Abstract
Mortality in traumatic brain injury (TBI) is thought to be pathology in the heart-brain axis but its effect on the prognosis of traumatic brain injury remains unclear. Our study aimed to investigate the relationship between cardiac troponin I (cTnI) level and prognosis in TBI patients. Between January 2017 and May 2021, 480 patients diagnosed with TBI, who applied to the emergency department, were retrospectively included in this multicentric study. The databases of the hospitals were examined comprehensively and the demographic, clinical, laboratory, radiological, and therapeutic data and results of the patients were obtained. The severity of trauma and clinical status was evaluated with AIS, Injury Severity Score (ISS), ASA physical status, and Glasgow Coma Scale (GCS). The severity of the trauma was evaluated with the ISS. The modified Rankin Scale (mRS) and the Glasgow Outcome Scale (GOS) at discharge were used to evaluate in-hospital clinical outcomes. cTnI levels were classified into three categories: normal (< 0.05 ng/ml), mildly elevated (0.05–0.99 ng/ml), and severely elevated (≥ 1 ng/ml). The mean age of the patients was 41.7 and 75.4% of them were men. It was observed that mortality among patients over 65 years (13.9%) increased. High cTnI was detected in 284 (59.1%) patients. Although it was not statistically significant regarding the elevation of cTnI in patients under 65 years of age (P = 0.62), the difference was significant for cTnI in patients over 65 years of age (P < 0.001). The relationship between cTnI elevation was found to be statistically significant (P < 0.001) as the severity of the trauma increased and when severe additional traumas (thoracic, abdominal, or pelvic) occurred. A high cTnI level is associated with poor prognosis in TBI patients. cTnI measurement is a useful tool for early risk stratification and accelerated care; however, further prospective studies are needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Traumatic brain injury (TBI) is a common cause of death and disability worldwide. Despite advances in technology, there is a significant increase in the incidence of TBI. Therefore, it is an important public health problem worldwide. Appropriate decision about primary diagnosis, medical care, and the prognosis is critical at an early stage [1,2,3].
Troponins C, T, and I are part of the troponin complex, which are proteins integrated into the contraction of heart muscles. They are expressed in skeletal and cardiac myocytes. Serum cardiac troponin I (cTnI) level is a sensitive biomarker routinely used in myocardial injury. It is known to increase cardiac pathologies such as myocardial infarction, heart failure, and atrial fibrillation [4, 5]. However, cTnI elevation has been observed in diseases such as renal failure, pulmonary embolism, and sepsis [6,7,8]. Its elevation has been defined in many systemic complications related to morbidity and mortality (e.g., hypotension, hypoxia, intracranial hypertension) and in non-cardiac surgery patients [9, 10]. Elevated cTnI was observed in 30% of severe trauma patients (cTnI blood levels were measured within 24 h). Most of these traumas are the result of blunt cardiac or blunt thoracic trauma. Accordingly, it is thought to be related to myocardial cell damage. However, this theory cannot explain the high cTnI observed in patients without blunt cardiac or blunt thoracic trauma [11,12,13,14].
Neurocardiac axis: although well described in various non-traumatic head injuries such as acute stroke, subarachnoid hemorrhage, seizure [15,16,17,18], and Guillain Barre syndrome [19], the effects of TBI on cardiac function has not been adequately demonstrated.
However, an increased risk of complications and mortality is associated with electrocardiographic changes in the absence of coronary artery disease, a transient left ventricular wall motion abnormality, and an elevation in cardiac enzymes [20]. However, its etiology and pathophysiology are unknown. It is thought to be due to a systemic inflammatory response or catecholamine storm [20,21,22].
In TBI, a prognostic evaluation can be made with biomarkers that can be applied both in the emergency rooms and in the intensive care (ICU), and accordingly, treatment can be obtained in the clinic. This study aims to investigate the relationship between cTnI as a marker for myocardial injury and prognosis in patients with TBI. Some animal studies support this hypothesis [23, 24].
Patients and Methods
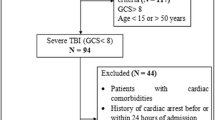
In this retrospective study, 480 patients diagnosed with TBI who applied to Adana City Hospital, Corlu State Hospital, and Seyhan State Hospital emergency between January 2017 and May 2021 were included. The databases of the hospitals were examined comprehensively and demographic, clinical, laboratory, radiological, and therapeutic data and results of the patients were obtained.
Patients aged 18 years or older, radiologically proven to have acute TBI, and whose cTnI blood levels were measured within 24 h of the posttraumatic period were included in the study. Patients who underwent cardiopulmonary resuscitation, had cardiac pathology, and had significant extracranial injuries, defined as Abbreviated Injury Scale (AIS) score ≥ 5, were excluded from the study.
Direct or secondary head traumas were included in the study. Trauma mechanisms were classified into three categories according to their etiology: (1) motor vehicle crash (MVC), (2) fall, and (3) other (sports injuries, penetrating injuries such as firearms).
The severity of the trauma and the clinical situation was evaluated according to AIS, Injury Severity Score (ISS), ASA physical status, and Glasgow Coma Scale (GCS) scores. Thoracic, abdominal, and pelvic traumas were classified by AIS, and brain traumas were classified by GCS. The severity of the trauma was evaluated with ISS and ASA physical status according to patients’ physical health status. The modified Rankin Scale (mRS) and the Glasgow Outcome Scale (GOS) at discharge were used to evaluate in-hospital clinical outcomes [25]. Cardiopulmonary parameters such as fever, pulse, arterial blood pressure, and blood oxygen levels were evaluated.
Plasma cTnI concentration was measured as ng/ml by the immunological method. cTnI levels were classified into three categories: normal (< 0.05 ng/ml), slightly elevated (0.05–0.99 ng/ml), and severely elevated (≥ 1 ng/ml). Electrocardiography and echocardiography were routinely performed in patients with elevated cTnI. Coronary angiography was performed when it seemed necessary. Patients with acute cardiac pathology were excluded from the study. Treatment regimens were grouped as medical treatment and surgery. While conservative treatment is applied in medical treatment; in surgical treatment, hematoma drainage, decompressive craniectomy, and external ventricular drainage were applied.
The student’s t-test was used for continuous variables, the Whitney U test for discontinuous variables, and χ2 for categorical variables. All analyzes were performed using R Statistical Software Version 3.3.2.
Results
In the multicentric study, 480 adult patients with TBI for 53 months were admitted. The mean age of the patients was 41.7 and 75.4% were men. It was observed that mortality over 65 years (13.9%) increased. The mean time between application and cTnI test was 1.8 h (10 min–24 h) (Table 1 Characteristics of the population).
The most common trauma mechanisms are; engine vehicle crash (MVC) (73.5%), fall (15.4%), and other (11%). All patients underwent a first CT scan. The most frequently defined cranial findings are; cerebral contusion, skull fractures, subdural hematoma, epidural hematoma, and pneumocephalus. In some patients, most of these pathologies were seen in combination (Fig. 1). The median GCS score was 6.85, and the cranial AIS score was 3.1. 182 (37.9%) patients were intubated. There was no statistically significant difference according to the cardiopulmonary data of the patients. However, there was a statistically significant difference between the severity of thoracic, abdominal, and pelvic injuries and the death rate (P < 0.001). The mean length of stay in the ICU was 5.22 days. The length of stay for general inpatient treatment of surviving patients (including patients who were followed up in the ward after ICU treatment) was 7.65 days. Median mRS was 2.4 and the GOS score was 4.3 at discharge. Of the 329 patients who died, 223 (67.8%) died in the ICU within the first 24 h. 248 (75.3%) patients died in the first 36 h, and 261 (79.3%) patients died in the first 48 h.
High cTnI was detected in 284 (59.1%) patients (Fig. 2). There was no significant difference between gender and cTnI elevation (P = 0.061). Although it was not statistically significant regarding the elevation of cTnI in patients under 65 years of age (P = 0.62), the difference was significant for cTnI in patients over 65 years of age (P < 0.001) (Fig. 3). The relationship between cTnI elevation was found to be statistically significant (P < 0.001) as the severity of the trauma increased and when severe additional traumas (thoracic or abdominal or pelvic) occurred. In the high cTnI group, a high mRS score (P < 0.001) and low GOS score at discharge (P < 0.001) were detected. (Table 2 Levels of cardiac cTnI).
Discussion
A retrospective study was conducted involving 480 patients with TBI. The relationship between cTnI and cardiopulmonary parameters was evaluated at the beginning of patient admission. In patients with initially high cTnI values; a higher ISS score, cranial AIS score, ASA physical status score, and a lower GCS score were detected. Although there was a significant relationship between high cTnI and white blood cell count, no significant difference was found in hemoglobin level. These findings appeared to be similar to previous studies [5, 21, 26]. Patients in the high cTnI group had low GCS at admission. Therefore, a high mRS score, a low GOS score, a high AIS score, and a high ASA score can be expected at discharge.
A positive correlation was found between the elevation of cTnI and the type of injury, the severity of the injury, and the risk of mortality. In age stratification, the elevation of cTnI is a particularly sensitive indicator of the risk of in-hospital mortality in patients over 65 years of age, but insignificant in patients younger than 65 years of age.
Another study [5] examined cTnI levels in 420 patients with blunt TBI. High cTnI was detected in 125 patients at the time of admission. 55 of these patients died. During the follow-up of the patients, high values were detected in an additional 48 patients in the serial cTnI test performed for up to 48 h, and 28 of these patients died. In addition, they found a significant relationship between cTnI values and cranial AIS scores in the study. In our study, it was shown that cTnI values were found at the highest levels (≥ 1 ng/ml) in patients who died within the first 24 h. A high correlation was observed between cTnI ≥ 1 ng/ml value and mortality. These patients generally had multiple intracerebral hemorrhages, intraventricular hemorrhages, and diffuse cerebral edema.
Although high cTnI in TBI seems to be a predictor of prognosis, it is associated with morbidity and mortality in various non-traumatic conditions. Other authors [26] found elevated cTnI values in patients with spontaneous intracerebral hematomas. They found a relationship between high cTnI and the patient’s age, gender, and volume of hemorrhage and mortality. Also, another study [27] found that the cTnI level was directly proportional to the severity of subarachnoid hemorrhage in patients with aneurysm with subarachnoid hemorrhage and it independently predicted poor functional recovery. In our study, we obtained similar results even though the brain injury was traumatic, unlike these studies. Although the etiology is not fully understood, various pathophysiological mechanisms have been suggested, such as microvascular spasm, coagulopathy, coronary artery spasm [28], and catecholamine storm triggering the acute inflammatory response [21, 28, 29]. Autopsy studies revealed subendocardial contractile band necrosis, which is unclear whether it is due to underlying cardiac ischemia or sympathetic discharge [30]. These cases may be explanatory for similar results.
In the cTnI high group; if there is no statistically significant increase in cardiopulmonary findings such as fever, pulse, mean blood pressure, and oxygen saturation, it may be that these patients are intubated under sedation.
This study has several limitations. First, this study is retrospective. It includes a small number of patients. The multicentric nature of this study brings a lack of standardization of indications and treatments among hospitals. Although ECG and continuous digital monitoring were performed in all patients, they could not be evaluated because monitoring could not be recorded. This important situation should be investigated in prospective studies. Despite the limitations, the findings of this study may help improve the quality of the intensive care unit. According to this study, a high cTnI value may be a useful serum biomarker. Patients with a high cTnI value can be monitored closely and get benefit from early intensive and invasive hemodynamic monitoring.
Conclusion
The cTnI level correlates with the severity of the head injury and can be used for early risk classification. Therefore, this cardiac marker can guide prognosis as an early biomarker in patients with TBI and can be used to improve treatment. For better monitoring and management of patients with TBI with high cTnI levels, further prospective studies should be supported.
References
Serri K, El Rayes M, Giraldeau G, Williamson D, Bernard F (2016) Traumatic brain injury is not associated with significant myocardial dysfunction: an observational pilot study. Scand J Trauma Resusc Emerg Med 24:31. https://doi.org/10.1186/s13049-016-0217-4
Picetti E, Iaccarino C, Servadei F (2017) Guidelines for the management of severe traumatic brain injury fourth edition. Neurosurgery 81(1):E2–E2
Hosseini SH, Ayyasi M, Akbari H, Gorji MAH (2017) Comparison of Glasgow coma scale, a full outline of unresponsiveness and acute physiology and chronic health evaluation in prediction of mortality rate among patients with traumatic brain injury admitted to intensive care unit. Anesthesiol Pain Med 7(5):e33653
Bhoi S, Verma P, Vankar S, Galwankar S (2014) High sensitivity troponins and conventional troponins at the bedside. Int J Crit Illn Inj Sci 4(3):253
Salim A, Hadjizacharia P, Brown C, Inaba K, Teixeira PG, Chan L et al (2008) Significance of troponin elevation after severe traumatic brain injury. J Trauma Acute Care Surg 64(1):46–52
Hamm CW, Giannitsis E, Katus HA (2002) Cardiac troponin elevations in patients without acute coronary syndrome. Am Heart Assoc 106(23):2871–2
Jeremias A, Gibson CM (2005) Narrative review: alternative causes for elevated cardiac troponin levels when acute coronary syndromes are excluded. Ann Intern Med 142(9):786–791
Roongsritong C, Warraich I, Bradley C (2004) Common causes of troponin elevations in the absence of acute myocardial infarction: incidence and clinical significance. Chest 125(5):1877–1884
Stein DM, Hu PF, Brenner M, Sheth KN, Liu K-H, Xiong W et al (2011) Brief episodes of intracranial hypertension and cerebral hypoperfusion are associated with poor functional outcome after severe traumatic brain injury. J Trauma Acute Care Surg 71(2):364–374
Zygun DA, Kortbeek JB, Fick GH, Laupland KB, Doig CJ (2005) Non-neurologic organ dysfunction in severe traumatic brain injury. Crit Care Med 33(3):654–660
Martin M, Mullenix P, Rhee P, Belzberg H, Demetriades D, Salim A (2005) Troponin increases in the critically injured patient: mechanical trauma or physiologic stress? J Trauma Acute Care Surg 59(5):1086–1091
Lindstaedt M, Germing A, Lawo T, von Dryander S, Jaeger D, Muhr G et al (2002) Acute and long-term clinical significance of myocardial contusion following blunt thoracic trauma: results of a prospective study. J Trauma Acute Care Surg 52(3):479–485
Salim A, Velmahos GC, Jindal A, Chan L, Vassiliu P, Belzberg H et al (2001) Clinically significant blunt cardiac trauma: role of serum troponin levels combined with electrocardiographic findings. J Trauma Acute Care Surg 50(2):237–243
Velmahos GC, Karaiskakis M, Salim A, Toutouzas KG, Murray J, Asensio J et al (2003) Normal electrocardiography and serum troponin I levels preclude the presence of clinically significant blunt cardiac injury. J Trauma Acute Care Surg 54(1):45–51
Hravnak M, Frangiskakis JM, Crago EA, Chang Y, Tanabe M, Gorcsan J III et al (2009) Elevated cardiac troponin I and relationship to persistence of electrocardiographic and echocardiographic abnormalities after aneurysmal subarachnoid hemorrhage. Stroke 40(11):3478–3484
Christensen H, Johannesen HH, Christensen AF, Bendtzen K, Boysen G (2004) Serum cardiac troponin I in acute stroke is related to serum cortisol and TNF-α. Cerebrovasc Dis 18(3):194–199
Putaala J, Lehto M, Meretoja A, Silvennoinen K, Curtze S, Kääriäinen J et al (2014) In-hospital cardiac complications after intracerebral hemorrhage. Int J Stroke 9(6):741–746
Sieweke N, Allendörfer J, Franzen W, Feustel A, Reichenberger F, Pabst W et al (2012) Cardiac troponin I elevation after epileptic seizure. BMC Neurol 12(1):1–6
Bernstein R, Mayer SA, Magnano A (2000) Neurogenic stunned myocardium in Guillain-Barré syndrome. Neurology 54(3):759–759
Nguyen H, Zaroff JG (2009) Neurogenic stunned myocardium. Curr Neurol Neurosci Rep 9(6):486
Cai SS, Bonds BW, Hu PF, Stein DM (2016) The role of cardiac troponin I in prognostication of patients with isolated severe traumatic brain injury. J Trauma Acute Care Surg 80(3):477
El-Menyar A, Goyal A, Latifi R, Al-Thani H, Frishman W (2017) Brain-heart interactions in traumatic brain injury. Cardiol Rev 25(6):279–288
Lee YL, Lim SW, Zheng HX, Chang WT, Nyam TTE, Chio CC et al (2020) The short-term effects of isolated traumatic brain injury on the heart in experimental healthy rats. Neurocrit Care 33(2):438–448
Lackner I, Weber B, Haffner-Luntzer M, Hristova S, Gebhard F, Lam C et al (2021) Systemic and local cardiac inflammation after experimental long bone fracture, traumatic brain injury and combined trauma in mice. J Orthop Transl 28:39–46
Yeatts SD, Martin RH, Meurer W, Silbergleit R, Rockswold GL, Barsan WG et al (2020) Sliding scoring of the glasgow outcome scale-extended as primary outcome in traumatic brain injury trials. J Neurotrauma 37(24):2674–2679
Hays A, Diringer MN (2006) Elevated troponin levels are associated with higher mortality following intracerebral hemorrhage. Neurology 66(9):1330–1334
Miketic JK, Hravnak M, Sereika SM, Crago EA (2010) Elevated cardiac troponin I and functional recovery and disability in patients after aneurysmal subarachnoid hemorrhage. Am J Crit Care 19(6):522–528
Chang P-C, Lee S-H, Hung H-F, Kaun P, Cheng J-J (1998) Transient ST elevation and left ventricular asynergy associated with normal coronary artery and Tc-99m PYP Myocardial Infarct Scan in subarachnoid hemorrhage. Int J Cardiol 63(2):189–192
Lee VH, Oh JK, Mulvagh SL, Wijdicks EF (2006) Mechanisms in neurogenic stress cardiomyopathy after aneurysmal subarachnoid hemorrhage. Neurocrit Care 5(3):243–249
Kono T, Morita H, Kuroiwa T, Onaka H, Takatsuka H, Fujiwara A (1994) Left ventricular wall motion abnormalities in patients with subarachnoid hemorrhage: neurogenic stunned myocardium. J Am Coll Cardiol 24(3):636–640
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
The study protocol was approved by the ethical committee of the İstanbul Medipol University (No: E-10840098–772.02–5618).
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sezer, C., Gokten, M. & Acıkalın, R. Troponin I New Biomarker in Traumatic Brain Injury. Indian J Surg 85, 120–126 (2023). https://doi.org/10.1007/s12262-022-03648-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12262-022-03648-1