Abstract
Objective
Electrocardiogram (ECG) patterns can change, especially in patients with central nervous system disorders such as spontaneous subarachnoid hemorrhage. However, the association between the prognosis of traumatic brain injury (TBI) and ECG findings is unknown. Therefore, this study aimed to compare and to analyze ECG findings to predict early mortality in patients with TBI.
Methods
This retrospective observational study included patients with severe trauma and TBI who were admitted to the emergency department (ED) between January 2018 and December 2020. TBI was defined as an abbreviated injury scale score of the head of ≥3. We examined ECG findings, including PR prolongation (≥ 200 ms), QRS complex widening (≥ 120 ms), corrected QT interval prolongation (QTP, ≥ 480 ms), ST-segment elevation, and ST-segment depression (STD) at ED arrival. The primary outcome was 48-h mortality.
Results
Of the total patients with TBI, 1024 patients were included in this study and 48-h mortality occurred in 89 patients (8.7%). In multivariate analysis, QTP (odds ratio [OR], 2.017; confidence interval [CI], 1.203–3.382) and STD (OR, 8.428; 95% CI, 5.019–14.152) were independently associated with 48-h mortality in patients with TBI. The areas under the curve (AUCs) of the revised trauma score (RTS), injury severity score (ISS), QTP, STD, and the combination of QTP and STD were 0.790 (95% CI, 0.764–0.815), 0.632 (95% CI, 0.602–0.662), 0.605 (95% CI, 0.574–0.635), 0.723 (95% CI, 0.695–0.750), and 0.786 (95% CI, 0.759–0.811), respectively. The AUC of the combination of QTP and STD significantly differed from that of ISS, QTP, and STD, but not RTS.
Conclusion
Based on the ECG findings, QTP and STD were associated with 48-h mortality in patients with TBI.
Similar content being viewed by others
Introduction
Traumatic brain injury (TBI) contributes to a substantial number of deaths and cases of permanent disability [1]. An estimated 2.8 million people experience TBI annually, leading to death in 50,000 and hospitalization in 282,000. TBI is a contributing factor to 30% of all injury-related deaths in the United States [1]. Owing to advances in trauma care, the risk of death from multiple organ dysfunction syndrome gradually decreases after 48 h [2]. In contrast, deaths within 48 h account for 61.9% of all trauma-related deaths, the causes of which include exsanguination and TBI [2, 3]. Therefore, it is important to assess risk factors early and to provide critical care for patients with a high risk of death within 48 h.
Many triaging tools for TBI have been developed, and several studies have investigated the effectiveness of these tools in predicting outcomes [4, 5]. The injury severity score (ISS) and revised trauma score (RTS) are commonly used tools in trauma, including TBI [4, 5].
TBI-related death is associated with the severity of brain injury, malignant cerebral edema, and extracranial pathologies, especially cardiac electrical dysfunction [6]. Cerebrogenic cardiovascular damage may result in sudden cardiac death through central autonomic dysfunction with elevated catecholamine levels, shift in the ratio of potassium ions to sodium ions after renin–angiotensin system activation, and cerebral injury–related inflammatory responses [6,7,8,9]. Thus, cerebrogenic cardiovascular damage, which results in abnormal electrocardiogram (ECG) findings, can have an important association with the outcome of patients with TBI. However, few studies have assessed the relationship between ECG findings and outcome in patients with TBI. Therefore, this study aimed to compare and to analyze the role of ECG findings in predicting early mortality in patients with TBI. Furthermore, this study examined the performance of ECG findings in predicting early mortality compared with previously reported prognostic tools such as RTS and ISS.
Methods
Study design and population
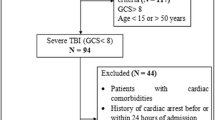
In this retrospective observational study, we enrolled patients with severe trauma and TBI who were admitted to the emergency department (ED) of Chonnam National University Hospital, Gwangju, South Korea, between January 2018 and December 2020. Severe trauma was defined as an ISS of > 15 [10]. TBI was defined as an abbreviated injury scale (AIS) score of the head of ≥3 [11]. Isolated TBI was defined as a head AIS score of ≥3 and any other AIS score of < 3 [12]. Combined TBI was defined as a head AIS score of ≥3 and at least one other AIS score of ≥3 [12]. The exclusion criteria were as follows: age < 18 years; burns, hanging, and drowning as specific trauma mechanisms; cardiac arrest after trauma before ED arrival; ECG data not measured or available at ED arrival; and missing data. The institutional review board at Chonnam National University Hospital approved the study protocol.
Data collection
The following data were obtained during the study period: age, trauma mechanism, sex, preexisting illness (previous percutaneous coronary intervention, hypertension, diabetes, renal impairment, and cerebrovascular accident), respiratory rate (RR), pulse rate, body temperature (BT), Glasgow Coma Scale (GCS) score, systolic blood pressure (SBP), ECG data on ED arrival, emergency operation, and 48-h mortality. RTS was calculated on the basis of the GCS score, SBP, and RR [13]. ISS was calculated on the basis of the AIS score [14]. To determine the presence of massive bleeding, we investigated whether massive transfusion (MT) was provided, which was defined as transfusion of > 10 units of PRCs within the first 24 h of admission or > 4 units in 1 h [15]. The primary outcome in this study was 48-h mortality in patients with TBI.
For analysis, posttrauma ECG data were obtained from the first interpretable 12-lead ECG within 1 h at ED arrival. ECGs were recorded at a speed of 25 mm/s and amplification of 10 mm/mV. We collected the PR interval (ms), QRS interval (ms), QT interval (ms), and ST-segment change as ECG data. The PR interval was the time from the onset of the P wave to the start of the QRS complex. It represents conduction through the atrioventricular node. PR prolongation was defined as a PR interval of ≥200 ms [16]. In patients with myocardial infarction or cardiomyopathy, the presence of heart scar tissue may slow down the electrical conduction between myocardial cells, resulting in widening of the QRS complexes [17]. QRS complex widening was defined as a QRS interval of ≥120 ms [17]. The QT interval was the time from the start point of the QRS complex, expressed as ventricular depolarization, to the return point (visualized) of the T wave, which results from ventricular repolarization. The corrected QT (QTc) interval was calculated after correcting for heart rate with the Bazett formula, as follows: QTc = QT/square root of the RR interval duration [18, 19]. QTc prolongation (QTP) was defined as a QTc interval of ≥480 ms [18, 19]. The ST segment was defined as the interval between ventricular depolarization and repolarization. In this study, we divided the recorded ST-segment changes into ST-segment elevation (STE) and ST-segment depression (STD). STE was defined as an elevation of ≥2 mm in a single lead, and STD was defined as a depression of ≥0.5 mm in two adjacent leads [20].
Statistical analyses
As continuous variables did not satisfy the normality test, median values are presented as interquartile ranges. Differences between continuous variables were analyzed using the Mann–Whitney U-test. Categorical variables are presented as frequencies and percentages. The chi-square test or Fisher’s exact test was used to analyze differences between categorical variables, as appropriate.
The results of multivariate analysis with logistic regression of covariates for 48-h mortality are expressed as odds ratios (ORs) and 95% confidence intervals (CIs). Variables with a P < 0.20 in univariate comparisons were included in the multivariate regression model. Using a step-by-step backward approach, variables with a threshold P > 0.10 were eliminated from the final adjusted regression model. Receiver operating characteristic curve analysis was performed to evaluate the prognostic performance of RTS, ISS, and ECG findings. The DeLong method was used for comparisons of area under the curve (AUC) values [21]. All analyses were performed using PASW/SPSS™ software (version 18; IBM Inc., Chicago, IL, USA) and MedCalc (version 19.0; MedCalc Software, bvba, Ostend, Belgium). A two-sided p-value of 0.05 was used to indicate statistical significance.
Results
Patient selection and characteristics
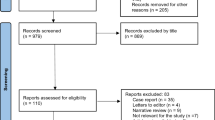
A total of 1190 patients with TBI who met the inclusion criteria were enrolled in this study. After applying the exclusion criteria, 1024 patients were finally included (Fig. 1). The number of male patients was 767 (74.9%), and the median patient age was 63.1 years (52.0–74.0 years). The 48-h mortality rate was 8.7% (n = 89). Of the 89 nonsurvivors, 79 (88.8%) died from brain herniation and 10 (11.25) died from massive hemorrhage.
Comparison of baseline and clinical characteristics between survivors and nonsurvivors
Table 1 compares the characteristics of survivors and nonsurvivors. Nonsurvivors had lower RTS, GCS score, and BT and higher ISS and pulse rate than survivors. No significant difference in SBP was observed between survivors and nonsurvivors. MT was more frequently performed in nonsurvivors than in survivors (Table 1).
A significant difference in PR interval was observed between survivors and nonsurvivors; however, there was no significant difference in the proportion of patients with PR prolongation (Table 2). Moreover, no significant difference was observed in the QRS interval between survivors and nonsurvivors, although the proportion of patients with widened QRS complexes showed a significant difference between groups (Table 1). The QTc interval was more prolonged in nonsurvivors than in survivors (460 [438–480] vs. 479 [456–512] ms, P < 0.001). The incidences of STE and STD in nonsurvivors were higher than those in survivors (Table 2).
In the isolated TBI group, survivors had higher RTS, GCS score, and BT values and lower ISS and PR values than non-survivors (Table 3). The PR interval, QTc interval, and incidence rates of QTP and STD were significantly different between survivors and nonsurvivors (Table 4).
In the combined TBI group, survivors had higher RTS, GCS score, SBP, and BT values and lower RR value than non-survivors (Table 3). The QTc interval and incidence rates of QRS widening, QTP, and STD were significantly different between survivors and nonsurvivors (Table 4).
Multivariate analysis for predicting 48-h mortality in patients with TBI
After adjusting for confounding factors, RTS (OR, 0.561; 95% CI, 0.483–0.650), BT (OR, 0.627; 95% CI, 0.413–0.953), emergency operation (OR, 0.363; 95% CI, 0.191–0.688), QTP (OR, 2.017; 95% CI, 1.203–3.382), and STD (OR, 8.428; 95% CI, 5.019–14.152) were independently associated with 48-h mortality in patients with TBI (Table 5).
In the isolated TBI group, QTP (OR, 2.098; 95% CI, 1.111–3.962) and STD (OR, 5.903; 95% CI, 3.146–11.076) were independently associated with 48-h mortality. In the combined TBI group, QTP (OR, 2.837; 95% CI, 1.011–7.958) and STD (OR, 15.430; 95% CI, 5.528–43.067) were associated with 48-h mortality (Table 6).
Prognostic performance of ECG variables for 48-h mortality in patients with TBI
On the basis of multivariate analysis, we set a limit to 48-h mortality in patients with TBI. The presence of STD (4 points) and/or QTP (1 point) was considered to predict 48-h mortality in patients with TBI. When QTP and STD were combined, the sum of scores ranged from 0 to 5, in which a higher score indicated a higher likelihood of 48-h mortality. The AUCs of RTS, ISS, QTP, STD, and the combination of QTP and STD were 0.790 (95% CI, 0.764–0.815), 0.632 (95% CI, 0.602–0.662), 0.605 (95% CI, 0.574–0.635), 0.723 (95% CI, 0.695–0.750), and 0.786 (95% CI, 0.759–0.811), respectively (Fig. 2). The AUC of the combination of QTP and STD was significantly different from that of ISS, STD, and QTP, but not RTS.
Receiver operating characteristic curve analyses of the RTS, ISS, QTP, STD, and the combination of QTP and STD for predicting 48-h mortality in patients with TBI. The AUCs of RTS, ISS, QTP, STD, and the combination of QTP and STD were 0.790 (95% CI, 0.764–0.815), 0.632 (95% CI, 0.602–0.662), 0.605 (95% CI, 0.574–0.635), 0.723 (95% CI, 0.695–0.750), and 0.786 (95% CI, 0.759–0.811), respectively. The AUC of the combination of QTP and STD was significantly different from that of ISS, STD, and QTP, but not RTS. RTS, revised trauma score; ISS, injury severity score; QTP, prolongation of corrected QT interval; STD, ST-segment depression; AUC, area under the curve; CI, confidence intervals
Discussion
Among the ECG variables, QTP and STD were independently associated with 48-h mortality in patients with TBI. The combination of QTP and STD had a similar performance to RTS in predicting 48-h mortality in patients with TBI. In both the isolated and combined TBI groups, QTP and STD were associated with 48-h mortality.
Krishnamoorthy et al. suggested that QTP (QTc > 440 ms) is related to cardiac dysfunction, including ejection fraction < 50% or regional wall motion abnormality on ECG, in patients with isolated TBI [22]. In patients with traumatic subarachnoid hemorrhage (SAH), the QTc interval was related to the severity of SAH based on computed tomography [18]. In another study on TBI, the QTc intervals of nonsurvivors were significantly more prolonged than those of survivors at hospital admission, consistent with the present study findings [23]. With respect to abnormal ECG findings in patients with TBI, the mechanism by which the brain affects the heart may be paroxysmal sympathetic hyperactivity [6]. In patients with SAH, the catecholamine level in cerebrospinal fluid was correlated with the severity of SAH [9].
Enhanced sympathetic activity can also induce ST-segment changes, including STE and STD, in severe neurological impairments such as TBI, SAH, or refractory seizures [24]. In a study on patients with SAH, 15% of patients with preoperative SAH developed STD [25]. ST-segment abnormalities were the most commonly reported ventricular repolarization disorders in TBI [26]. Even in the present study, STD occurred in 15.4% of the total patients with TBI, and the proportion of patients with STD with poor outcome was higher than that of patients with good outcome. Several studies have investigated the association between STD and the severity of head injury. In pediatric patients with TBI, STD led to hemodynamic instability or cardiac arrest, which improved only after surgery or other procedures [27]. In aneurysmal SAH, STD appears to have a significant relationship to the neurological outcome [28]. Manninen et al. [29] reported that the incidence of ECG abnormalities was statistically higher in patients with increased amounts of intracranial blood or intracerebral clots observed on computed tomography. STD in TBI is considered to cause myocardial ischemia due to severe sympathetic stimulation and elevated intracranial pressure.
Some studies have assessed the occurrence of STE in TBI [30,31,32]. However, none of the studies evaluated the frequency of STE or its association with outcomes such as mortality in TBI. Similar to STD, STE can also cause coronary vasospasm along with myocardial dysfunction with elevated intracranial pressure and consequent sympathetic activation. These sequential effects may eventually influence the outcome of TBI. In the present study, the proportion of patients with STE was higher among nonsurvivors than among survivors; however, there was no significant association with 48-h mortality in multivariate analysis. In the case of SAH, STE appeared to be related to delayed cerebral ischemia, although this relationship is still controversial [33]. Prospective multicenter studies on STE are needed to elucidate this issue.
In the present study, RTS was associated with 48-h mortality in TBI. We considered that the GCS score, a component of RTS, plays an important role in predicting 48-h mortality. Several studies have demonstrated that the GCS score is related to mortality in patients with TBI [34, 35]. In a study by Han et al., a GCS score of ≤5 was associated with mortality in TBI, and the GCS score of nonsurvivors in the present study was 4 (3–9) [35]. However, there are several barriers to determining the GCS score. The verbal GCS score may show variability in intubated patients, and the GCS score may be influenced by other factors that affect the level of consciousness, such as alcohol or sedative use. In addition, the reliability of the GCS score evaluated immediately after resuscitation is controversial. In contrast, in ECG measurement, there is no difference in score between evaluators, the effect of alcohol or sedatives is less than that on the GCS score, and the ECG results are hardly affected by different procedures.
This study had some limitations. First, because this was a retrospective study conducted in a single center, our results cannot be immediately generalized to the entire population. Further multicenter studies with larger sample sizes and a prospective design are needed to substantiate our findings. Second, we did not obtain data for all ECG variables in patients with TBI. However, as there was no difference in mortality between patients for whom ECG was available and those who did not (8.7% vs. 14.3%; p = 0.087), we can conclude that ECG abnormalities frequently occur in patients with severe TBI and that ECG abnormalities are associated with 48-h mortality. Third, we included ECG data alone at ED arrival. Thus, we could not investigate the serial changes in ECG findings or timing of ECG measurement that best reflects the prognosis of TBI. Fourth, we are unsure whether all potential factors, which can cause ECG changes, such as hypotension or hypothermia, were excluded. Although the confounding factors including systolic BP and hypothermia determined based on RTS and BT were adjusted, the factors that were overlooked may have influenced the ECG changes. For example, plasma hyperosmolarity was associated with QTP and atrial fibrillation [36]. In addition, disorders in electrolyte ions, including calcium and sodium, can influence the ECG changes including QTP or ST segment change [37, 38]. Fifth, we did not analyze the effect of medication history in patients with TBI. Data about the medication history were inaccurate and insufficient; hence, it could not be included in the analysis of our study. Finally, external or internal validation was not performed in this study. Thus, the ECG results including QTP and STD were not considered as predictors of the outcome of patients with TBI. In previous studies that focused on the phenomenon of ECG change after TBI, our study demonstrated the association between the acute phase prognosis of TBI and ECG in terms of 48-h mortality. It may be difficult to directly apply it to clinical practice. However, we believe that at least QTP and STD could reflect the condition of patients with TBI, which could serve as basis for developing the treatment guidelines.
Conclusion
In this study, QTP and STD were independently associated with 48-h mortality in patients with TBI. The combination of QTP and STD had a similar performance to RTS in predicting 48-h mortality. Based on the ECG findings, QTP and STD were associated with 48-h mortality in patients with TBI.
Availability of data and materials
The data used and analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- TBI:
-
Traumatic brain injury
- ISS:
-
Injury severity score
- RTS:
-
Revised trauma score
- ECG:
-
Electrocardiogram
- ED:
-
Emergency department
- AIS:
-
Abbreviated injury scale
- RR:
-
Respiratory rate
- PR:
-
Pulse rate
- BT:
-
Body temperature
- GCS:
-
Glasgow Coma Scale
- SBP:
-
Systolic blood pressure
- MT:
-
Massive transfusion
- QTc:
-
Corrected QT interval
- QTP:
-
QTc prolongation
- STE:
-
ST-segment elevation
- STD:
-
ST-segment depression
- OR:
-
Odds ratio
- CI:
-
Confdence interval
- AUC:
-
Area under the curve
References
Taylor CA, Bell JM, Breiding MJ, Xu L. Traumatic brain injury-related emergency department visits, hospitalizations, and deaths - United States, 2007 and 2013. MMWR Surveill Summ. 2017;66(9):1–16. https://doi.org/10.15585/mmwr.ss6609a1 PMID: 28301451; PMCID: PMC5829835.
Evans JA, van Wessem KJ, McDougall D, Lee KA, Lyons T, Balogh ZJ. Epidemiology of traumatic deaths: comprehensive population-based assessment. World J Surg. 2010;34(1):158–63. https://doi.org/10.1007/s00268-009-0266-1 PMID: 19882185.
Rauf R, von Matthey F, Croenlein M, Zyskowski M, van Griensven M, Biberthaler P, et al. Changes in the temporal distribution of in-hospital mortality in severely injured patients-an analysis of the TraumaRegister DGU. PLoS One. 2019;14(2):e0212095. https://doi.org/10.1371/journal.pone.0212095 PMID: 30794579; PMCID: PMC6386341.
Powers AY, Pinto MB, Tang OY, Chen JS, Doberstein C, Asaad WF. Predicting mortality in traumatic intracranial hemorrhage. J Neurosurg. 2019;132(2):552–9. https://doi.org/10.3171/2018.11.JNS182199 PMID: 30797192.
Mahadewa TGB, Golden N, Saputra A, Ryalino C. Modified revised trauma-Marshall score as a proposed tool in predicting the outcome of moderate and severe traumatic brain injury. Open Access Emerg Med. 2018;10:135–9. https://doi.org/10.2147/OAEM.S179090 PMID: 30349408; PMCID: PMC6183729.
Silvani A, Calandra-Buonaura G, Dampney RA, Cortelli P. Brain-heart interactions: physiology and clinical implications. Philos Trans A Math Phys Eng Sci. 2016;374(2067):20150181. https://doi.org/10.1098/rsta.2015.0181 PMID: 27044998.
Zipes DP, Rubart M. Neural modulation of cardiac arrhythmias and sudden cardiac death. Heart Rhythm. 2006;3(1):108–13. https://doi.org/10.1016/j.hrthm.2005.09.021 PMID: 16399065; PMCID: PMC2566299.
Ha Y, Jeong JA, Kim Y, Churchill DG. Sodium and potassium relating to Parkinson’s disease and traumatic brain injury. Met Ions Life Sci. 2016;16:585–601. https://doi.org/10.1007/978-3-319-21756-7_16 PMID: 26860312.
Moussouttas M, Lai EW, Khoury J, Huynh TT, Dombrowski K, Pacak K. Determinants of central sympathetic activation in spontaneous primary subarachnoid hemorrhage. Neurocrit Care. 2012;16(3):381–8. https://doi.org/10.1007/s12028-012-9673-5 PMID: 22311230.
Baker SP, O'Neill B, Haddon W Jr, Long WB. The injury severity score: a method for describing patients with multiple injuries and evaluating emergency care. J Trauma. 1974;14(3):187–96 PMID: 4814394.
Mellick D, Gerhart KA, Whiteneck GG. Understanding outcomes based on the post-acute hospitalization pathways followed by persons with traumatic brain injury. Brain Inj. 2003;17(1):55–71. https://doi.org/10.1080/0269905021000010159 PMID: 12519648.
Dübendorfer C, Billeter AT, Seifert B, Keel M, Turina M. Serial lactate and admission SOFA scores in trauma: an analysis of predictive value in 724 patients with and without traumatic brain injury. Eur J Trauma Emerg Surg. 2013;39(1):25–34. https://doi.org/10.1007/s00068-012-0212-z PMID: 26814920.
Champion HR, Sacco WJ, Copes WS, Gann DS, Gennarelli TA, Flanagan ME. A revision of the trauma score. J Trauma. 1989;29(5):623–9. https://doi.org/10.1097/00005373-198905000-00017 PMID: 2657085.
Foreman BP, Caesar RR, Parks J, Madden C, Gentilello LM, Shafi S, et al. Usefulness of the abbreviated injury score and the injury severity score in comparison to the Glasgow coma scale in predicting outcome after traumatic brain injury. J Trauma. 2007;62(4):946–50. https://doi.org/10.1097/01.ta.0000229796.14717.3a PMID: 17426553.
American College of Surgeons Committee on Trauma. Advanced trauma life support (ATLS) student course manual. 10th ed. Chicago: American College of Surgeons; 2018.
Aro AL, Anttonen O, Kerola T, Junttila MJ, Tikkanen JT, Rissanen HA, et al. Prognostic significance of prolonged PR interval in the general population. Eur Heart J. 2014;35(2):123–9. https://doi.org/10.1093/eurheartj/eht176 PMID: 23677846.
Katritsis DG, Brugada J. Differential diagnosis of wide QRS Tachycardias. Arrhythmia Electrophysiol Rev. 2020;9(3):155–60. https://doi.org/10.15420/aer.2020.20 PMID: 33240511; PMCID: PMC7675136.
Collier BR, Miller SL, Kramer GS, Balon JA, Gonzalez LS 3rd. Traumatic subarachnoid hemorrhage and QTc prolongation. J Neurosurg Anesthesiol. 2004;16(3):196–200. https://doi.org/10.1097/00008506-200407000-00003 PMID: 15211156.
Johnson JN, Ackerman MJ. QTc: how long is too long? Br J Sports Med. 2009;43(9):657–62. https://doi.org/10.1136/bjsm.2008.054734 PMID: 19734499; PMCID: PMC3940069.
Kim YJ, Min SY, Lee DH, Lee BK, Jeung KW, Lee HJ, et al. The role of post-resuscitation electrocardiogram in patients with ST-segment changes in the immediate post-cardiac arrest period. JACC Cardiovasc Interv. 2017;10(5):451–9. https://doi.org/10.1016/j.jcin.2016.11.046 PMID: 28279312.
DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837–45 PMID: 3203132.
Krishnamoorthy V, Prathep S, Sharma D, Gibbons E, Vavilala MS. Association between electrocardiographic findings and cardiac dysfunction in adult isolated traumatic brain injury. Indian J Crit Care Med. 2014;18(9):570–4. https://doi.org/10.4103/0972-5229.140144 PMID: 25249741; PMCID: PMC4166872.
Dabrowski W, Schlegel TT, Wosko J, Rola R, Rzecki Z, Malbrain MLNG, et al. Changes in spatial QRS-T angle and QTc interval in patients with traumatic brain injury with or without intra-abdominal hypertension. J Electrocardiol. 2018;51(3):499–507. https://doi.org/10.1016/j.jelectrocard.2017.12.038 PMID: 29310923.
Prasad Hrishi A, Ruby Lionel K, Prathapadas U. Head rules over the heart: cardiac manifestations of cerebral disorders. Indian J Crit Care Med. 2019;23(7):329–35. https://doi.org/10.5005/jp-journals-10071-23208 PMID: 31406441; PMCID: PMC6686577.
Rudehill A, Olsson GL, Sundqvist K, Gordon E. ECG abnormalities in patients with subarachnoid haemorrhage and intracranial tumours. J Neurol Neurosurg Psychiatry. 1987;50(10):1375–81. https://doi.org/10.1136/jnnp.50.10.1375 PMID: 3681317; PMCID: PMC1032467.
Lenstra JJ, Kuznecova-Keppel Hesselink L, la Bastide-van GS, Jacobs B, Nijsten MWN, van der Horst ICC, et al. The association of early electrocardiographic abnormalities with brain injury severity and outcome in severe traumatic brain injury. Front Neurol. 2021;11:597737. https://doi.org/10.3389/fneur.2020.597737 PMID: 33488498; PMCID: PMC7819976.
Dash M, Bithal PK, Prabhakar H, Chouhan RS, Mohanty B. ECG changes in pediatric patients with severe head injury. J Neurosurg Anesthesiol. 2003;15(3):270–3. https://doi.org/10.1097/00008506-200307000-00017 PMID: 12826977.
Sakr YL, Lim N, Amaral AC, Ghosn I, Carvalho FB, Renard M, et al. Relation of ECG changes to neurological outcome in patients with aneurysmal subarachnoid hemorrhage. Int J Cardiol. 2004;96(3):369–73. https://doi.org/10.1016/j.ijcard.2003.07.027 PMID: 15301889.
Manninen PH, Ayra B, Gelb AW, Pelz D. Association between electrocardiographic abnormalities and intracranial blood in patients following acute subarachnoid hemorrhage. J Neurosurg Anesthesiol. 1995;7(1):12–6. https://doi.org/10.1097/00008506-199501000-00003 PMID: 7881235.
Bhagat H, Narang R, Sharma D, Dash HH, Chauhan H. ST elevation--an indication of reversible neurogenic myocardial dysfunction in patients with head injury. Ann Card Anaesth. 2009;12(2):149–51. https://doi.org/10.4103/0971-9784.53446 PMID: 19602742.
Bhagat H, Chauhan H, Dash HH. ST elevation in a head-injured patient for emergency neurosurgery: do we routinely need a cardiac evaluation? Ann Card Anaesth. 2010;13(1):73–4. https://doi.org/10.4103/0971-9784.58843 PMID: 20075544.
Hashemian AM, Ahmadi K, Taherinia A, Sharifi MD, Ramezani J, Jazayeri SB, et al. ECG changes of cardiac origin in elderly patients with traumatic brain injury. Med J Islam Repub Iran. 2015;29:306 PMID: 26913269.
Schuiling WJ, Algra A, de Weerd AW, Leemans P, Rinkel GJ. ECG abnormalities in predicting secondary cerebral ischemia after subarachnoid haemorrhage. Acta Neurochir. 2006;148(8):853–8. https://doi.org/10.1007/s00701-006-0808-3 PMID: 16791433. discussion 858.
Demetriades D, Kuncir E, Velmahos GC, Rhee P, Alo K, Chan LS. Outcome and prognostic factors in head injuries with an admission Glasgow coma scale score of 3. Arch Surg. 2004;139(10):1066–8. https://doi.org/10.1001/archsurg.139.10.1066 PMID: 15492144.
Han JX, See AAQ, Gandhi M, King NKK. Models of mortality and morbidity in severe traumatic brain injury: an analysis of a Singapore Neurotrauma database. World Neurosurg. 2017;108:885–93.e1. https://doi.org/10.1016/j.wneu.2017.08.147 PMID: 28867312.
Dabrowski W, Siwicka-Gieroba D, Robba C, Badenes R, Bialy M, Iwaniuk P, et al. Plasma Hyperosmolality prolongs QTc interval and increases risk for atrial fibrillation in traumatic brain injury patients. J Clin Med. 2020;9(5):1293. https://doi.org/10.3390/jcm9051293 PMID: 32365845.
Landstrom AP, Dobrev D, Wehrens XHT. Calcium signaling and cardiac arrhythmias. Circ Res. 2017;120(12):1969–93. https://doi.org/10.1161/CIRCRESAHA.117.310083 PMID: 28596175.
Watanabe H, Koopmann TT, Le Scouarnec S, Yang T, Ingram CR, Schott JJ, et al. Sodium channel β1 subunit mutations associated with Brugada syndrome and cardiac conduction disease in humans. J Clin Invest. 2008;118(6):2260–8. https://doi.org/10.1172/JCI33891 PMID: 18464934.
Acknowledgements
None.
Funding
The authors declared that this study has received no financial support.
Author information
Authors and Affiliations
Contributions
JH Lee and DH Lee designed the study and conceived the framework of this article. YS Cho and DK Kim collected the clinical data. BK Lee and YH Jung conducted the statistical analysis. Then, JH Lee and DH Lee wrote the first draft of this manuscript. All of the authors made contributions to the final version of the manuscript. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study approved by the Chonnam National University Hospital Institutional Review Board (CNUH-2021-064). The requirement for informed consent was waived because the analysis was retrospective and anonymized. The requirement for consent to participate was waived because the analysis was retrospective and anonymized.
Consent for publication
The requirement for consent for publication was waived because the analysis was retrospective and anonymized.
Competing interests
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Lee, J.H., Lee, D.H., Lee, B.K. et al. Role of electrocardiogram findings in predicting 48-h mortality in patients with traumatic brain injury. BMC Neurol 22, 190 (2022). https://doi.org/10.1186/s12883-022-02717-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12883-022-02717-y