Abstract
Background
Sepsis-associated acute kidney injury (SA-AKI) has high mortality rates. The osteoprotegerin (OPG)/receptor activator of nuclear factor-κB ligand (RANKL)/receptor activator of nuclear factor-κB (RANK)/Toll-like receptor 4 (TLR4) pathway and its potential role in SA-AKI pathogenesis remain to be fully understood. Herein, we addressed this issue using mouse models.
Methods
An SA-AKI mouse model was established using the cecal ligation and puncture method (CLP). Mice were grouped into sham, CLP model, CLP + recombinant RANKL, and CLP + anti-RANKL groups. Serum creatinine (Scr) and blood urea nitrogen (BUN) levels were measured to assess kidney function. ELISA was used to detect serum IL-1β, TNF-α, and IL-6 levels. Real-time quantitative PCR and Western blot were used to detect the mRNA and protein expression levels of OPG, RANKL, RANK, and TLR4 in kidney tissues. HE staining was performed to evaluate the pathological changes.
Results
The CLP model group showed higher levels of Scr and BUN, indicating impaired kidney function in SA-AKI, compared to the sham group. Treatment with recombinant RANKL in the CLP + recombinant RANKL group reduced Scr and BUN levels, while anti-RANKL treatment in the CLP + anti-RANKL group elevated their levels. Moreover, the CLP model group had significantly increased IL-1β, TNF-α, and IL-6 than the sham group, indicating elevated inflammation in SA-AKI. The CLP + recombinant RANKL group demonstrated decreased cytokine levels, whereas the CLP + anti-RANKL group showed an increase. Additionally, the histopathological evaluation revealed distinct kidney tissue damage in the CLP model group. Recombinant RANKL treatment reduced this damage, while anti-RANKL treatment exacerbated it. Mechanically, the mRNA and protein expression of RANKL were significantly decreased, while those of OPG, RANK, and TLR4 were significantly increased in the CLP model group and the CLP + anti-RANKL group. Interestingly, treatment with recombinant RANKL reversed these changes, as evidenced by significantly increased RANKL but decreased OPG, RANK, and TLR4.
Conclusion
The OPG/RANKL/RANK/TLR4 pathway is involved in SA-AKI pathogenesis. Recombinant RANKL treatment attenuates the inflammatory response and kidney tissue damage in SA-AKI, possibly via regulating this pathway. This pathway shows promise as a therapeutic target for SA-AKI.
Similar content being viewed by others
Background
Sepsis is a life-threatening clinical syndrome that is prone to complications such as septic shock and acute kidney injury (AKI) [1]. It is a leading cause of mortality in intensive care unit patients [2], resulting in a considerable rise in mortality rates [3]. Sepsis-related acute kidney injury (SA-AKI) accounts for over 50% of clinical AKI cases [4]. In cases where sepsis is complicated by AKI, the mortality rate of the patient may reach 75% [5]. The primary treatment of SA-AKI is kidney replacement therapy. Kidney replacement therapy is limited to symptomatic treatment and is not correlated with the early diagnosis of AKI. Identifying new targets for the prevention and treatment of SA-AKI will effectively reduce the mortality rate of critically ill patients. Therefore, understanding the pathogenesis of SA-AKI and exploring novel treatment strategies are of particular significance [6].
Osteoprotegerin (OPG) is a secreted protein involved in the regulation of bone resorption, and it is distributed in organs including the heart, kidneys, liver, bones, and blood vessels in the human body. The receptor activator of nuclear factor-κB ligand (RANKL) is a type II transmembrane protein, while the receptor activator of nuclear factor-κB (RANK) is a type I transmembrane protein that belongs to the tumor necrosis factor receptor superfamily. RANKL is presently the sole known ligand that binds to the RANK membrane receptor, leading to receptor trimerization and subsequently initiating the activation of TNF receptor-associated factors (TRAF) and downstream signaling pathways [7]. OPG, also identified as the decoy receptor for RANKL, competes with RANKL for binding and the interaction of these three components constructs a regulatory system [8]. The involvement of the OPG/RANKL/RANK pathway in pathological angiogenesis, cell survival, bone metabolism, prevention and treatment of acute inflammatory diseases, development of breast epithelial cells, immune function, and cancer has been reported [9, 10]. However, there is limited information on whether this signaling pathway contributes to the development of SA-AKI through participation in the imbalance of inflammatory reactions and endothelial dysfunction.
Toll-like receptor 4 (TLR4) is a natural immune receptor, and the excessive activation of TLR4 triggers the production of various inflammatory factors, playing a crucial role in the induction of inflammatory responses [11, 12]. It is reported that the binding of RANKL to RANK can inhibit the activation of TLR4 in macrophages, promote the binding between TRAF6 (a key activator of TLR4) and RANK, and affect upstream events of nuclear factor-kappa B activation, thereby reducing the expression of pro-inflammatory factors [10].
Herein, our study investigated whether it is possible to mitigate the inflammatory response of SA-AKI and improve SA-AKI by regulating the OPG/RANKL/RANK/TLR4 signaling pathway. We established an SA-AKI model through the cecal ligation and puncture (CLP) method, administered recombinant RANKL and anti-RANKL pretreatment, and detected the levels of inflammatory factors in four groups. The mRNA and protein expression levels of OPG, RANKL, RANK, and TLR4 were measured, while kidney tissue damage was observed. Our findings may provide experimental evidence for the development of therapeutic targets for SA-AKI.
Methods
Study animals
Twenty-four healthy male C57BL/6 mice, 8–10 weeks old with a body weight of 20–25 g, were purchased from the Animal Experimental Center of Xinjiang Medical University (animal license number: SCXK (Xin) 2018-0001). The mice were acclimated in the animal room with a constant temperature (22–24 °C) and a light/dark cycle (12 h) for 3 days before the experiment. They were fasted for 8 h before the experimental procedure but had access to water. All methods were performed following the relevant guidelines and regulations. The experimental protocol was reviewed and approved by the Experimental Animal Ethics Committee of the Xinjiang Medical University (Approval No.: IACUC-20230217-18). The study is reported in accordance with ARRIVE guidelines.
Model establishment and animal grouping
Mice were randomly divided into the sham group, CLP model group, CLP + recombinant RANKL group, and CLP + anti-RANKL group, with 6 mice in each group. The SA-AKI model was established using the CLP method [13]. Briefly, after anesthetizing with 3% pentobarbital sodium (1 mL/kg) by intraperitoneal injection, a 1–1.5 cm incision was made along the midline of the lower abdomen. The cecum was ligated with a size 4 surgical suture 1 cm away from the end of the cecum. An 18-gauge needle was used to puncture the ligation site on both sides of the cecal wall twice, and a small amount of feces was squeezed out. After confirming the patency of the puncture site, the cecum was returned to the abdominal cavity, and the abdomen was closed in layers. The sham group only underwent laparotomy and closure of the abdomen without cecal ligation and puncture. The CLP + recombinant RANKL group and CLP + anti-RANKL group were intraperitoneally injected with recombinant RANKL (5 µg per mouse; Abclonal, Wuhan, China) and anti-RANKL (200 µg per mouse; Bio X cell, West Lebanon, NH, USA), respectively, 2 h before modeling, while the sham group and CLP model group were intraperitoneally injected with an equal amount of sterile water for injection. After the surgical procedure, the mice were immediately given 30 ml/kg of normal saline subcutaneously on the back of the neck for fluid resuscitation and kept warm while waiting for anesthesia recovery.
Sample collection
At 24 h after modeling, blood was taken from the orbital vein, and serum was obtained after centrifugation (3,000 r/min, 15 min). The serum was stored at -80 °C until further analysis. After blood collection, the mice were euthanized by cervical dislocation. The right kidney tissue was collected and stored at -80 °C until further analysis. The left kidney tissue was also collected and fixed in 4% paraformaldehyde.
Detection of serum creatinine (scr) and blood Urea Nitrogen (BUN)
Scr level was determined using the creatinine oxidase method, and BUN level was determined using the urease method, strictly following the instructions of the creatinine assay kit and urea nitrogen assay kit (Nanjing Jiancheng Bioengineering Institute, China).
ELISA
The levels of IL-1β, TNF-α, and IL-6 in the serum were measured with corresponding ELISA kits (ELK Biotechnology CO., LTD, Wuhan, China), following the kit instructions.
Real-time quantitative PCR (RT-qPCR)
Total RNA was extracted from the right kidney tissues. The cDNA was obtained after reverse transcription. The RT-qPCR was performed. The mRNA expression levels of OPG, RANKL, RANK, and TLR4 were calculated using the 2−ΔΔCT method.
Western blotting
The right kidney tissues were subjected to tissue lysis and then the total protein was extracted after centrifugation at 12,000 rpm for 5 min at 4 °C. The protein concentration was detected with the BCA method. After electrophoresis separation, the proteins were transferred to the membrane, which was blocked with 5% skim milk for 2 h. Subsequently, the membrane was incubated overnight at 4 °C with the primary antibodies against OPG (1:1000; Affinity Bioscience, OH, USA), RANKL (1:3000; Proteintech Group, Inc, Wuhan, China), RANK (1:500; Abcam, Cambridge, UK), TLR4 (1:500; Proteintech Group, Inc), and β-actin (1:10000) (Tiandeyue (Beijing) Biotechnology Co., Ltd, China). The next day, the membrane was washed with TBST buffer and then incubated with the horseradish peroxidase-conjugated sheep anti-rabbit or anti-mouse secondary antibody (1:10000; Wuhan Aspen Biotechnology Co., Ltd, China). Lastly, the relative expression levels of the target proteins were analyzed using Image J and normalized to β-actin.
Hematoxylin and Eosin (HE) staining
The left kidney tissue was fixed in 4% paraformaldehyde for 24 h, rinsed in running water for 2 h, dehydrated in graded ethanol, cleared, immersed in paraffin for 1 h, embedded, sectioned, stained with HE, mounted with neutral gum, and observed under a microscope for morphological changes. The histopathological scoring criteria were as follows: no apparent lesions (0 points), interstitial congestion or glomerular atrophy (1 point), tubular epithelial necrosis (1 point), tubular lumen dilation (1 point), and interstitial inflammatory cell infiltration (1 point), with a total score of 5 points [14].
Statistical analysis
Data analysis was performed using SPSS 26.0 and GraphPad Prism 9.0 software. Normally distributed quantitative data are expressed as mean ± standard deviation. The t-test was used for comparisons between two groups, and analysis of variance was used for comparisons among multiple groups. A P value of < 0.05 was considered statistically significant.
Results
Levels of Scr and BUN
To investigate whether OPG/RANKL/RANK is related to the occurrence and development of SA-AKI, the levels of Scr and BUN were detected. The results revealed that the CLP model group showed a significant increase in the levels of Scr (Fig. 1A) and BUN (Fig. 1B) compared to the sham group (P < 0.05). In contrast to the CLP model group, the CLP + recombinant RANKL group exhibited a significant decrease in Scr and BUN levels, whereas the CLP + anti-RANKL group showed a significant increase (P < 0.05). This indicates the involvement of OPG/RANKL/RANK in the occurrence and development of SA-AKI.
Changes in serum cytokine levels
We further detected the serum levels of IL-1β, TNF-α, and IL-6 by using ELISA to examine whether inflammatory response is involved in the occurrence and development of SA-AKI. Compared with the sham group, the serum levels of IL-1β (Fig. 2A), TNF-α (Fig. 2B), and IL-6 (Fig. 2C) in the CLP model group were significantly increased (P < 0.05). Compared with the CLP model group, the levels of the above factors were significantly decreased in the CLP + recombinant RANKL group but were significantly increased in the CLP + anti-RANKL group (P < 0.05). This suggests the involvement of inflammatory response in the pathogenesis of SA-AKI.
The expression levels of OPG, RANKL, RANK, and TLR4 mRNA in kidney tissues
To determine the expression of OPG/RANKL/RANK/TLR4 in SA-AKI, we conducted RT-qPCR. In kidney tissues, the mRNA expression levels of OPG (Fig. 3A), RANKL (Fig. 3B), RANK (Fig. 3C), and TLR4 (Fig. 3D) differed among groups. Specifically, RANKL mRNA expression was significantly decreased in the CLP model group compared to the sham group, while OPG, RANK, and TLR4 mRNA expression was significantly increased (P < 0.05). Moreover, the CLP + recombinant RANKL group exhibited contrasting results compared to the CLP model group for these indicators (P < 0.05). Additionally, the CLP + anti-RANKL group demonstrated increased levels of OPG, RANK, and TLR4 mRNA while decreasing RANKL mRNA expression compared to the CLP model group (P < 0.05).
The expression levels of OPG, RANKL, RANK, and TLR4 proteins in kidney tissues
We further performed the Western blot to measure the expression levels of OPG, RANKL, RANK, and TLR4 proteins in kidney tissues (Fig. 4). Compared with the sham group, the protein expression of RANKL (Fig. 4A and C) in the kidney tissues of the CLP model group was significantly decreased, while the protein expression of OPG (Fig. 4A and B), RANK (Fig. 4A and D), and TLR4 (Fig. 4A and E) was significantly increased (P < 0.05). There were significantly higher levels of RANKL, but significantly lower levels of OPG, RANK, and TLR4 proteins in the CLP + recombinant RANKL group than in the CLP model group (P < 0.05). On the contrary, the CLP + anti-RANKL group had higher levels of OPG, RANK, and TLR4 proteins but lower RANKL than the CLP model group (P < 0.05). This data is consistent with those of RT-qPCR, indicating the involvement of OPG/RANKL/RANK/TLR4 in the occurrence and development of SA-AKI.
The expression levels of OPG, RANKL, RANK, and TLR4 protein in kidney tissues. Western blot detected the protein expression levels in each group. (A) Representative Western blot results. (B) Relative level of OPG. (C) Relative level of RANKL. (D) Relative level of RANK. (E) Relative level of TLR4. *P < 0.05, **P < 0.01, ****P < 0.0001
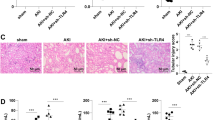
Histopathological changes in the kidney tissues
The histopathological changes in the kidney tissues were detected with HE staining and the results are presented in Fig. 5. The histopathological scores of each group were calculated and compared. Following 24 h of modeling, no evident abnormalities were detected in the kidney tissue of the sham group (Fig. 5A). The kidney tissue structure in the CLP model group exhibited damage, including partial ischemic contraction of the kidney glomerulus, enlargement of the capsular space, necrosis, and shedding of kidney tubular epithelial cells into the tubular lumen, tubular dilation, kidney interstitial edema, infiltration of inflammatory cells, and a markedly higher histopathological score than the sham group. In the CLP + recombinant RANKL group, the extent of kidney tissue damage was decreased compared to the CLP model group, resulting in a significantly lower histopathological score (P < 0.05) (Fig. 5B). Conversely, the CLP + anti-RANKL group exhibited increased kidney tissue damage compared to the CLP model group, leading to a significantly higher histopathological score (P < 0.05). These results demonstrate that recombinant RANKL attenuates kidney tissue damage, whereas the use of anti-RANKL results in an exacerbation of the damage.
Discussion
Sepsis has a rapid onset, quick progression, and poor prognosis, and can involve multiple organs throughout the body (such as blood vessels, kidneys, lungs, liver, heart, and brain) [15]. According to research, the mortality rate of sepsis in China is as high as 67 per 100,000 [16]. The mortality rate of sepsis is closely related to the development of organ dysfunction, with AKI being the most common, most severe, and highest mortality complication [17]. AKI progresses rapidly, is highly critical, and has a high degree of disability and fatality [18]. In this study, a sepsis mouse model was established using the CLP method. At 24 h after the surgery, the mice showed signs of lethargy, reduced activity, increased heart rate, and decreased urine output in the CLP group (data not shown). Laboratory tests showed a significant increase in Scr, BUN, IL-1β, TNF-α, and IL-6 and aggravated pathological changes in the kidneys of the CLP group, indicating the successful establishment of a mouse model with SA-AKI.
OPG, a member of the tumor necrosis factor superfamily, has been shown to directly participate in the inflammatory process associated with inflammatory bowel disease [19]. In AKI secondary to liver cirrhosis, serum OPG levels increase as the injury progresses, thereby exacerbating its severity [20]. Additionally, two recent clinical studies have identified OPG as a predictor of mortality in patients with systemic inflammatory response syndrome in intensive care units [21, 22]. Moreover, circulating OPG significantly increases in the serum of patients with SA-AKI, leading to an exacerbation of the inflammatory cascade reaction during SA-AKI [22]. Consistently, we found that the levels of inflammatory factors and the mRNA and protein expression of OPG in the serum of the CLP group mice were significantly higher than those in the Sham group. Moreover, there was inflammatory cell infiltration in the CLP group. After pretreatment with recombinant RANKL and anti-RANKL, the mRNA and protein expression of OPG showed a decrease and increase, respectively, and the degree of kidney tissue injury showed a reduction and exacerbation. These findings indicate that OPG is involved in the process of SA-AKI and may exacerbate SA-AKI by increasing the inflammatory cascade reaction.
It has been reported that RANKL plays a key role in many immune-inflammatory disorders [23]. Upon binding of RANKL to the receptor RANK, it can exert potent anti-inflammatory effects [24]. External application of recombinant RANKL protein can increase the expression of IL-10, thereby exerting anti-inflammatory effects [25]. The presence of RANKL in the nervous system can reduce neuronal death caused by microglia during stroke. The augmentation of RANKL/RANK signaling with recombinant RANKL protein can downregulate inflammatory factors, thereby alleviating the inflammatory response after stroke [26]. Similarly, in a mouse model pretreated with LPS, soluble RANKL was identified in the serum, and the level of RANKL was significantly reduced [27]. It was also found that RANKL inhibited the production of pro-inflammatory cytokines in macrophages [27]. In this study, we obtained similar results. Compared with the Sham group, the mRNA and protein expression of RANKL in the CLP group were significantly downregulated. The level of inflammatory factors was significantly increased, and the kidney tissue structure was damaged. In contrast, in the group pre-treated with recombinant RANKL, there was upregulated expression of RANKL at mRNA and protein levels. This may be attributed to the exogenous supplementation of RANKL. However, the underlying mechanism remains to be further determined. Moreover, the inflammatory response and kidney pathological damage were significantly reduced. Conversely, the anti-RANKL pre-treatment group showed an aggravation compared to the CLP group, suggesting that recombinant RANKL enhances the signaling of RANK, thereby alleviating the inflammatory response and kidney function damage of SA-AKI. Therefore, the mechanism by which RANKL exerts anti-inflammatory effects after binding to RANK may be related to the downregulation of OPG expression.
TLR4 is an important member of the TLR family, which triggers a cascade reaction upon binding to lipopolysaccharide, promoting the production of TNF, IL-1β, IL-6, and other pro-inflammatory cytokines, thereby initiating a pro-inflammatory immune response [28,29,30]. Inhibiting TLR4 signaling can significantly reduce the severity of SA-AKI in mice [31, 32]. The results of this study revealed a significant increase in TLR4 mRNA and protein expression in the CLP group, accompanied by significant inflammation, indicating that TLR4 is involved in the occurrence and development of SA-AKI. Therefore, inhibiting TLR4 signaling can mitigate the inflammatory response, thereby improving SA-AKI. Interestingly, the mRNA and protein expression levels of TLR4 in the recombinant RANKL pretreatment group were significantly downregulated, and the levels of inflammatory factors and the degree of kidney injury were significantly reduced compared to the CLP group. However, the results were abolished in the anti-RANKL pretreatment group and were more significant than the CLP group. These findings indicate that the combination of RANKL with RANK can inhibit the activation of TLR4, thereby alleviating the inflammatory response during SA-AKI and reducing the degree of kidney injury.
There are some limitations in this study. For example, due to limited funding, we did not comprehensively assess the involvement of inflammasome NLRP3, cell death mechanisms, or macrophages in SA-AKI, nor did we explore their relationship with the OPG/RANKL/RANK/TLR4 signaling pathway. Further studies are warranted.
A schematic diagram illustrating the OPG/RANKL/RANK/TLR4 signaling pathway. RANKL competes with OPG for binding to RANK. Upon binding of RANKL to RANK, it triggers the interaction of RANK with TRAF6, leading to the suppression of the TLR4 signaling pathway, the activation of which is initiated upon the binding of LPS with CD14. This in turn inhibits the activation of the NF-κB signaling pathway and the subsequent release of inflammatory mediators
Conclusion
In summary, our results demonstrate that RANKL plays an important role in preventing sepsis-associated AKI. Its mechanism may involve increasing the signal transduction after RANKL binds to RANK and reducing the expression of OPG and TLR4, thereby inhibiting the inflammatory response, reducing kidney tissue damage, and alleviating kidney dysfunction (Fig. 6). Therefore, the OPG/RANKL/RANK/TLR4 signaling pathway may become a potential therapeutic target for SA-AKI, providing a theoretical basis and intervention potential for clinical diagnosis and treatment. In the future, we will further investigate the mechanisms underlying the role of the OPG/RANKL/RANK/TLR4 signaling pathway in SA-AKI.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AKI:
-
Acute kidney injury
- SA-AKI:
-
Sepsis-related acute kidney injury
- OPG:
-
Osteoprotegerin
- RANKL:
-
Receptor activator of nuclear factor-κB ligand
- RANK:
-
Receptor activator of nuclear factor-κB
- TRAF:
-
TNF receptor-associated factors
- TLR4:
-
Toll-like receptor 4
- CLP:
-
Cecal ligation and puncture
References
Wang X, Wang Y, Kong M, Yang J. MiR-22-3p suppresses sepsis-induced acute kidney injury by targeting PTEN. Biosci Rep 2020, 40(6).
Perner A, Cecconi M, Cronhjort M, Darmon M, Jakob SM, Pettila V, van der Horst ICC. Expert statement for the management of hypovolemia in sepsis. Intensive Care Med. 2018;44(6):791–8.
May CN, Bellomo R, Lankadeva YR. Therapeutic potential of megadose vitamin C to reverse organ dysfunction in sepsis and COVID-19. Br J Pharmacol. 2021;178(19):3864–8.
Zarbock A, Nadim MK, Pickkers P, Gomez H, Bell S, Joannidis M, Kashani K, Koyner JL, Pannu N, Meersch M, et al. Sepsis-associated acute kidney injury: consensus report of the 28th Acute Disease Quality Initiative workgroup. Nat Rev Nephrol. 2023;19(6):401–17.
Poston JT, Koyner JL. Sepsis associated acute kidney injury. BMJ. 2019;364:k4891.
Petejova N, Martinek A, Zadrazil J, Kanova M, Klementa V, Sigutova R, Kacirova I, Hrabovsky V, Svagera Z, Stejskal D. Acute Kidney Injury in Septic Patients Treated by Selected Nephrotoxic Antibiotic Agents-Pathophysiology and Biomarkers-A Review. International journal of molecular sciences 2020, 21(19).
Xiao J, Yang Q, Zhang Y, Xu H, Ye Y, Li L, Yang Y, Jin S. Maresin conjugates in tissue regeneration-1 suppresses ferroptosis in septic acute kidney injury. Cell Bioscience. 2021;11(1):221.
van Dam PA, Verhoeven Y, Trinh XB, Wouters A, Lardon F, Prenen H, Smits E, Baldewijns M, Lammens M. RANK/RANKL signaling inhibition may improve the effectiveness of checkpoint blockade in cancer treatment. Crit Rev Oncol Hematol. 2019;133:85–91.
Dufresne SS, Dumont NA, Boulanger-Piette A, Fajardo VA, Gamu D, Kake-Guena SA, David RO, Bouchard P, Lavergne É, Penninger JM, et al. Muscle RANK is a key regulator of Ca2 + storage, SERCA activity, and function of fast-twitch skeletal muscles. Am J Physiol Cell Physiol. 2016;310(8):C663–672.
Mota RF, Cavalcanti de Araujo PH, Cezine MER, Matsuo FS, Metzner RJM, Oliveira de Biagi Junior CA, Peronni KC, Hayashi H, Shimamura M, Nakagami H et al. RANKL Impairs the TLR4 Pathway by Increasing TRAF6 and RANK Interaction in Macrophages. BioMed research international 2022, 2022:7740079.
Tsukamoto H, Takeuchi S, Kubota K, Kobayashi Y, Kozakai S, Ukai I, Shichiku A, Okubo M, Numasaki M, Kanemitsu Y, et al. Lipopolysaccharide (LPS)-binding protein stimulates CD14-dependent toll-like receptor 4 internalization and LPS-induced TBK1-IKKϵ-IRF3 axis activation. J Biol Chem. 2018;293(26):10186–201.
Xu W, Xie J, Chen X. H2S alleviates urinary Sepsis-Induced Acute kidney Injury by inhibiting the TLR4/MyD88/PI3K signaling pathway. Chin J Pathophysiology. 2019;35(2):243–7.
Yang J, Xu H, Wang B, Wang Y, Cao S. Study on platelet activation status and IL-18 expression in septic rats. Practical J Shock. 2018;2(2):83–6.
Ying J, Wu J, Yao L. Protective effect of Ligustrazine on endothelial cells in mice with acute kidney Injury Induced by Sepsis. Zhejiang Med. 2021;43(2):138–42.
Markwart R, Saito H, Harder T, Tomczyk S, Cassini A, Fleischmann-Struzek C, Reichert F, Eckmanns T, Allegranzi B. Epidemiology and burden of sepsis acquired in hospitals and intensive care units: a systematic review and meta-analysis. Intensive Care Med. 2020;46(8):1536–51.
Weng L, Zeng XY, Yin P, Wang LJ, Wang CY, Jiang W, Zhou MG, Du B, China Critical Care Clinical Trials G. Sepsis-related mortality in China: a descriptive analysis. Intensive Care Med. 2018;44(7):1071–80.
Wijayaratne D, Wijewickrama E. Acute kidney injury in sepsis. Sri Lanka J Surg. 2017;35(6):15–25.
Fan H, Zhao Y, Chen GD, Sun M, Zhu JH. Health insurance status and risk factors of mortality in patients with septic acute kidney injury in Ningbo, China. J Int Med Res. 2019;47(1):370–6.
De Voogd FA, Gearry RB, Mulder CJ, Day AS. Osteoprotegerin: a novel biomarker for inflammatory bowel disease and gastrointestinal carcinoma. J Gastroenterol Hepatol. 2016;31(8):1386–92.
Xue W. Serum OPG levels and their clinical significance in patients with cirrhosis complicated by Acute kidney Injury. Lab Med Clin. 2020;17(14):2080–1.
Kemperman H, Schrijver IT, Roest M, Kesecioglu J, van Solinge WW, de Lange DW. Osteoprotegerin is higher in Sepsis Than in Noninfectious SIRS and predicts 30-Day mortality of SIRS patients in the Intensive Care. J Appl Lab Med. 2019;3(4):559–68.
Schaalan M, Mohamed W. Predictive ability of circulating osteoprotegerin as a novel biomarker for early detection of acute kidney injury induced by sepsis. Eur Cytokine Netw. 2017;28(2):52–62.
Melagraki G, Leonis G, Ntougkos E, Rinotas V, Papaneophytou C, Mavromoustakos T, Kontopidis G, Douni E, Kollias G, Afantitis A. Current status and future prospects of small-molecule protein-protein Interaction (PPI) inhibitors of Tumor Necrosis factor (TNF) and receptor activator of NF-kappaB ligand (RANKL). Curr Top Med Chem. 2018;18(8):661–73.
Francisconi CF, Vieira AE, Azevedo MCS, Tabanez AP, Fonseca AC, Trombone APF, Letra A, Silva RM, Sfeir CS, Little SR, et al. RANKL triggers treg-mediated Immunoregulation in Inflammatory Osteolysis. J Dent Res. 2018;97(8):917–27.
Kobayashi-Sakamoto M, Maeda T, Yusa J, Kato Y, Kiyoura Y. RANK-RANKL signaling upregulates Il-10 mRNA expression in mucosal Candida infection in vivo. Microb Pathog. 2020;149:104285.
Rochette L, Meloux A, Rigal E, Zeller M, Malka G, Cottin Y, Vergely C. The role of Osteoprotegerin in vascular calcification and bone metabolism: the basis for developing new therapeutics. Calcif Tissue Int. 2019;105(3):239–51.
Maruyama K, Takada Y, Ray N, Kishimoto Y, Penninger JM, Yasuda H, Matsuo K. Receptor activator of NF-kappa B ligand and osteoprotegerin regulate proinflammatory cytokine production in mice. J Immunol. 2006;177(6):3799–805.
Wang Y, Zhang S, Li H, Wang H, Zhang T, Hutchinson MR, Yin H, Wang X. Small-molecule modulators of toll-like receptors. Acc Chem Res. 2020;53(5):1046–55.
Kuzmich NN, Sivak KV, Chubarev VN, Porozov YB, Savateeva-Lyubimova TN, Peri F. TLR4 signaling pathway modulators as potential therapeutics in inflammation and Sepsis. Vaccines. 2017;5(4):34.
Swanson L, Katkar GD, Tam J, Pranadinata RF, Chareddy Y, Coates J, Anandachar MS, Castillo V, Olson J, Nizet V, et al. TLR4 signaling and macrophage inflammatory responses are dampened by GIV/Girdin. Proc Natl Acad Sci U S A. 2020;117(43):26895–906.
Peerapornratana S, Manrique-Caballero CL, Gomez H, Kellum JA. Acute kidney injury from sepsis: current concepts, epidemiology, pathophysiology, prevention and treatment. Kidney Int. 2019;96(5):1083–99.
Valles PG, Gil Lorenzo AF, Garcia RD, Cacciamani V, Benardon ME, Costantino VV. Toll-like receptor 4 in Acute kidney Injury. Int J Mol Sci 2023, 24(2).
Acknowledgements
Not applicable.
Funding
This study was funded by the Natural Science Foundation of Xinjiang Uygur Autonomous Region (2022D01C604).
Author information
Authors and Affiliations
Contributions
Xinrong Niu designed the study, collected the funds, and wrote the manuscript. Xinrong Niu, Caihong Wang, Hui Li, and Weilin Chen performed the experiments and collected the data. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All methods were performed following the relevant guidelines and regulations. The experimental protocol was reviewed and approved by the Experimental Animal Ethics Committee of the Xinjiang Medical University (Approval No.: IACUC-20230217-18). The study is reported in accordance with ARRIVE guidelines.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Niu, X., Wang, C., Li, H. et al. Role of OPG/RANKL/RANK/TLR4 signaling pathway in sepsis-associated acute kidney injury. BMC Nephrol 25, 205 (2024). https://doi.org/10.1186/s12882-024-03648-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12882-024-03648-1