AF, et al. Recent Myocardial Infarction is Associated With Increased Risk in Older Adults With Acute Ischemic Stroke Receiving Thrombolytic Therapy. J Am Heart Assoc. 2019;8(15):e012450. https://doi.org/10.1161/jaha.119.012450.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
NX and CP designed the study. NX wrote the manuscript. NX and CP collected, analyzed, and interpreted the data. CP critically reviewed, edited, and approved the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The requirement of ethical approval for this was waived by the Institutional Review Board of Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, because the data was accessed from MIMIC-III database (a publicly available database). Written informed consent was not required because of its retrospective design. All methods were performed in accordance with the relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Xu, N., Peng, C. Association between red cell distribution width-to-platelet ratio and short-term and long-term mortality risk in patients with acute ischemic stroke. BMC Neurol 23, 191 (2023). https://doi.org/10.1186/s12883-023-03219-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12883-023-03219-1