Abstract
Background
Immunoglobulin type A (IgA) nephropathy is the most common primary glomerulonephritis (GN) worldwide with higher rates in East and Pacific Asia compared to North America and Europe. Despite high reported prevalence of IgAN in these countries, the overall disease prevalence across Asia is not available. Treatment patterns of IgAN patients across Asian countries have also not been summarized. The aim of this study was to review and summarize evidence on IgA nephropathy prevalence, treatment patterns, and humanistic and economic burden in mainland China, Taiwan, South Korea, Japan, and Australia.
Methods
A targeted literature review was conducted in PubMed and local databases in China (including Taiwan), South Korea, Japan, and Australia between January 2010-December 2021. Website literature searches were conducted using Google Scholar and Baidu.
Results
Sixty-nine publications and 3 clinical guidelines were included. Incidence ranged from 0 to 10.7 per 100 000 people per year in Australia, Japan, and Taiwan, and ranged from 6.3 to 24.70% among patients who underwent renal biopsy in mainland China. Prevalence and diagnosis rates ranged from 0 to 72.1% in mainland China, South Korea, Taiwan, Japan, and Australia. Mortality rates in mainland China, South Korea, and Japan varied widely. The top 3 commonly used therapies were angiotensin-converting enzyme inhibitor/angiotensin receptor blockers (0.9-99.6%), corticosteroids (3.5-100%), and immunosuppressants (1.6-85.5%) in Japan, mainland China, and South Korea. Patient quality of life was measured by different tools, and annual hospitalization costs ranged from $1 284.73 to $2 252.12 (2015–2018) in China.
Conclusions
The prevalence of IgA nephropathy among the general population in select countries/regions is not commonly available, despite evidence from studies and clinical guidelines. In addition, it is observed across geographic regions that heterogeneity exists in prevalence rates, and large variations exist in treatment patterns. There is need to fill in these gaps to understand the contributing factors behind the differences through population-based, multi-center, and real-world studies.
Similar content being viewed by others
Background
Immunoglobulin type A nephropathy (IgAN), also known as Berger’s disease, is a kidney disease caused by kidney deposition of immunoglobulin type A (IgA) complexes involving galactose-deficient IgA [1] and resulting in inflammatory tissue damage [2]. IgAN affects the kidneys by attacking the glomeruli and is characterized by persistent urinary abnormalities including microscopic hematuria, gross hematuria, and/or proteinuria [2, 3]. IgAN is the most common form of biopsy-proven primary glomerulonephritis (PGN) worldwide [3] and is one of the leading causes of chronic kidney disease (CKD) and end-stage renal disease (ESRD) [4].
Primary treatments for IgAN include angiotensin-converting enzyme inhibitor/angiotensin receptor blockers (ACEIs/ARBs), corticosteroids, and immunosuppressants [1, 4]. These treatments aim to address symptoms and manifestations of IgAN but not the underlying cause. Nearly one-third of IgAN patients develop ESRD within 10 years [5]. On average, patients with IgAN die 6 years earlier than the general population [6]. In addition, patients’ quality of life (QoL) is greatly impacted due to pain, fatigue, and poor mental health [4], and indirect caregiver burden is high due to time spent caring for patients who progress to ESRD. Thus, caregivers’ QoL and psychological well-being can also be negatively impacted [7].
IgAN prevalence is highest in Asia, intermediate in Europe and the US, and lower in African countries [8]. The overall global incidence is approximately 2.5 per 100,000 people per year [2]. A higher prevalence of IgAN is seen in countries where routine screening is practiced [4]. While geographic variations of IgAN have been studied previously [3, 9], few recent studies have focused on regional disease burden differences and treatment patterns in among IgAN patients across Asian countries/regions and Australia.
This review aimed to summarize the disease burden and treatment patterns of IgAN in select countries/regions in the Asia-Pacific region, specifically mainland China, Taiwan, South Korea, Japan, and Australia.
Methods
Data sources and search strategy
A targeted literature review (TLR) was conducted to identify relevant literature published from January 2010 to December 2021 for mainland China, Taiwan, South Korea, Australia, and Japan. The earliest year of publication was expanded from 2010 to 2001 to capture evidence more comprehensively on outcomes of interest. Medline and Embase were the primary databases for publications in English. For publications in local languages, WANFANG and China National Knowledge Infrastructure (CNKI) databases were searched for publications in Chinese, Korean Medical Database and Korean Information Service System (KISS) databases were searched for publications in Korean, and Scholarly and Academic Information Navigator (CiNii) was searched for publications in Japanese. Supplementary searches for clinical guidelines, conference proceedings, and websites of governmental and non-governmental organizations were conducted using Google, Baidu (for Chinese sources), and Naver (for Korean sources). Publications cited as references were also considered for screening.
Search terms included IgA nephropathy, Berger’s disease, incidence, prevalence, mortality, quality of life, cost, burden, and treatment. Observational studies, reviews, and registry studies were included in the search. Publications that reported prevalence, incidence, mortality, treatment patterns, guidelines, economic, and humanistic burden were included for data extraction. Search terms in English and local languages are listed in Supplementary Table S1.
Study selection and data extraction
After the search was conducted and duplicates were removed, the title, abstract, and full texts of the remaining publications were screened. A second reviewer conducted the validation and finalization for publications to be included in the data extraction phase. During screening, the inclusion and exclusion criteria mainly focused on outcomes. Systematic reviews, observational studies including registry/database studies and other real-world studies, annual reports were considered for inclusion. Publications that reported evidence regarding epidemiology (incidence, prevalence, and mortality), humanistic and economic burden, and treatment patterns (treatment guidelines, duration, adherence, persistence, switching, and discontinuation) were included for data extraction. Studies that did not include outcomes of interest were excluded, as were studies with a small sample size (< 25). Strict predefined population, intervention, comparators, outcomes, and study design (PICOS) selection criteria and a Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) diagram were not used in this study. Study characteristics, patient characteristics, epidemiological outcomes, disease burden, and treatment patterns were extracted.
Study quality assessment
All eligible studies went through a quality assessment (QA) using a recommended checklist, according to the Center for Reviews and Dissemination Guidance for Undertaking Reviews in Health Care recommendations [10]. Quality assessment was performed for all eligible articles by two reviewers. The checklist consisted of 9 items excluding basic information for the included studies. Because all publications included in this study were observational studies or reviews, only the non-randomized clinical trial checklist was used for observational studies.
Results
Sixty-nine publications were included for this review, among which 38 were from mainland China (2015–2021) [11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48], 15 from Japan (2003–2021) [49,50,51,52,53,54,55,56,57,58,59,60,61,62,63], 10 from South Korea (2010–2020) [64,65,66,67,68,69,70,71,72,73], 3 from Taiwan (2014–2019) [74,75,76], and 3 from Australia (2001–2021) [77,78,79]; characteristics of the studies are shown in Supplementary Table S2. Approximately 83% the publications reported a retrospective study design (n = 57). For publications from mainland China, sample sizes ranged from 74 [37] to 4,367,829 [47], and male percentages ranged from 37.5% [17] to 97.3% [32]. For publications from Japan, sample sizes ranged from 52 [53] to 270,902 [63]; the male percentage ranged from 37.1% [58] to 56.96% [52]. For publications from South Korea, sample sizes ranged from 25 [64] to 5,114 [67]; the male percentage ranged from 36% [64] to 66.6% [73]. For publications from Taiwan, sample sizes ranged from 91 [75] to 7,073 [76]; the male percentage ranged from 45.9% [76] to 52.7% [75]. For publications from Australia, sample sizes ranged from 1,147 [78] to 2,457 [79]; the male percentage ranged from 60% [77] to 69.7% [79]. The Kidney Disease: Improving Global Outcomes (KDIGO) Clinical Practice Guideline [1] and 2 country-specific guidelines [80, 81] were also included for evidence on treatment patterns.
Sixty-eight journal articles were assessed for study quality (all details of the quality assessment are shown in Supplementary Table S3); one white paper was not included in the study quality assessment. Approximately 75% (51/68 articles) were deemed to be of good quality (i.e., without inherent flaws). Few studies reported the incidence/prevalence of IgAN directly and percentage of IgAN were extracted from included studies. The appropriateness of the statistical analysis conducted was not clear or not specified in 5 studies, as they did not define P values and the level of significance for all observations. Across studies, outcome measures were generally considered reliable. However, 33 articles stated that the results could be generalized to routine practice. In one case-control study, the similarity of both groups at the outset of the study was not clear.
Incidence
Six publications provided evidence on IgAN incidence [30, 61, 63, 74, 77, 78] in Australia (n = 2), Japan (n = 2), mainland China (n = 1), and Taiwan (n = 1). Most were cross-sectional observational studies (n = 4), and sample sizes ranged from 156 [74] to 270,902 [63].
In Australia, IgAN incidence was estimated to be 1.41–10.5 per 100,000 people per year [77, 78]. According to Briganti 2001 [78], IgAN incidence in Australia was lowest (0.0 per 100,000 per year) among male children and highest (10.7 per 100,000 per year) among male adults [78]. In Japan, only 2 studies reporting incidence data among children were identified. Utsunomiya 2003 [63] reported an incidence rate of 4.5 per 100,000 per year among 270,902 junior high and elementary school students; Kajiwara 2020 [61] reported a rate of 3.3 per 100,000 per year among 60,816 junior high and elementary school students. Both publications collected urine samples through a school urinary screening system in students 6 to 15 years old. In mainland China, the incidence rate of IgAN was estimated to be 6.3% among elderly patients who underwent renal biopsy and 24.7% among non-elderly patients who underwent renal biopsy [30]. In Taiwan, IgAN incidence was estimated to be 5.5 per million per year among the general population (around 23.5 million between 2014 and 2016), based on 1,445 renal biopsy records from a registry database [74]. In general, IgAN incidence was higher in males (5.7 per 100,000 per year) compared with females (2.9 per 100,000 per year) [78]. IgAN incidence was not reported in Korean populations.
Prevalence and diagnosis rate
IgAN prevalence among the general population was not reported in the included publications. But one cross-sectional study (n = 3,623) reported an IgAN prevalence rate of 0.03% among the general Chinese pediatric population [34]. Thirty-five publications were identified with diagnosis rates among 2 populations: patients who received renal biopsies and PGN patients [13, 14, 17,18,19, 21, 22, 24, 30, 31, 33,34,35,36, 39, 40, 43,44,45,46,47,48, 52, 59, 67,68,69,70,71,72, 74,75,76, 79]. Twenty-one publications were from mainland China [13, 14, 17,18,19, 21, 24, 30, 31, 33,34,35,36, 39, 40, 43,44,45,46,47,48], 6 from South Korea [67,68,69,70,71,72], 3 from Taiwan [74,75,76], 3 from Japan [52, 55, 59], and 1 from Australia [79]. The majority (88%) were cohort studies (n = 17) [13, 21, 31, 33, 35, 36, 39, 40, 43,44,45,46, 52, 68,69,70,71] and cross-sectional studies (n = 13) [14, 17,18,19, 21, 24, 34, 37, 47, 59, 67, 72, 74, 79], with the remainder being an annual report [76], a registry study [55] and a chart review [75]. Sample sizes ranged from 33 [70] to 43,67,829 [47].
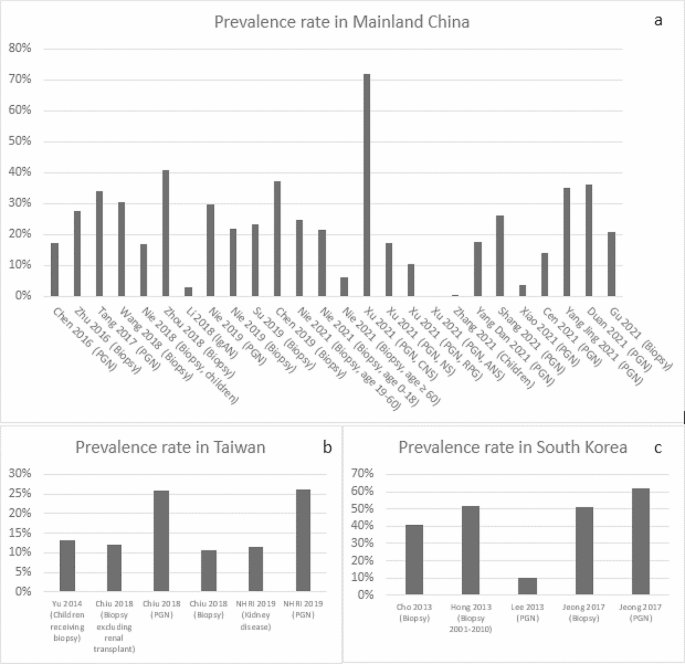
In mainland China, the mean diagnosis rate of IgAN was estimated to be 24.1% among patients undergoing renal biopsies (median: 23.0%; range: 6.3-40.9%) [13, 19, 21, 22, 24, 30, 46] and 27.3% (median: 27.9%; range: 0-72.1%) [14, 19, 21, 33, 36, 40, 43,44,45, 48] among PGN patients (Fig. 1a); The mean IgAN diagnosis rate was estimated to be 21.7% (median: 17.5%; 17-30.4%) among children who underwent renal biopsy [17, 18, 35]. In Taiwan, the mean diagnosis rate of IgAN was 12.1% (median: 12.2%; range: 10.8-13.2%) among patients undergoing renal biopsies [74, 75] and was reported similar (26%) among PGN patients [74, 76] (Fig. 1b). In South Korea, the mean diagnosis rate was 41% (median: 38.1%; range: 25.8-61.9%) among patients undergoing renal biopsies [67, 69, 71, 72] and around 51.6% (average of 51.3% and 51.9%) among PGN patients [68, 70] (Fig. 1c). In Japan, Hattori 2016 reported a mean estimated IgAN diagnosis rate of 23% (median: 22.9%) among CKD patients [59]. In addition, the reported IgAN diagnosis rate among patients who underwent renal biopsy was 31%, with 6.9% in patients aged 65 to 80 years old and 10.5% in patients aged 80 years or older [52, 55]. In Australia, Lee 2020 reported an IgAN diagnosis rate of 13% among patients undergoing renal biopsy [79].
IgAN Prevalence in Mainland China, Taiwan and South Korea (Abbreviation: ANS, acute nephritic syndrome; CNS, chronic nephrotic syndrome; NHRI, National Health Research Institute & Taiwan Society of Nephrology; NS, nephritis syndrome(e; PGN, primary glomerulonephritis; RPG, rapidly progressive glomerulonephritis)
Disease progression and mortality
Among included studies, all-cause mortality was mainly reported as deaths due to ESRD. Seven publications from mainland China [23, 26,27,28,29, 41, 42], 7 from Korea [64,65,66, 68, 70, 71, 73], 4 from Japan [50, 51, 57, 62], and 1 from Taiwan [75] reported rate of progression to ESRD in IgAN. These studies varied in the definition of endpoint, patient characteristics, and follow-up duration. In China, the median rate of progression to ESRD was 4.1% [28] over 6 months, ranged from 1.3 to 15.8% (median: 1.3%) over 40–45 months [29, 41], ranged from 6.6 to 15% (median: 8.3%) over 4–10 years [23, 27, 42], and 33% over 15 years [42]. In Korea, the median rate of progression to ESRD ranged from 2.5 to 39.7% (median: 19%) from 60 to 100 months [64,65,66, 68, 70, 71, 73].
Regarding direct reports on mortality, in mainland China, 0.7% of adult IgAN patients progressed to death according to 1 study of 944 patients from 2003 to 2014 with a median follow-up of 4.2 years [23]. In South Korea, the median death rate was 5.3% (range: 4.4-5.9%) [65, 66, 68] for 1,364 IgAN patients with a median follow-up of 100 months. In addition, 2 publications reported a standard mortality ratio (expressed as the ratio between the observed and the expected number of deaths in the general population) of 1.43 (95% confidence interval:1.04–1.92) among 1,364 IgAN patients in relation to the general population [65, 68]. In Japan, IgAN mortality was estimated to be 0.3 per 100 person-years among non-smokers [51], 1.3 per 100 person-years among smokers [51] and 1.2 per 100 person-years among patients who received kidney replacement therapy [53] based on 2 retrospective studies [51, 53]. No mortality data was found among IgAN patients in Taiwan or Australia.
Treatment patterns
Twenty publications [1, 11, 15, 26, 27, 29, 42, 49, 50, 54, 56,57,58, 60, 62, 64, 68, 71, 73, 81] and 3 clinical guidelines reported treatment patterns. Nine from mainland China [11, 15, 26,27,28,29, 32, 41, 42], 8 from Japan [49, 50, 54, 56,57,58, 60, 62], and 4 from South Korea [64, 68, 71, 73]. 80% publications were retrospective studies (n = 16) [11, 15, 26, 27, 29, 42, 49, 56,57,58, 60, 62, 64, 68, 71, 73]. Sample sizes ranged from 25 [64] to 2,283 [50]. The KDIGO [1] and 2 country-specific treatment guidelines, 1 from mainland China [80] and 1 from Japan [81], were identified. No treatment guidelines were identified in Taiwan, South Korea, or Australia.
The KDIGO guidelines (2021 version) provide treatment recommendations for adults and children with IgAN [1]. The guidelines state that the management of IgAN should be multifaceted, optimized with supportive care, and include ACEIs/ARBs as tolerated or allowed, control blood pressure, minimize cardiovascular risk, and adherence to lifestyle changes including dietary counseling, smoking cessation, weight control, and exercise, as appropriate. The guidelines provide specific treatment recommendations according to the variant forms of IgAN, the level of proteinuria, and high-risk rate for progression after maximal supportive care. The main treatment regimens include ACEIs and ARBs, immunosuppressants, cyclophosphamide, tonsillectomy, and lifestyle modification [1]. Similar to the KDIGO guidelines, the primary treatment recommendations in the Chinese 2017 guidelines for children with IgAN were glucocorticoids, immunosuppressants, and ACEIs/ARBs [80]. Japanese 2020 guidelines covered children and adults, with different treatment recommendations based on symptoms and subtype of IgAN (the subgroup classification for adults was based on estimated glomerular filtration rate and proteinuria; symptoms among children were classified as mild or severe) [81].
In mainland China, 6 studies investigated adult populations [15, 26, 28, 29, 32, 42] (Table 1) and 3 investigated pediatric populations [11, 27, 41] (Table 2). For drug usage among adult patients, ACEIs/ARBs had the largest median percentage at 66.7% (range: 38-90%) [15, 26, 28, 29, 32, 42], followed by steroids, with median of 36% (corticosteroids/prednisone/intravenous methylprednisolone injection, range: 10-100%) [15, 26, 28, 29, 32, 42] and immunosuppressants (including in combination with steroids), with median of 25.9% (cyclophosphamide, tacrolimus and tripterygium wilfordii, range: 1.6-72%) [15, 26, 28, 29, 32, 42]. Among pediatric patients, immunosuppressants (cyclophosphamide/mycophenolate /Tripterygium wilfordii /leflunomide) were the common drugs recommended, with a median of 64% (range: 1.7–72.2%) [11, 27, 41], followed by ACEIs/ARBs, with a median of 49.5% (range: 2.5-70%) [11, 27, 41] and steroids with a median of 45% (range: 25.3-69.3% as sum of oral prednisone and intravenous methylprednisolone) [11, 27, 41].
In South Korea, 3 publications on adult IgAN patients [64, 68, 71] (Table 1) and 1 publication among pediatric patients [73] (Table 2) were identified. Among adults, ACEIs/ARBs were the most common treatments (27.7-83.4%) [68, 71, 73], followed by ACEIs/ARBs and corticosteroid combinations (33.9%) [64] and corticosteroids alone (12.4-28.8%) [68, 71, 73]. Among pediatric patients, the frequency of immunosuppressant use was 50.2% [73].
In Japan, 7 publications reported IgAN treatment patterns among adults [50, 54, 56,57,58, 60, 62] (Table 1) and 2 publications [49, 54] among pediatric patients (Table 2). Among adults, ACEIs/ARBs were the most common treatment (25-99.6%) [50, 54, 56,57,58, 60, 62], followed by antiplatelet agents (58.1-96.8%) [54] and corticosteroid-immunosuppressant combination therapy (1.5-74%) [62]. Notably, the rate of administering steroid-immunosuppressant combination was only 1.5% in a retrospective cohort study that sampled 1,012 IgAN patients with a mean age of 32.96 ± 12 years [56]. Among pediatric patients, ACEIs/ARBs were the most frequently administered treatments (0.9-95.7%) [49, 54], followed by antiplatelet agents (range: 1.2-82.6%) [49, 54] and immunosuppressants (range: 4.6-68.5%) [49]. The frequency of administering treatments varied greatly across different subgroups. For example, the frequency of administering ACEIs/ARBs ranged from 0.9% for the diffuse mesangial proliferation subgroup (n = 108) to 50.9% for the focal mesangial proliferation subgroup (n = 173) in 1 retrospective study in Japanese children with IgAN from 1990 to 2004 [49]. Tonsillectomy or tonsillectomy combined with steroid was mostly reported in Japanese studies, with frequencies ranging from 1 to 66.2% across publications (Table 1). This is in accordance with the KDIGO 2021 guidelines’ evidence that supports the routine use of tonsillectomy in Japanese high-risk patients with IgAN [1]. No publications reporting IgAN treatment patterns were identified for Taiwan or Australia.
Humanistic burden
Four publications in China reported QoL, measured by the 36-Item Short Form Health Survey (SF-36) [16, 25], Daily Living Ability Rating Scale (DLARS) [37], and QoL scale (QOLs) combined with Self-Rating Anxiety Scale (SAS) and Self-Rating Depression Scale (SDS) [38]. SF-36 scores reflect physical and mental health based on 8 health concepts, including physical and social functioning, role limitations due to physical and emotional problems, mental health, vitality, bodily pain, and general health (GH) perception [82]. Two publications evaluated the effects of individualized nursing intervention (INI, one improved nursing intervention which costs more time than routine nursing intervention [RNI]) on the psychological mood and QoL among IgAN patients [16, 25]. There were two subgroups, the patients in the control group received RNI and patients in the intervention group received INI [16, 25]. The mean GH score was 32.16 [16] among total IgAN patients (n = 98; mean age: 32.74 years; male percentage: 50%) in 2017 and 80.15 increasing from 69.93 at baseline [25] after intervention among total IgAN patients (n = 84; mean age: 33.57 years; male percentage: 60.7%) in 2019. In both publications, the intervention groups had higher mean GH scores than that in the control groups (39.47 vs. 24.84 [16] and 85.73 vs. 74.56 [25], respectively). Two other prospective studies assessed the effect of INI for IgAN patients [37, 38]. Results showed that both mean DLARS and QOLs scores were higher among the intervention group compared to the control group (88.5 vs. 75.7 and 39.5 vs. 24.8, respectively) [37, 38]. SAS and SDS scores were also evaluated by Qi 2021 [38], the mean SAS score decreased more in the intervention group (49.2 ± 6.3 decreased from 62.1 ± 5.8) than that in the control group (57 ± 4.9 decreased from 62.4 ± 6.1) from baseline. Similarly, the mean SDS score decreased more in the intervention group (43.3 ± 5.2 decreased from 56.2 ± 6) than in the control group (52.6 ± 6.4 decreased from 57 ± 6.2) from baseline [38].
Economic burden
No publications reported indirect costs, but 3 retrospective studies reported hospitalization costs for IgAN patients in China (see Supplementary Figure S1) [12, 20, 47]. Hospitalization cost per patient per year is ¥14,900 ($2,252.12; exchange rate of Chinese Yuan [CNY] and US dollar in 2018 was 6.616 [83]) as reported by Zheng 2018 [20], and between ¥9,618 ($1,532.26; exchange rate of CNY and US dollar in 2015 is 6.227 [83]) and ¥10,019 ($1,608.96) as reported by Peng 2015 [12]. One large database study covering 54.1% of tertiary hospitals in 31 Chinese provinces from 2010 to 2015 reported a hospitalization cost of ¥8,000/$1,284.73 (¥6,000-¥12,000) [47]. Drug costs accounted for 28.39% of total hospitalization costs, followed by diagnostic testing costs [12]. Length of stay per patient per year in China ranged from 10 to 14.3 days across 3 publications [12, 20, 47].
Discussion
To our knowledge, this is the first TLR to summarize the evidence on IgAN disease burden and treatment patterns in mainland China, Taiwan, South Korea, Japan, and Australia. The findings of this review revealed evidence gaps in IgAN epidemiology and humanistic and economic burden. No incidence data was identified in South Korea; no mortality data was identified in Taiwan and Australia; no country/region-specific treatment guidelines were found for Taiwan, South Korea, or Australia; no evidence on treatment patterns from the publications was identified for Taiwan or Australia; and no humanistic burden or economic data was identified except for mainland China.
The IgAN incidence rates among Japanese, Taiwanese, and Australian populations ranged from 0 to 10.7 per 100,000 people per year, higher than the incidence rate reported in a recent systematic literature review (SLR) by Kwon 2021 [84] (1.29 per 100,000 people per year). Kwon 2021 [84] is an SLR focusing on US epidemiology, health-related QoL, and the economic burden of IgAN (the included studies were published from January 2010 to June 2020), similar to our study’s objective. Incidence rates among children and teenagers (0-4.5 per 100,000 per year) were similar to the incidence rate in Venezuela (0.03 per 100,000 per year) [85] and in Italy (0.31 per 100,000 per year) [86]. The overall prevalence and diagnosis rates of IgAN were similar across selected countries/regions. The diagnosis rates in this review differed from those found in PGN patients and patients who received renal biopsy in Kwon 2021 [84]; diagnosis rates of IgAN from our results were higher in PGN patients compared with patients who received renal biopsies since renal biopsies were often performed on PGN patients before diagnosis. This applied to both adult and pediatric populations. Compared to the US population in Kwon 2021 [84], the diagnosis rate among PGN populations in this review was higher (26-72.1% vs. 9.4-19.7%). The diagnosis rate among populations with renal biopsies was also higher (6.3-61.9% vs. 6.3-14.3%). Notably, though not covered by this review, the pathological profile such as Oxford Classification/MEST classification could also shed light upon disease burden, which could be further explored by future studies.
IgAN treatments primarily consisted of ACEIs/ARBs, and high utilization of steroids was found despite mixed evidence on their benefits and safety. There is limited data on IgAN treatment patterns from Taiwan and Australia. Among the publications that reported treatment patterns, few specified drugs’ generic names. The primary treatment patterns reported among select countries/regions in this study are similar to those in US as reported by Kwon 2021 (frequently used therapies were immunosuppressives, corticosteroids, and ACEIs/ARBs) [84]. Immunosuppressives were used more by children than adults based on data from mainland China, South Korea, and Japan. According to the KDIGO guideline regarding glomerular diseases, the immunosuppressive therapies including azathioprine, cyclophosphamide, calcineurin inhibitors, and rituximab are not recommended for treating IgAN. Mycophenolate mofetil is recommended in Chinese patients and tonsillectomy is recommended to be used in Japanese IgAN patients [1]. Only Chinese studies reporting SF-36 scores and other metrics were identified. Therefore, more studies on QoL in IgAN patients and caregivers in other regions are warranted.
Evidence of economic burden was identified only from studies in mainland China; Li 2018 was one retrospective national inpatient database study, which included the major hospitals that covers multiple geographic locations [47], other two studies used the data from one hospital. The mean cost per patient per year reported by Li 2018 is $1,284.73, while one Canadian retrospective study for costs and healthcare resource utilization reported a mean outpatient medication cost per patient per year of Canadian dollar (CAD) $221 in 2016 [87]. To control medical costs, hospitals in China are undergoing clinical pathway optimization programs [12].
Publications reported heterogeneous sample populations where IgAN prevalence/diagnosis rates were evaluated. Among 22 publications that reported IgAN prevalence/diagnosis rates, 15 measured IgAN prevalence for patients who underwent renal biopsy and 9 measured IgAN prevalence for patients diagnosed with PGN. Heterogeneity in IgAN prevalence/diagnosis rates may be attributed to differences in study years, patient race/ethnicity, patient age, treatment method, risk factors, diagnosis, and follow-up duration. Other study design–related factors that could introduce bias include sample size and gender composition.
Finally, differences in IgAN prevalence across regions should be noted. County/region-specific healthcare infrastructure and policies influence the epidemiological evidence of IgAN. systematic urine screening programs among individuals with asymptomatic, persistent microscopic hematuria with/without mild proteinuria are commonly implemented in certain countries/regions. These programs facilitate detection of IgAN patients who would otherwise receive a delayed diagnosis or none at all. Countries/regions where screening programs are performed may therefore have higher reported IgAN prevalence. Screening programs play a crucial role in early diagnosis and early treatment [88].
To our knowledge, this is the first TLR for IgAN in mainland China, Taiwan, South Korea, Japan, and Australia. However, several limitations should be noted. Due to the targeted nature of this review, the search focused on the most relevant literature, and the publications included in this study were prioritized, which potentially have led to an incomplete picture of IgAN-related epidemiology, treatment patterns and disease burden. Across included publications, the sample sizes varied widely and were not always reported. Additionally, this TLR did not weigh the data from included publications; therefore, biases should be considered when comparing outcomes. Studies came from primarily single institutions, and national-level data was not always available for the selected countries/regions. Moreover, this review only covered select Asia-Pacific countries/regions; future reviews and studies in other countries and regions within Asia-Pacific are therefore warranted. Despite these limitations, the evidence gathered in this literature review may help provide a preliminary understanding of the disease burden of IgAN in the Asia-Pacific region.
This TLR summarized evidence on Immunoglobulin type A nephropathy (IgAN) prevalence, treatment patterns, and humanistic and economic burden. Our results suggest that despite the overall scarcity of information in general, evidence on disease burden and treatment patterns has been reported by some studies and several clinical guidelines. The prevalence of IgAN among the general population is not commonly available, while that among patients receiving renal biopsies and diagnosed with PGN is more frequently reported. Heterogeneity in prevalence rates across geographic regions might be explained by differences in initial diagnosis in some regions due to variation in local screening policy and disease management. There is a need to understand how the disease progression differs by those practices. Treatment patterns have been reported mainly in studies from some Asia areas, but geographic variations are noticeable. There is also a need to generate more evidence to shed light upon the possible explanation to the differences in the treatment patterns across geographic regions. In sum, more real-world studies at national levels across select countries/regions are warranted to fill the evidence gaps, particularly regarding incidence, humanistic burden, and economic burden.
Conclusion
The prevalence of IgA nephropathy among the general population in select APAC countries/regions is not commonly available, despite evidence from studies and clinical guidelines. In addition, it is observed across geographic regions that heterogeneity exists in prevalence rates, and large variations exist in treatment patterns. Future studies are needed to fill in these gaps to understand the contributing factors behind the differences through population-based, multi-center, and real-world studies.
Data availability
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Abbreviations
- ACEI:
-
Angiotensin-converting enzyme inhibitor
- ANS:
-
Acute nephritic syndrome
- APAC:
-
Asia Pacific
- ARBs:
-
Angiotensin receptor blockers
- CAD:
-
Canadian dollar
- CKD:
-
Chronic kidney disease
- CNKI:
-
China National Knowledge Infrastructure
- CNS:
-
Chronic nephrotic syndrome
- CNY:
-
Chinese Yuan
- CTX:
-
Cyclophosphamide
- DLARS:
-
Daily Living Ability Rating Scale
- DMP:
-
Diffuse mesangial proliferation
- EMBASE:
-
Excerpta Medica Database
- ESRD:
-
End-stage kidney failure
- FMP:
-
Focal mesangial proliferation
- GH:
-
General health
- INI:
-
Individualized nursing intervention
- KDIGO:
-
The Kidney Disease: Improving Global Outcomes
- KISS:
-
Korean Information Service System
- MMF:
-
Mycophenolate mofetil
- NR:
-
Not reported
- NS:
-
Nephritis syndrome
- PGN:
-
Primary glomerulonephritis
- PICOS:
-
Population, intervention, comparators, outcomes, and study design
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- QA:
-
Quality assessment
- RNI:
-
Routine nursing intervention
- RPG:
-
Rapidly progressive glomerulonephritis
- SAS:
-
Self-Rating Anxiety Scale
- SD:
-
Standard deviation
- SDS:
-
Self-Rating Depression Scale
- SF-36:
-
36-Item Short Form Health Survey
- SLR:
-
Systematic literature review
- TLR:
-
Targeted literature review
- TSN:
-
Taiwan Society of Nephrology
- WGNSSDTCRD:
-
Working Group for National Survey on Status of Diagnosis and Treatment of Childhood Renal Diseases
References
Kidney Disease. Improving global outcomes (KDIGO) Glomerular Diseases Work Group. KDIGO 2021 clinical practice guideline for the management of glomerular diseases. Kidney Int. 2021;100(4S):S1–276.
National Institute of Diabetes and Digestive and Kidney Diseases. IgA nephropathy [Available from: https://www.niddk.nih.gov/health-information/kidney-disease/iga-nephropathy.
Schena FP, Nistor I. Epidemiology of IgA nephropathy: a global perspective. Semin Nephrol. 2018;38(5):435–42.
Hassler JR. IgA nephropathy: a brief review. Semin Diagn Pathol. 2020;37(3):143–7.
Zhang H. KDIGO Zhinan Jiedu: IgA shenbing zhiliao[KDIGO guideline interpretation: treatment of IgA nephropathy. Chin J Practical Intern Medicine]. 2012;32(12):925–7.
Jarrick S, Lundberg S, Welander A, Carrero JJ, Hoijer J, Bottai M, et al. Mortality in IgA nephropathy: a nationwide population-based cohort study. J Am Soc Nephrol. 2019;30(5):866–76.
Adejumo OA, Iyawe IO, Akinbodewa AA, Abolarin OS, Alli EO. Burden, psychological well-being and quality of life of caregivers of end stage renal disease patients. Ghana Med J. 2019;53(3):190–6.
Woo KT, Chan CM, Mooi CY, LC H, Tan HK, Foo M, et al. The changing pattern of primary glomerulonephritis in Singapore and other countries over the past 3 decades. Clin Nephrol. 2010;74(5):372–83.
Coppo R. Pediatric IgA Nephropathy in Europe. Kidney Dis (Basel). 2019;5(3):182–8.
Centre for Reviews and Dissemination. University of York. Systematic Reviews: CRD’s guidance for undertaking reviews in health care. 2009.
Working Group for National Survey on Status Diagnosis and Treatment of Childhood Renal Diseases. [Multicenter investigation of therapeutic status of children with IgA nephropathy in China]. Zhonghua Er Ke Za Zhi. 2013;51(7):486–90.
Peng Q, Xu G, Zhang C, Fang P. Wuhan Mou sanjia Yiyuan IgA shenbing Shen Chuanci huojian huanzhe linchuang lujing shishi xiaoguo pingjia[Evaluation of clinical pathway implementation effect in patients with IgA nephropathy renal puncture biopsy in a tertiary hospital in Wuhan]. Med Soc. 2015;28(10):18–20.
Zhu Z, Zou Q, Chen Y, Hu F, Bai J, Chao Q, et al. 224 Li Shen Huoti Zuzhi Jiancha De Linchuang lujing Yu Bingli fenxi[Analysis of the clinical pathway and pathologic features of 224 cases of renal biopsy]. Huaxi Med. 2016;31(5):845–9.
Tang L, Yao J, Kong X, Sun Q, Wang Z, Zhang Y, et al. Increasing prevalence of membranous nephropathy in patients with primary glomerular diseases: a cross-sectional study in China. Nephrol (Carlton). 2017;22(2):168–73.
Zhou S, Fu J, Liu M, Yang S, Zhou Q, Yu X, et al. The prevalence and risk factors of abnormal circadian blood pressure in patients with IgA nephropathy. Clin Nephrol. 2017;88(12):344–53.
Lu H, Xiao L, Lu X, Liang J. Gexinghua huli moshi dui IgA shenbing huanzhe qingxu ji shenghuo zhiliang yingxiang de yanjiu [The effect of personalized nursing mode on the emotion and quality of life of patients with IgA nephropathy]. Contemp Med. 2017;23(5):30–3.
Wang N, Zhu T, Tao Y. Clinicopathological features of pediatric renal biopsies in the plateau regions of China. J Int Med Res. 2018;46(11):4539–46.
Nie S, He W, Huang T, Liu D, Wang G, Geng J, et al. The spectrum of biopsy-proven glomerular diseases among children in China: a national, cross-sectional survey. Clin J Am Soc Nephrol. 2018;13(7):1047–54.
Zhou Q, Yang X, Wang M, Wang H, Zhao J, Bi Y, et al. Changes in the diagnosis of glomerular diseases in east China: a 15-year renal biopsy study. Ren Fail. 2018;40(1):657–64.
Zheng X, Zhang J, Lu C. Xinjiang Weiwuerzu Zizhiqu renmin Yiyuan 2012 ~ 2017nian manxing shenzangbing huanzhe de jibing goucheng ji yiliao feiyong de hengduanmian diaocha [Disease composition and medical expenses of chronic kidney disease in people’s hospital of Xinjiang Uygur Autonomous Region from 2012 to 2017: a cross-sectional survey]. Chin J Evid-Based Med. 2018;18(9):903–6.
Nie P, Chen R, Luo M, Dong C, Chen L, Liu J et al. Clinical and pathological analysis of 4910 patients who received renal biopsies at a single center in Northeast China. Biomed Res Int 26 Mar 2019;2019:6869179.
Su S, Yu J, Wang Y, Wang Y, Li J, Xu Z. Clinicopathologic correlations of renal biopsy findings from northeast China: a 10-year retrospective study. Med (Baltim). 2019;98(23):e15880.
Cai Q, Shi S, Wang S, Ren Y, Hou W, Liu L, et al. Microangiopathic lesions in IgA nephropathy: a cohort study. Am J Kidney Dis. 2019;74(5):629–39.
Chen L, Luodelete M, Dong C, Li B, Zhang W, Nie P, et al. Pathological spectrum of glomerular disease in patients with renal insufficiency: a single-center study in northeastern China. Ren Fail. 2019;41(1):473–80.
Huang L. Tanjiu dui huanyou butong dengji IgAshenbing de huanzhe yuyi gexinghua huli ganyu duiyu xinli qingxu ji shenghuo zhiliang de yingxiang [Investigation of the effect of personalized nursing interventions on psychological, emotional and quality of life in patients with different grades of IgA nephropathy (IgAN)]. J Gen Pract Dentistry (Electronic Version). 2019;6(25):110–4.
Tian S, Yang X, Luo J, Guo H. Clinical and prognostic significance of C1q deposition in IgAN patients-a retrospective study. Int Immunopharmacol. 2020;88:106896.
Wu H, Xia Z, Gao C, Zhang P, Yang X, Wang R, et al. The correlation analysis between the Oxford classification of Chinese IgA nephropathy children and renal outcome– a retrospective cohort study. BMC Nephrol. 2020;21(1):247.
Liu Y, Wei W, Yu C, Xing L, Wang M, Liu R, et al. Epidemiology and risk factors for progression in Chinese patients with IgA nephropathy. Med Clin (Barc). 2021;157(6):267–73.
Wen D, Tang Y, Tan L, Tan J, Chen D, Zhang Y, et al. Sex disparities in IgA nephropathy: a retrospective study in Chinese patients. Int Urol Nephrol. 2021;53(2):315–23.
Nie P, Lou Y, Wang Y, Bai X, Zhang L, Jiang S, et al. Clinical and pathological analysis of renal biopsies of elderly patients in Northeast China: a single-center study. Ren Fail. 2021;43(1):851–9.
Feng S, Wang L, Liu X, Luo W, Xie M, Yang Q. 1002 li manxing shenzangbing huaner linchuang Ji Bingli fenxi[Clinical and pathological analysis of 1002 children with chronic kidney disease]. J Clin Pediatr. 2021;39(02):87–90.
Zhu L, Huang X, Zhang J, Li W, Chen E, Guo N. 102 Li Yizhishen IgA shenbing de huli tihui [Nursing experience of 102 cases of IgA nephropathy in transplanted kidneys]. Gen Pract Nurs. 2021;19(04):513–5.
Xu Z, Xiong Z. 3554 li shenzang bingli yu linchuang xiangguanxing fenxi [Analysis of renal pathology and clinical correlation in 3554 cases] [Shuoshi, https://doi.org/10.26921/d.cnki.ganyu.2021.001127]: M.S., Anhui Medical University; 2021.
Zhang P, Chen Z, Liu M. Huizhoushi dayawan diqu xuelingqian ertong niaoye shaicha fenxi [Analysis of urine screening in preschool children in Dayawan, Huizhou]. World’s Newest Med Inform Digest. 2021;21(84).
Yang D, Xie Y, He Z, Li Y, Li C. Qinhuangdaoshi 1459 Li xueling ertong shenzang jibing linchuang Yu Bingli fenxi [Clinical and pathological analysis of 1459 cases of renal disease in school-age children in Qinhuangdao]. Chin Healing Med. 2021;30(06):640–3.
Shang R, Zhu Y, Lin Z, Ma D, Ma Y, Ji M, et al. Yu Qiong liangdi yuanfaxing shenxiaoqiubing bingli leixing de bianqian duibi ji linchuang fenxi[Comparison and clinical analysis of pathological types of primary glomerular diseases in North Henan and Hainan]. J Clin Nephrol. 2021;21(2):111–8.
Lu X. Zhendui butong fenji IgA shenbing huanzhe kaizhan gexinghua huli moshi de linchuang xiaoguo guancha [Clinical effects of personalized care model for patients with different grades of IgA nephropathy]. Essent Health Readings. 2021;8:125.
Qi S. Zhendui butong fenji IgA shenbing kaizhan gexinghua huli moshi de linchuang xiaoguo guancha [Clinical effects of personalized care model for patients with different grades of IgA nephropathy]. Diet Health Care. 2021;8.
Pan Q, Ye Z, Zeng C, Ning W. Feishenbingxing tefaxing moxing shenbing Yu feishenbingxing IgA shenbing de linchuang tedian bijiao [Clinical comparative analysis of non-nephrotic idiopathic membranous nephropathy and non-nephrotic IgA nephropathy]. Anhui Med. 2021;25(2):268–70.
Xiao L, Wang J, Zhang M, He X, Gao J, Xi C. Yufangxing kangning zai budui guanbing shenbing zonghezheng zhiliao zhong de yingyong xiaoguo yanjiu [Study on the effect of preventive anticoagulation in the treatment of nephrotic syndrome in army officers and soldiers]. Northwest J De?F Med. 2021;42(01):30–6.
Zhao JL, Wang JJ, Huang GP, Feng CY. Primary IgA nephropathy with nephrotic-range proteinuria in Chinese children. Med (Baltim). 2021;100(21):e26050.
Le W, Liang S, Deng K, Hu Y, Zeng C, Liu D. 1126 li zhongguo hanzu chengren IgA shenbing huanzhe de changqi yuhou ji weixian yinsu fenxi [Long-term prognosis and risk factor analysis of 1126 Chinese Han adult patients with IgA nephropathy]. J Nephrol Dialysis Ren Transplantation. 2011;20(02):101–8.
Cen J, Hu H, Cheng Y, Liu Y, Wu S, Qin W, et al. Guangxi duominzu juju diqu dan zhongxin shenhuojian bingli ziliao ji minzu tedian fenxi [Pathological data of single-center kidney biopsy and analysis of ethnic characteristics in a multi-ethnic area of Guangxi]. J Chengdu Med Coll. 2021;16(04):482–5.
Yang J, Zhang L, Wang Y. Manxing Shenzangbing Shen Chuanci huojian bingli tezheng fenxi [Analysis of pathological features of renal puncture biopsy in chronic kidney disease]. Tibetan Med. 2021;42(05):49–51.
Duan Y, Lie C, Zhang L, AYiJiaKen K, Guo W, Li Y, et al. Xinjiang Weiwuer Zizhiqu 10 684 Li Shen huojian bingli ziliao Yu Liuxingbingxue tedian fenxi [Analysis of pathological data and epidemiological characteristics of 10 684 kidney biopsies in Xinjiang Uygur Autonomous Region]. Chin J Nephrol. 2021;37(06):490–8.
Gu C, Li Q, Liang W, Bi H, Xie M, Wu D. Guilin he jining liangsuo yiyuan 1370 Li Shen huojian jibing fenbu tezheng [Characteristics of disease distribution in 1370 kidney biopsies from two hospitals in Guilin and Jining]. J Cent South Univ (Medical Edition). 2021;46(09):974–82.
Li J, Cui Z, Long J, Huang W, Wang J, Zhang H, et al. Primary glomerular nephropathy among hospitalized patients in a national database in China. Nephrology, Dialysis, transplantation: Official Publication of the European Dialysis and Transplant Association -. Eur Ren Association. 2018;33(12):2173–81.
Chen S, Tang Z, Xiang H, Li X, Chen H, Zhang H, et al. Etiology and outcome of crescentic glomerulonephritis from a single center in China: a 10-year review. Am J Kidney Dis. 2016;67(3):376–83.
Yata N, Nakanishi K, Shima Y, Togawa H, Obana M, Sako M, et al. Improved renal survival in Japanese children with IgA nephropathy. Pediatr Nephrol. 2008;23(6):905–12.
Goto M, Wakai K, Kawamura T, Ando M, Endoh M, Tomino Y. A scoring system to predict renal outcome in IgA nephropathy: a nationwide 10-year prospective cohort study. Nephrology, Dialysis, transplantation: Official Publication of the European Dialysis and Transplant Association -. Eur Ren Association. 2009;24(10):3068–74.
Yamamoto R, Nagasawa Y, Shoji T, Iwatani H, Hamano T, Kawada N, et al. Cigarette smoking and progression of IgA nephropathy. Am J Kidney Dis. 2010;56(2):313–24.
Yokoyama H, Sugiyama H, Sato H, Taguchi T, Nagata M, Matsuo S, et al. Renal disease in the elderly and the very elderly Japanese: analysis of the Japan Renal Biopsy Registry (J-RBR). Clin Exp Nephrol. 2012;16(6):903–20.
Komatsu H, Kikuchi M, Nakagawa H, Fukuda A, Iwakiri T, Toida T, et al. Long-term survival of patients with IgA nephropathy after dialysis therapy. Kidney Blood Press Res. 2013;37(6):649–56.
Matsuzaki K, Suzuki Y, Nakata J, Sakamoto N, Horikoshi S, Kawamura T, et al. Nationwide survey on current treatments for IgA nephropathy in Japan. Clin Exp Nephrol. 2013;17(6):827–33.
Sugiyama H, Yokoyama H, Sato H, Saito T, Kohda Y, Nishi S, et al. Japan Renal Biopsy Registry and Japan kidney Disease Registry: Committee Report for 2009 and 2010. Clin Exp Nephrol. 2013;17(2):155–73.
Moriyama T, Tanaka K, Iwasaki C, Oshima Y, Ochi A, Kataoka H, et al. Prognosis in IgA nephropathy: 30-year analysis of 1,012 patients at a single center in Japan. PLoS ONE. 2014;9(3):e91756.
Sato R, Joh K, Komatsuda A, Ohtani H, Okuyama S, Togashi M, et al. Validation of the Japanese histologic classification 2013 of immunoglobulin A nephropathy for prediction of long-term prognosis in a Japanese single-center cohort. Clin Exp Nephrol. 2015;19(3):411–8.
Oshima Y, Moriyama T, Itabashi M, Takei T, Nitta K. Characteristics of IgA nephropathy in advanced-age patients. Int Urol Nephrol. 2015;47(1):137–45.
Hattori M, Iwano M, Sako M, Honda M, Okada H, Akioka Y, et al. Transition of adolescent and young adult patients with childhood-onset chronic kidney disease from pediatric to adult renal services: a nationwide survey in Japan. Clin Exp Nephrol. 2016;20(6):918–25.
Kaihan AB, Yasuda Y, Katsuno T, Kato S, Imaizumi T, Ozeki T, et al. The Japanese histologic classification and T-score in the Oxford classification system could predict renal outcome in Japanese IgA nephropathy patients. Clin Exp Nephrol. 2017;21(6):986–94.
Kajiwara N, Hayashi K, Fujiwara M, Nakayama H, Ozaki Y. Identification of children with chronic kidney disease through school urinary screening using urinary protein/creatinine ratio measurement: an observational study. Clin Exp Nephrol. 2020;24(5):450–7.
Miyabe Y, Karasawa K, Akiyama K, Ogura S, Takabe T, Sugiura N, et al. Grading system utilising the total score of Oxford classification for predicting renal prognosis in IgA nephropathy. Sci Rep. 2021;11(1):3584.
Utsunomiya Y, Koda T, Kado T, Okada S, Hayashi A, Kanzaki S, et al. Incidence of pediatric IgA nephropathy. Pediatr Nephrol. 2003;18(6):511–5.
Lee S, Choi S, Se-bin S, Kyunghwan J, Taewon L. Relative risk factors of prognosis in IgA nephropathy patients with depressed renal functions. Korean J Nephrol. 2010;29(2):198–207.
Lee H, Kim DK, Oh KH, Joo KW, Kim YS, Chae DW, et al. Mortality of IgA nephropathy patients: a single center experience over 30 years. PLoS ONE. 2012;7(12):e51225.
Lee Ha-Jung. Long-term patient and renal survivals and their predictable factor analyses in IgA nephropathy patients [Thesis]: College of Medicine, Seoul National University; 2012.
Cho BS, Hahn WH, Cheong HI, Lim I, Ko CW, Kim SY, et al. A nationwide study of mass urine screening tests on Korean school children and implications for chronic kidney disease management. Clin Exp Nephrol. 2013;17(2):205–10.
Lee H, Kim DK, Oh KH, Joo KW, Kim YS, Chae DW, et al. Mortality and renal outcome of primary glomerulonephritis in Korea: observation in 1,943 biopsied cases. Am J Nephrol. 2013;37(1):74–83.
Bae HJ, Moon KR, Kim YJ, Choi DE, Na KR, Lee KW, et al. Clinical and histopathological analysis of the kidney biopsies of 2,450 patients seen over 30 years at Chungnam National University Hospital. Korean J Med. 2015;84(3):379–88.
Jeong EG, Hyoun S, Lee SM, An WS, Kim SE, Son YK. Clinical outcomes of nephrotic syndrome in immunoglobulin a nephropathy. Saudi J Kidney Dis Transplantation. 2017;28(6):1314–20.
Kee YK, Yoon CY, Kim SJ, Moon SJ, Kim CH, Park JT, et al. Determination of the optimal target level of proteinuria in the management of patients with glomerular diseases by using different definitions of proteinuria. Med (Baltim). 2017;96(44):e8154.
Shin HS, Cho DH, Kang SK, Kim HJ, Kim SY, Yang JW, et al. Patterns of renal disease in South Korea: a 20-year review of a single-center renal biopsy database. Ren Fail. 2017;39(1):540–6.
Suh JS, Jang KM, Hyun H, Cho MH, Lee JH, Park YS, et al. Remission of proteinuria may protect against progression to chronic kidney disease in pediatric-onset IgA nephropathy. J Clin Med. 2020;9(7):2058.
Chiu HF, Chen HC, Lu KC, Shu KH. Taiwan Society of Nephrology. Distribution of glomerular diseases in Taiwan: preliminary report of National Renal Biopsy Registry-publication on behalf of Taiwan Society of Nephrology. BMC Nephrol. 2018;19(1):6.
Yu MC, Lee F, Huang WH, Hsueh S. Percutaneous ultrasound-guided renal biopsy in children: the need for renal biopsy in pediatric patients with persistent asymptomatic microscopic hematuria. Biomedical J. 2014;37(6):391–7.
National Health Research Institute & Taiwan Society of Nephrology. 2019 Annual Report on Kidney Disease in Taiwan.
Jegatheesan D, Nath K, Reyaldeen R, Sivasuthan G, John GT, Francis L, et al. Epidemiology of biopsy-proven glomerulonephritis in Queensland adults. Nephrol (Carlton). 2016;21(1):28–34.
Briganti EM, Dowling J, Finlay M, Hill PA, Jones CL, Kincaid-Smith PS, et al. The incidence of biopsy-proven glomerulonephritis in Australia. Nephrology, Dialysis, transplantation: Official Publication of the European Dialysis and Transplant Association. - Eur Ren Association. 2001;16(7):1364–7.
Lee AYS, Lin M-W. Do IgA nephropathy presentations display any seasonality? J Nephropathology. 2021;10(3):e33–e.
Subspecialty Group of Renal Diseases of the Society of Pediatrics Chinese Medical Association. [Evidence-based guidelines for diagnosis and treatment of primary IgA nephropathy (2016)]. Zhonghua Er Ke Za Zhi. 2017;55(9):643–6.
[Health, Labor and Welfare Scientific Research Grant Policy Research Project for Intractable Diseases. (Intractable Disease Policy Research Project)].[IgA Nephropathy 2020 Clinical Practice Guidelines].[Tokyo Igakusha]. 2021.
LoMartire R, Ang BO, Gerdle B, Vixner L. Psychometric properties of short Form-36 Health Survey, EuroQol 5-dimensions, and hospital anxiety and Depression Scale in patients with chronic pain. Pain. 2020;161(1):83–95.
OECD. Conversion rates - Exchange rates - OECD Data [Available from: http://data.oecd.org/conversion/exchange-rates.htm.
Kwon CS, Daniele P, Forsythe A, Ngai C. A systematic literature review of the epidemiology, health-related quality of life impact, and economic burden of immunoglobulin A nephropathy. J Health Econ Outcomes Res. 2021;8(2):36–45.
Orta-Sibu N, Lopez M, Moriyon JC, Chavez JB. Renal diseases in children in Venezuela, South America. Pediatr Nephrol. 2002;17(7):566–9.
Coppo R, Gianoglio B, Porcellini MG, Maringhini S. Frequency of renal diseases and clinical indications for renal biopsy in children (report of the Italian National Registry of Renal biopsies in Children). Group of Renal Immunopathology of the Italian Society of Pediatric Nephrology and Group of Renal Immunopathology of the Italian Society of Nephrology. Nephrology, Dialysis, transplantation: Official Publication of the European Dialysis and Transplant Association -. Eur Ren Association. 1998;13(2):293–7.
Barbour S, Lo C, Espino-Hernandez G, Sajjadi S, Feehally J, Klarenbach S, et al. The population-level costs of immunosuppression medications for the treatment of glomerulonephritis are increasing over time due to changing patterns of practice. Nephrology, Dialysis, transplantation: Official Publication of the European Dialysis and Transplant Association -. Eur Ren Association. 2018;33(4):626–34.
Shen P, He L, Li Y, Wang Y, Chan M. Natural history and prognostic factors of IgA nephropathy presented with isolated microscopic hematuria in Chinese patients. Nephron Clin Pract. 2007;106(4):c157–61.
Acknowledgements
This work was presented as an abstract at the ISN World Congress of Nephrology 2022 meeting.
Funding
This work was supported by Otsuka Pharmaceutical Development & Commercialization, Inc., Princeton, NJ.
Author information
Authors and Affiliations
Contributions
Research conception and/or design: Kristin Pareja, Sandipan Bhattacharjee, Omer Zaidi, Fen Du, and Zhaoli Tang; Literature searching strategy: Omer Zaidi, Fen Du, and Zhaoli Tang; literature screening and data extraction and analysis: Fen Du and Zhaoli Tang; All authors were involved in the drafting and /or substantial revision of manuscript; All authors accept accountability for their contributions and agree as a condition of authorship to ensure resolution of questions about the work. All authors approved the submitted version.
Corresponding author
Ethics declarations
Competing interests
Kristin Pareja and Sandipan Bhattacharjee are employees of Otsuka Pharmaceutical Development & Commercialization, Inc., Princeton, NJ, United States. Omer Zaidi, Fen Du, and Zhaoli Tang are employees of OPEN Health and were paid consultants by Otsuka.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zaidi, O., Du, F., Tang, Z. et al. Review on epidemiology, disease burden, and treatment patterns of IgA nephropathy in select APAC countries. BMC Nephrol 25, 136 (2024). https://doi.org/10.1186/s12882-024-03555-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12882-024-03555-5