Abstract
Aim
Our aim in this study was primarily to assess the relationship between visceral adiposity index (VAI) and the risk of kidney stones and kidney stone recurrence in US adults.
Methods
We used data from the National Health and Nutrition Examination Survey (NHANES) 2007–2014 for our analysis. VAI was calculated from waist circumference (WC), body mass index (BMI), triglycerides (TG), and high-density lipoprotein-cholesterol (HDL-C). Kidney stones and recurrence of kidney stones were obtained from questionnaire interview data. We used multivariate logistic regression analysis to explore the correlation between VAI and the risk of kidney stone and kidney stone recurrence. In addition, we performed subgroup analysis, interaction tests, and restricted cubic spline (RCS) analysis.
Results
A total of 9886 participants were included in this study, with a prevalence of 9.24% for kidney stones and 2.97% for recurrence of kidney stones. The prevalence of kidney stones and kidney stone recurrence increased with higher quartiles of VAI. We observed a significantly positive correlation between VAI and the risk of kidney stone and kidney stone recurrence. Participants with the highest VAI quartiles had a 48% (OR: 1.48, 95%CI: 1.08–2.02) and 52% (OR: 1.52, 95%CI: 0.86–2.71) increased risk of kidney stones and kidney stone recurrence, respectively, compared to participants with the lowest VAI quartiles. Subgroup analysis and interaction tests demonstrated this positive association independent of different subgroup factors.
Conclusion
Visceral fat accumulation may be associated with an increased risk of kidney stones and kidney stone recurrence.
Similar content being viewed by others
Introduction
Kidney stones are a major public health problem worldwide and bring a heavy economic burden [1]. A UK cohort study found a rising trend in the cost of kidney stone treatment [2]. The prevalence of kidney stones has been reported to be approximately 10.1% among adults in the United States, and the prevalence of stones is significantly higher in men than in women [3]. In Europe, the prevalence of kidney stones is around 5%-14% [4, 5]. Besides, the recurrence rate of kidney stones within five years is about 50% [6]. Patients with kidney stones have a significantly increased risk of end-stage renal disease and chronic kidney disease compared to patients without kidney stones, even if the patient has only one episode of kidney stones [7]. Patients with recurrent symptomatic kidney stones have a higher risk of end-stage renal disease [8].
Obesity is also a global public health problem that threatens the health of all humans, with the prevalence of obesity increasing, with nearly 600 million adults reported to be obese worldwide in 2015 [9]. In addition, it is expected that by 2030, nearly 50% of U.S. adults will be obese [10]. Obese individuals are reported to be at greater risk of kidney stones, and the risk of kidney stones is further increased when obesity is combined with metabolic syndrome [11]. Another study that evaluated the association between kidney stones and obesity indicators found that body mass index (BMI), waist circumference (WC), waist-to-height ratio (WHtR), and waist-to-hip ratio (WHR), all of which are obesity-related indices, were significantly associated with an increased risk of kidney stones [12].
Traditional obesity-related indices such as BMI, WC, and WHtR are difficult to differentiate between subcutaneous and visceral fat accumulation. Visceral adiposity index (VAI), a sex-specific index based on WC, BMI, triglycerides, and HDL Cholesterol, can accurately identify and assess visceral adiposity function [13]. One study found that the positive predictive value of VAI for diabetes was significantly higher than that of BMI and WC [14]. VAI was also associated with a significantly increased risk of type 2 diabetes in older Chinese adults [15]. A 10-year follow-up study found that VAI was associated with a significant increase in cardiovascular disease prevalence [16]. Another study also confirmed that VAI was independently associated with an increased risk of angina, hypertension, and coronary atherosclerotic heart disease [17]. In hemodialysis patients, the investigators found that the predictive value of VAI for all-cause mortality was similar to that of WC and WHtR, while VAI was significantly better than WC in predicting the occurrence of cardiovascular events [18]. In addition, VAI can be used to predict renal function in patients with type 2 diabetes because it is significantly and positively correlated with the patient's urinary albumin level [19]. Another study also confirmed that VAI is closely associated with the manifestation of decreased renal function such as albuminuria. However, the association of VAI with the risk of kidney stones and kidney stone recurrence has been rarely reported before, so we used data from the National Health and Nutrition Examination Survey (NHANES) to assess the association between VAI and the risk of kidney stones and kidney stone recurrence.
Materials and methods
Study population
Data for our study were obtained from the National Health and Nutrition Examination Survey ( NHANES), a national cross-sectional survey study designed to assess the nutritional and health status of the U.S. population. The study recruited a highly representative sample due to the stratified, multi-stage, and probability sampling design approach of NHANES. All NHANES study protocols were approved by the National Center for Health Statistics (NCHS) Research Ethics Review Board and all participants signed written informed consent. A detailed description of the NHANES study and its data can be accessed online at https://www.cdc.gov/nchs/nhanes/.
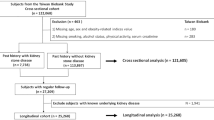
We obtained data from four consecutive cycles of NHANES 2007–2014, for which complete data on VAI, renal stones, and renal stone recurrence were available during the four consecutive survey cycles. A total of 40,617 subjects were initially included in our study, and after excluding participants < 18 years of age, and with missing data on VAI (n = 14,300) and kidney stones (n = 546), a total of 9886 participants were included in our final analysis (Fig. 1).
Exposure and outcome definitions
VAI was designed as an exposure variable in our study. Visceral Adiposity Index (VAI) is a sex-specific index based on waist circumference (WC), body mass index (BMI), total triglycerides (TG), and high-density lipoproteins (HDL-C). The VAI was calculated as follows [13]: For males: VAI = WC/(39.68 + (1.88 × BMI)) × (TG/1.03) × (1.31/HDL-C); For females: VAI = WC/(36.58 + (1.89 × BMI)) × (TG/0.81) × (1.52/HDL-C). The units of TG and HDL are mmol/L, while the units of WC are cm. VAI can be used to assess visceral fat function. A higher VAI score represents a greater amount of visceral fat and the patient may be at higher risk for cardiovascular disease.
The incidence of kidney stones and the recurrence of kidney stones were designed as outcome variables. The status of kidney stones was assessed by the following two questions. These questions were "Have you ever had a kidney stone?" and "How many times have you passed a kidney stone?" Participants who answered yes were assumed to have kidney stones. Participants who had experienced two or more kidney stones were assumed to have a recurrence of kidney stones.
Covariates
Our covariates included in these studies included age, sex, race, educational levels, BMI, physical activity, diabetes (DM) and hypertension. BMI was classified as < 25, 25–29.9, and ≥ 30 kg/m2, which corresponded to normal weight, overweight, and obese population for all participants. Hypertension was defined based on a self-reported diagnosis of hypertension, diastolic blood pressure ≥ 90 mmHg or systolic blood pressure ≥ 140 mmHg, or the use of anti-hypertensive medications [20]. DM was defined base on a self-reported diagnosis of diabete mellitus, 2-h plasma glucose ≥ 200 mg/dL in an oral glucose tolerance test, HbAlc ≥ 6.5%, use of oral hypoglycemic agents, or fasting glucose ≥ 126 mg/dL [21]. Physical activity (PA) status was collected through questionnaire interviews, which assessed PA in the past 30 days through questionnaires, mainly in the categories of walk, bicycle, work activity, and recreational activity. Staff calculated the MET minutes per week of each activity by multiplying the standard MET value of each activity by the total number of minutes per week of each activity. In accordance with previous studies [22], different groups were differentiated based on PA levels, where participants with PA < 150 MET-min/week were defined as Very low PA, participants with PA between 150–960 MET-min/week were defined as Low PA group, participants with PA between 960–1800 MET-min/week were defined as Medium PA, and those with PA > 1800 MET-min/week were defined as High PA group. All detailed measurement processes of these variables are publicly available at www.cdc.gov/nchs/nhanes/.
Statistical analysis
All statistical analyses were performed according to Centers for Disease Control and Prevention (CDC) guidelines, with appropriate NHANES sampling weights applied and a complex multistage cluster survey design considered in the analysis. Continuous variables were presented as means with standard error, and categorical variables were presented as proportions. The difference between subjects grouped by VAI quartiles was evaluated by a weighted Student's t-test (for continuous variables) or weighted Chi-Square test (for categorical variables). Multivariable logistic regression was used in three different models to investigate the association between VAI and the risk of kidney stone and kidney stone recurrence. In Model 1, no covariates were adjusted. Model 2 was adjusted for age, gender, race, educational levels, and BMI (continuous variable). Model 3 was adjusted for age, sex, race, educational levels, BMI (continuous variable), PA, DM, and hypertension. We tested the correlation between VAI and the risk of kidney stone and kidney stone recurrence under different stratification factors by subgroup analysis. In addition, these stratification factors were also considered as potential modifiers, and an interaction term was added to test for heterogeneity between the subgroups. The analysis of potential nonlinear relationships employed restricted cubic spline (RCS) analysis, employing three piecewise points, to flexibly explore associations between VAI and the risk of kidney stone and kidney stone recurrence. All analyses were performed using R version 4.2.1 (The R Foundation). P < 0.05 was considered statistically significant.
Results
Participants' characteristics at baseline
A total of 9886 participants were included in this study with a mean age of 47.38 ± 0.31 years, of which 51.69% were females and 48.31% were males. The mean VAI index was 2.09 ± 0.04. The mean prevalence of kidney stones was 9.24% overall and increased with the increasing VAI quartiles (Quartile 1: 6.08%, Quartile 2: 8.57%, Quartile 3: 9.95%, Quartile: 12.40%). The recurrence rate of kidney stones in all participants was 2.97% and individuals with higher VAI quartile tended to have a higher risk of renal stone recurrence (Quartile 1: 1.78%, Quartile 2: 1.86%, Quartile 3: 3.78%, Quartile: 4.51%). Among the four VAI quartiles, statistically significant differences were found in age, total cholesterol, HDL-C, triglycerides, waist circumference, race, PIR, education, physical activity, BMI, alcohol consumption, smoking status, hypertension, and diabetes mellitus (p < 0.05). Compared to the lowest VAI quartile, participants in the increased VAI group had higher levels of total cholesterol, triglycerides, waist circumference, BMI, and lower levels of HDL-C, and were more likely to develop hypertension and diabetes. In addition, participants in the increased VAI group were more likely to be Mexican American and Non-Hispanic White, less physically active, and more likely to be current smokers and non-drinkers There was no statistical significance between quartiles in sex (p > 0.05) (Table 1).
The association of visceral adiposity index and the risk of kidney stone
The association between VAI and the risk of kidney stones was indicated in Table 2. The authors observed that higher VAI was associated with an increased risk of kidney stones. In the crude model (Model 1), we observed a positive association between increased VAI and kidney stone risk. This positive correlation remained significant in Model 2 adjusted for age, sex, and race. In the fully adjusted model (Model 3), the positive correlation between VAI and kidney stone risk remained robust. The authors identified a 2% increase in the risk of kidney stones for each unit increase in VAI (OR = 1.02, 95%CI: 1.00–1.04). This positive association remained statistically significant when we considered VAI as quartiles. Participants in the highest quartile of VAI had a significant 41% increased risk of kidney stones compared to those in the lowest quartile of VAI (OR: 1.41, 95%CI: 1.06–1.87) (Table 2).
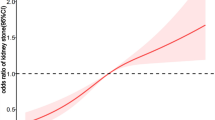
To further investigate whether there was a nonlinear relationship between VAI and kidney stones, we performed an RCS analysis. Our results revealed no nonlinear relationship between VAI and kidney stones (P nonlinear = 0.1140) (Fig. 2).
The association of visceral adiposity index and the risk of kidney stone recurrence
For the risk of kidney stone recurrence, We also observed a positive association between VAI and the likelihood of kidney stone recurrence. A significant positive association between VAI and the risk of kidney stone recurrence was observed in Model 1 and Model 2 (Model 1: OR: 1.03, 95%CI: 1.01–1.06; Model 2: OR: 1.02, 95%CI: 1.00–1.04). After full adjustment, we observed that each 1-unit increase in VAI increased the risk of kidney stone recurrence by 2% (Model 3: OR: 1.02, 95%CI: 1.01–1.04).
When we treated VAI as quartiles, we could still observe a positive association between VAI and the risk of kidney stone recurrence. Subjects in the highest quartile had a significantly increased risk of kidney stone recurrence by 63% compared to subjects in the lowest VAI quartile (OR: 1.63, 95%CI: 1.43–2.76) (Table 3).
In addition, we also explored the non-linear relationship between VAI and the risk of kidney stone recurrence by RCS analysis. Our results showed that the relationship between VAI and kidney stone recurrence was nonlinear (P nonlinear = 0.0087) (Fig. 2).
Subgroup analysis
For the correlation between VAI and kidney stones, we observed a positive association among participants stratified by age. Each unit increase in VAI was significantly associated with a 4.7% increase in the risk of kidney stones in participants aged 60 years or older (OR: 1.047, 95% CI: 1.003–1.093). However, in the interaction test, we did not find an effect of age on the correlation between VAI and kidney stones (P for interaction = 0.604) (Fig. 3).
In addition, we evaluated the association between VAI and the risk of renal stone recurrence by subgroup analysis. A significant association was observed in participants who had diabetes (OR: 1.044, 95%CI: 1.010–1.079). Interaction terms were also used to test the heterogeneities among each subgroup, and our results showed that no significant difference was revealed among diabetes status (P for interaction = 0.079), indicating that this association was not dependent on diabetes status (Fig. 4). Furthermore, no significant differences were indicated by the interaction test across other stratifications, suggesting that this positive association between VAI and kidney stone recurrence was not significantly influenced by gender, age, hypertension, and BMI, and could be applied in a variety of population settings.
Multivariate logistic regression models of kidney stone and kidney stone recurrence
Age, gender, race, educational levels, BMI, physical activity, and hypertension remained significantly associated with the risk of kidney stones in the fully adjusted model (Supplemental Table 1). We observed that each 1 unit increase in age was associated with a 2% increase in the risk of kidney stones (OR: 1.02, 95%CI: 1.01–1.05). Men had a 29% increased odds of kidney stones compared to women (OR: 1.29, 95%CI: 1.08–1.53). Compared to Mexican American, participants who were non-Hispanic Black and non-His panic White had a 45% lower risk (OR: 0.55, 95%CI: 0.43–0.72) and a 43% elevated risk (OR: 1.43, 95%CI: 1.13–1.82) of kidney stones, respectively. Compared with those who had a high PA, the odds of kidney stones were elevated by 6% and 13% in participants who had a low PA (OR = 1.06, 95%CI: 1.02–1.36) and very low PA (OR = 1.13, 95%CI: 1.01–1.40), respectively. Per unit increase in BMI, the odds of kidney stones were increased by 3%. Besides, compared with participants without hypertension, those with hypertension had a 26% increased risk of kidney stones (OR = 1.26, 95%CI: 1.01–1.57).
We also analyzed the possible factors associated with the risk of kidney stone recurrence by multifactorial analysis in Supplemental Table 2. Compared with female participants, male participants had a 59% increased risk of kidney stone recurrence(OR:1.59, 95%CI: 1.21–2.09). Compared with Mexican American, the risk of kidney stone recurrence in non-Hispanic White and non-Hispanic Black were lowered by 65% (OR = 0.35, 95%CI: 0.17–0.75) and elevated by 78% (OR = 1.78, 95%CI: 1.09–2.90), respectively. Per unit increase in BMI, the odds of kidney stone recurrence were increased by 6% (OR = 1.06, 95%CI: 1.03–1.09). The odds of kidney stone recurrence were increased by 6% and 45% in hypertension (OR = 1.06, 95%CI: 1.02–1.18) and diabetes (OR = 1.45, 95%CI: 1.05–2.20) populations compared with their counterparts.
Discussion
This study, which recruited 9886 participants, showed that participants with higher VAI were more likely to develop kidney stones and recurrence of kidney stones. The results of our subgroup analysis and interaction tests also illustrated that this positive association was independent of sex, age, BMI, hypertension, diabetes, and smoking status. Management targeting visceral fat distribution may reduce the incidence of kidney stones, and clinicians should pay more attention to this indicator.
Although fewer studies have reported the association between VAI and the risk of kidney stones and recurrence of kidney stones, previous studies have described the relationship between VAI and kidney function. One study found that in older adults, VAI was associated with an increased risk of chronic kidney disease (CKD)-related events such as decreased glomerular filtration rate and rapid kidney function decline [23]. VAI can be a good predictor of CKD, with a prediction rate of 77% [24]. Excessive visceral fat deposition in patients with CKD increases the risk of all-cause mortality [25]. VAI was also independently associated with a significantly higher urinary albumin creatinine ratio in patients with prediabetes [26]. VAI also plays an important value in predicting the development of end-stage renal disease in patients with type 2 diabetes [27, 28]. Kim et al. found that visceral fat accumulation could be a better predictor of CKD severity than BMI, regardless of the patient's gender [29]. Another study in Korean adults showed a positive association of VAI with CKD incidence in men only, and this association was no longer significant in women [30]. Otunctemur et al. suggested that VAI levels are associated with the progression of renal cell carcinoma [31]. Fukuhara et al. also revealed VAI levels in patients after renal transplantation, with increasing visceral fat accumulation as the time after transplantation increased [32].
One study found that a BMI > 30 kg/m2 was independently associated with an increased risk of kidney stone disease [33]. An almost twofold increased risk of kidney stones was also observed in pediatric kidney stone patients with a BMI > 30 kg/m2 compared to those with a BMI in the normal range [34]. A US study found a 3% prevalence of kidney stones in participants without comorbid metabolic syndrome and a significant increase in the prevalence of kidney stones in participants with comorbid metabolic syndrome (hyperlipidemia, hypertriglyceridemia, hyperglycemia, hypertension, and abdominal obesity), with a 9.8% prevalence of kidney stones in those with five comorbid metabolic syndrome features [35]. Significant dyslipidemia has been identified in patients with kidney stones [36]. Another study found that dyslipidemia was associated with altered chemical composition in the urine and this chemical composition played a key role in stone formation, such as patients with high triglycerides exhibited increased uric acid excretion and significantly lower urinary pH, those with high total cholesterol had significantly higher urinary calcium levels, and patients with reduced high-density lipoprotein (HDL) showed significantly higher levels of oxalic acid and uric acid in their urine [37]. Mosli et al. also analyzed the composition of kidney stones in overweight and obese patients and found that their stones were predominantly composed of calcium oxalate and uric acid [38]. Hypercalciuria and hyperoxaluria were identified as high risk factors for calcium stone formation, while excessive uric acid excretion and decreased urinary pH were the main abnormal factors contributing to uric acid stone formation [39]. Furthermore, in obese patients, the accumulation of fat in proximal tubular cells reduced their ability to secrete ammonia, resulting in urine acidification, which could contribute to stone progression [40] Significantly increased levels of osteopontin and biomarkers associated with inflammatory responses and accumulation of pro-inflammatory macrophages were also observed in animal experiments in high-fat-fed mice, ultimately leading to crystal and mineral deposition in the tubular lumen of the kidneys and resulting in the progression of kidney stones [41]. These mice also exhibited a notable increase in oxalic acid levels in their urine [41]. In contrast, the administration of statin treatment in high-fat diet-fed mouse models led to increased renal osteopontin expression and reduced deposition of calcium oxalate stones [42]. The strong association between dyslipidemia, one of the important indicators for calculating the visceral fat accumulation index, and the progression of kidney stones explains the increased risk of kidney stones due to high VAI levels. However, Besiroglu et al. discovered a significantly elevated risk of urolithiasis in patients with high triglyceride (TG) and low high-density lipoprotein (HDL) levels [43]. Furthermore, the authors observed a robust correlation between any lipid profile disorders and the risk of urolithiasis. However, the association between TG and urolithiasis was found to be more consistent when compared to the association between HDL and urolithiasis. Cai et al. observed that non-stone-forming individuals in the TG group had increased urinary oxalate excretion, while those in the high TC group exhibited increased urinary calcium excretion. These constituents are known to be associated with urinary lithogenesis [44]. In contrast, individuals in the high low-density lipoprotein (LDL) group had decreased urinary calcium and oxalate excretion, whereas those in the low high-density lipoprotein (HDL) group displayed increased urinary citrate and magnesium excretion, which are protective factors against stone formation [44]. The physiological mechanisms underlying why specific components of dyslipidemia have paradoxical effects on urinary changes related to stone formation remain unclear at present. Future studies should also prioritize investigating potential connections between lipid metabolic profiles and different types of stone formations. Additionally, well-designed prospective randomized controlled trials or cohort studies are required to provide a more comprehensive understanding of the causal relationship between dyslipidemia and urolithiasis.
This study had several strengths. First, this study was based on data from NHANES, and because NHANES was a national population-based sample survey, the sample size obtained was fully representative. Second, we adjusted for confounding covariates to reduce confounding bias and to make our results more reliable.
Our study also had some limitations. First, the type of our study was a cross-sectional study, and the design of this type of study prevented us from obtaining a clear causal relationship between VAI and kidney stones. Second, although we have adjusted for several potential covariates, we cannot ensure that we have excluded all covariates that could lead to confounding, such as the use of some drugs. Finally, because the NHANES sample was specific to the US population only, our results may not be generalizable to the world.
Conclusion
Elevated VAI showed a positive association with the risk of kidney stones and kidney stone recurrence. Our present study highlighted the importance of visceral fat accumulation management in patients at risk for kidney stones. However, further large prospective studies are needed to confirm the causal relationship between VAI and kidney stones.
Availability of data and materials
Data described in the manuscript, codebook, and analytic code will be made publicly and freely available without restriction at www.cdc.gov/nchs/nhanes/.
References
Türk C, Petřík A, Sarica K, Seitz C, Skolarikos A, Straub M, et al. EAU Guidelines on Interventional Treatment for Urolithiasis. Eur Urol. 2016;69(3):475–82.
Geraghty RM, Cook P, Walker V, Somani BK. Evaluation of the economic burden of kidney stone disease in the UK: a retrospective cohort study with a mean follow-up of 19 years. BJU Int. 2020;125(4):586–94.
Chewcharat A, Curhan G. Trends in the prevalence of kidney stones in the United States from 2007 to 2016. Urolithiasis. 2021;49(1):27–39.
Rukin NJ, Siddiqui ZA, Chedgy ECP, Somani BK. Trends in Upper Tract Stone Disease in England: Evidence from the Hospital Episodes Statistics Database. Urol Int. 2017;98(4):391–6.
Sorokin I, Mamoulakis C, Miyazawa K, Rodgers A, Talati J, Lotan Y. Epidemiology of stone disease across the world. World J Urol. 2017;35(9):1301–20.
D’Costa MR, Haley WE, Mara KC, Enders FT, Vrtiska TJ, Pais VM, et al. Symptomatic and Radiographic Manifestations of Kidney Stone Recurrence and Their Prediction by Risk Factors: A Prospective Cohort Study. J Am Soc Nephrol. 2019;30(7):1251–60.
Alexander RT, Hemmelgarn BR, Wiebe N, Bello A, Morgan C, Samuel S, et al. Kidney stones and kidney function loss: a cohort study. BMJ. 2012;345: e5287.
Dhondup T, Kittanamongkolchai W, Vaughan LE, Mehta RA, Chhina JK, Enders FT, et al. Risk of ESRD and Mortality in Kidney and Bladder Stone Formers. Am J Kidney Dis. 2018;72(6):790–7.
Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, Lee A, et al. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N Engl J Med. 2017;377(1):13–27.
Ward ZJ, Bleich SN, Cradock AL, Barrett JL, Giles CM, Flax C, et al. Projected U.S. State-Level Prevalence of Adult Obesity and Severe Obesity. N Engl J Med. 2019;381(25):2440–50.
Ye Z, Wu C, Xiong Y, Zhang F, Luo J, Xu L, et al. Obesity, metabolic dysfunction, and risk of kidney stone disease: a national cross-sectional study. Aging Male. 2023;26(1):2195932.
Lee MR, Ke HL, Huang JC, Huang SP, Geng JH. Obesity-related indices and its association with kidney stone disease: a cross-sectional and longitudinal cohort study. Urolithiasis. 2022;50(1):55–63.
Amato MC, Giordano C, Galia M, Criscimanna A, Vitabile S, Midiri M, et al. Visceral Adiposity Index: a reliable indicator of visceral fat function associated with cardiometabolic risk. Diabetes Care. 2010;33(4):920–2.
Wei J, Liu X, Xue H, Wang Y, Shi Z. Comparisons of visceral adiposity index, body shape index, body mass index and waist circumference and their associations with diabetes mellitus in adults. Nutrients. 2019;11(7):1580.
Yu J, Yi Q, Chen G, Hou L, Liu Q, Xu Y, et al. The visceral adiposity index and risk of type 2 diabetes mellitus in China: A national cohort analysis. Diabetes Metab Res Rev. 2022;38(3): e3507.
Kouli GM, Panagiotakos DB, Kyrou I, Georgousopoulou EN, Chrysohoou C, Tsigos C, et al. Visceral adiposity index and 10-year cardiovascular disease incidence: The ATTICA study. Nutr Metab Cardiovasc Dis. 2017;27(10):881–9.
Zhang Y, He Q, Zhang W, Xiong Y, Shen S, Yang J, et al. Non-linear Associations Between Visceral Adiposity Index and Cardiovascular and Cerebrovascular Diseases: Results From the NHANES (1999–2018). Front Cardiovasc Med. 2022;9: 908020.
Chen HY, Chiu YL, Chuang YF, Hsu SP, Pai MF, Yang JY, et al. Visceral adiposity index and risks of cardiovascular events and mortality in prevalent hemodialysis patients. Cardiovasc Diabetol. 2014;13:136.
Wen J, Yuan H. Independent association between the visceral adiposity index and microalbuminuria in patients with newly diagnosed type 2 diabetes. Diabetes Metab Res Rev. 2020;36(1): e3198.
Fryar CD, Ostchega Y, Hales CM, Zhang G, Kruszon-Moran D. Hypertension Prevalence and Control Among Adults: United States, 2015–2016. NCHS Data Brief. 2017;289:1–8.
Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and Trends in Diabetes Among Adults in the United States, 1988–2012. JAMA. 2015;314(10):1021–9.
Perez-Lasierra JL, Casajus JA, González-Agüero A, Moreno-Franco B. Association of physical activity levels and prevalence of major degenerative diseases: Evidence from the national health and nutrition examination survey (NHANES) 1999–2018. Exp Gerontol. 2022;158: 111656.
Lei L, Dai L, Zhang Q, Lu J, Tang Y, Xiao M, et al. The Association Between Visceral Adiposity Index and Worsening Renal Function in the Elderly. Front Nutr. 2022;9: 861801.
Fang T, Zhang Q, Wang Y, Zha H. Diagnostic value of visceral adiposity index in chronic kidney disease: a meta-analysis. Acta Diabetol. 2023;60(6):739–48.
Yan LJ, Zeng YR, Chan-Shan Ma RN, Zheng Y. J-shaped association between the visceral adiposity index and all-cause mortality in patients with chronic kidney disease. Nutrition. 2022;103–104: 111832.
Wang J, Jin X, Chen K, Yan W, Wang A, Zhu B, et al. Visceral adiposity index is closely associated with urinary albumin-creatinine ratio in the Chinese population with prediabetes. Diabetes Metab Res Rev. 2021;37(7): e3424.
Ou YL, Lee MY, Lin IT, Wen WL, Hsu WH, Chen SC. Obesity-related indices are associated with albuminuria and advanced kidney disease in type 2 diabetes mellitus. Ren Fail. 2021;43(1):1250–8.
Zhou C, Zhang Y, Yang S, He P, Wu Q, Ye Z, et al. Associations between visceral adiposity index and incident nephropathy outcomes in diabetic patients: Insights from the ACCORD trial. Diabetes Metab Res Rev. 2023;39(3): e3602.
Kim B, Kim GM, Oh S. Use of the visceral adiposity index as an indicator of chronic kidney disease in older adults: comparison with body mass index. J Clin Med. 2022;11(21):6297.
Seong JM, Lee JH, Gi MY, Son YH, Moon AE, Park CE, et al. Gender difference in the association of chronic kidney disease with visceral adiposity index and lipid accumulation product index in Korean adults: Korean National Health and Nutrition Examination Survey. Int Urol Nephrol. 2021;53(7):1417–25.
Otunctemur A, Dursun M, Ozer K, Horsanali O, Ozbek E. Renal Cell Carcinoma and Visceral Adipose Index: a new risk parameter. Int Braz J Urol. 2016;42(5):955–9.
Fukuhara H, Nishida H, Nawano T, Takai S, Narisawa T, Kanno H, et al. Changes in Visceral and Subcutaneous Adipose Tissue and Body Composition in Kidney Transplant Recipients at 1, 3, and 5 Years After Kidney Transplant. Transplant Proc. 2022;54(2):351–4.
Semins MJ, Shore AD, Makary MA, Magnuson T, Johns R, Matlaga BR. The association of increasing body mass index and kidney stone disease. J Urol. 2010;183(2):571–5.
Alfandary H, Haskin O, Davidovits M, Pleniceanu O, Leiba A, Dagan A. Increasing Prevalence of Nephrolithiasis in Association with Increased Body Mass Index in Children: A Population Based Study. J Urol. 2018;199(4):1044–9.
West B, Luke A, Durazo-Arvizu RA, Cao G, Shoham D, Kramer H. Metabolic syndrome and self-reported history of kidney stones: the National Health and Nutrition Examination Survey (NHANES III) 1988–1994. Am J Kidney Dis. 2008;51(5):741–7.
Sönmez MG, Kılınç MT, Göksoy İ, Kozanhan B, Aydın A, Balasar M, et al. Visceral adiposity index in kidney stone patients who have undergone surgery. Cent European J Urol. 2022;75(2):191–8.
Hung JA, Li CH, Geng JH, Wu DW, Chen SC. Dyslipidemia increases the risk of incident kidney stone disease in a large Taiwanese population follow-up study. Nutrients. 2022;14(7):1339.
Mosli HA, Mosli HH, Kamal WK. Kidney stone composition in overweight and obese patients: a preliminary report. Res Rep Urol. 2013;5:11–5.
Khan SR, Pearle MS, Robertson WG, Gambaro G, Canales BK, Doizi S, et al. Kidney stones Nat Rev Dis Primers. 2016;2:16008.
Kelly C, Geraghty RM, Somani BK. Nephrolithiasis in the Obese Patient. Curr Urol Rep. 2019;20(7):36.
Taguchi K, Okada A, Hamamoto S, Iwatsuki S, Naiki T, Ando R, et al. Proinflammatory and Metabolic Changes Facilitate Renal Crystal Deposition in an Obese Mouse Model of Metabolic Syndrome. J Urol. 2015;194(6):1787–96.
Liu CJ, Tsai YS, Huang HS. Atorvastatin decreases renal calcium oxalate stone deposits by enhancing renal osteopontin expression in hyperoxaluric stone-forming rats fed a high-fat diet. Int J Mol Sci. 2022;23(6):3048.
Besiroglu H, Ozbek E. Association between blood lipid profile and urolithiasis: A systematic review and meta-analysis of observational studies. Int J Urol. 2019;26(1):7–17.
Cai C, Mai Z, Deng T, Zhao Z, Zhu W, Wen Y, et al. Impact of dyslipidemia on 24-h urine composition in adults without urolithiasis. Lipids Health Dis. 2018;17(1):250.
Acknowledgements
Not applicable.
Funding
The First batch of key Disciplines On Public Health in Chongqing.
Author information
Authors and Affiliations
Contributions
DL and CL analyzed and interpreted the data and wrote the paper. MY reviewed the manuscript. All authors approved the final submission.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The studies involving human participants were reviewed and approved by the NCHS Ethics Review Board. Written informed consent was obtained from all participants in this study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplemental Table 1.
Multivariate logistic regression models of kidney stones.
Additional file 2: Supplemental Table 2.
Multivariate logistic regression models of kidney stone recurrence.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Liang, D., Liu, C. & Yang, M. The association of visceral adiposity index with the risk of kidney stone and kidney stone recurrence. BMC Nephrol 24, 368 (2023). https://doi.org/10.1186/s12882-023-03421-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12882-023-03421-w