Abstract
Background
Acute kidney disease (AKD) evolves a spectrum of acute and subacute kidney disease requiring a global strategy to address. The present study aimed to explore the impact of AKD on the prognosis of ischemic stroke.
Methods
The Third China National Stroke Registry (CNSR-III) was a nationwide registry of ischemic stroke or transient ischemic attack between August 2015 and March 2018. As a subgroup of CNSR-III, the patients who had serum creatinine (sCr) and serum cystatin C (sCysC) centrally tested on admission and at 3-month, and with 1-year follow-up data were enrolled. Modified AKD criteria were applied to identify patients with AKD during the first 3 months post stroke according to the guidelines developed by the Kidney Disease: Improving Global Outcomes in 2012. The primary clinical outcome was 1-year all-cause death, and secondary outcomes were stroke recurrence and post stroke disability.
Results
Five thousand sixty-five patients were recruited in the study. AKD was identified in 3.9%, 6.7%, 9.9% and 6.2% of the patients by using sCr, sCr-based estimated glomerular filtration rate (eGFRsCr), sCysC-based eGFR (eGFRsCysC), and combined sCr and sCysC-based eGFR (eGFRsCr+sCysC) criteria, respectively. AKD defined as sCr or eGFRsCr criteria significantly increased the risk of all-cause mortality (adjusted HR 2.67, 95% CI: 1.27–5.61; adjusted HR 2.19, 95% CI: 1.17–4.10) and post stroke disability (adjusted OR 1.60, 95% CI: 1.04–2.44; adjusted OR 1.51, 95% CI: 1.08–2.11). AKD diagnosed by eGFRsCysC or eGFRsCr+sCysC criteria had no significant impact on the risk of all-cause death and post stroke disability. AKD, defined by whichever criteria, was not associated with the risk of stroke recurrence in the adjusted model.
Conclusions
AKD, diagnosed by sCr or eGFRsCr criteria, were independently associated with 1-year all-cause death and post stroke disability in Chinese ischemic stroke patients.
Similar content being viewed by others
Background
Acute kidney disease (AKD) was first proposed by the Kidney Disease: Improving Global Outcomes (KDIGO) Clinical Practice Guideline for Acute Kidney Injury (AKI) in 2012 [1]. The definition of AKD includes AKI, or a glomerular filtration rate (GFR) < 60 mL/min/1.73m2 for < 3 months, or a decrease in GFR by ≥ 35% for < 3 months, or an increase in serum creatinine (sCr) by > 50% for < 3 months, or any kidney damage lasting < 3 months [1]. The conceptual frame work of AKD is intended to cover the entire spectrum of acute and subacute stages which might not fulfill the strict criteria for AKI or chronic kidney disease (CKD), but still requires medical attention to prevent adverse outcomes. Up to now, limited clinical studies have characterized AKD [2, 3]. Accumulated evidence demonstrates that stroke patients with AKI or CKD would have higher risk of mortality, stroke recurrence and disability, but less is known about the impact of AKD on the prognosis of ischemic stroke [4,5,6,7].
The Third China National Stroke Registry (CNSR-III) was a cohort study in patients with acute ischemic stroke or transient ischemic attack (TIA) [8]. Two renal function markers, sCr and serum cystatin C (sCysC), were centrally tested on admission and at 3-month follow-up. Therefore, valuable data were available for initial estimation of a specific type of AKD, which focused on the changes in kidney function during the 3-month after stroke attack. We attempted to show whether stroke patients who developed AKD would be at higher risk of subsequent worse stroke outcomes.
Methods
CNSR-III was a national, hospital-based, prospective study between August 2015 and March 2018, designed to evaluate the aetiology, imaging and biological markers for the prognosis of ischemic stroke or TIA. Patients older than 18 years old, and within 7 days from the onset of symptoms were enrolled. Special personnel were assigned to control the quality of data during the implementation of the project. Data clean was also conducted. Details of the CNSR-III cohort were described elsewhere [8]. In this subgroup study of CNSR-III, the population included those with sCr and sCysC centrally tested on admission and at 3-month after admission, and with 1-year follow-up data from CNSR-III. Patients’ baseline information was collected within 24 h after admission through a face-to-face interview by research coordinators. Demographic information included age, gender, body mass index (BMI) calculated as weight/height (kg/m2). Medical history included a history of diabetes, hypertension, dyslipidemia, and coronary heart disease. Patients were classified into different subtypes according to the TOAST criteria (Trial of Org 10,172 in Acute Stroke Treatment): large artery atherosclerosis, cardioembolism, small artery occlusion, other determined etiology and undetermined etiology [9]. Stroke severity was assessed by the National Institute of Health Stroke Scale (NIHSS) and disability was assessed by the modified Rankin Scale (mRS) within 24 h after admission [8]. The medications used during hospitalization, including dehydrant and angiotensin converting enzyme inhibitors/angiotensin receptor blockers (ACEI/ARBs), were collected.
In the present study, serum biomarkers for renal function evaluation included sCr and sCysC, and there were two time points for evaluating renal function. The blood samples were collected on the first day of enrolment and at 3-month, and stored in cryotube at − 80 °C refrigerator at clinical sites. The samples were transported through cold chain to the central laboratory in Beijing Tiantan Hospital, where all serum specimens were stored at − 80 °C until testing was performed. The value of sCr was measured by enzymatic method (sarcosine oxidase-PAP) using a commercial kit (Beckman Coulter, Brea, CA, USA) according to the manufacturer’s protocol. The value of sCysC was measured by the immunoturbidimetric method (Roche cobas c501 analyzer with Cystatin C assay), which had an approximate coefficient of variation of 2%. Study technicians running the assays were blinded to the participant’s clinical information. In order to capture more people who truly had AKD but did not have renal function data before the stroke attack, modified KDIGO AKD criteria was applied using a similar methodology to AKI studies [1, 10, 11]. (1) AKD defined by sCr: sCr measured at 3-month increases or decreases by > 50% of the value on admission; (2) AKD defined by estimated GFR (eGFR): eGFR measured at 3-month increases or decreases by ≥ 35% of the value on admission. eGFR were calculated as sCr-based eGFR (eGFRsCr), sCysC-based eGFR (eGFRsCysC), and combined sCr and sCysC-based eGFR (eGFRsCr+sCysC) using the equations from the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) [12].
Primary outcome was all-cause death which occurred from 3-month to 1-year. Secondary outcomes included stroke recurrence defined as new ischemic stroke and haemorrhagic stroke from 3-month to 1-year, and post stroke disability defined by scores on the mRS range from 3 to 6 at 1-year.
The SAS 9.4 (SAS Institute Inc., Cary, NC) statistical analysis software was used for data processing. Continuous variables were expressed as mean with standard deviation (SD) or median with interquartile range (IQR); categorical variables were expressed as number with percentage. Continuous variables between groups were compared using t test, or Mann–Whitney U test; categorical variables were compared using χ2 test. Survival curves were estimated by the Kaplan–Meier method and compared by the log rank test. Cox proportional hazard regression model or multivariate logistic regression analysis was used for assessment of variables that were associated with all-cause death, stroke recurrence or stroke disability by calculating hazard ratios / odds ratio (HR/OR) and 95% confidence interval (CI). P value less than 0.05 was considered statistically significant. The proportional hazard assumption for the Cox regression model was examined by including a time-dependent covariate with interaction of AKD into the model. Multicollinearity analysis, and analysis of the interaction between AKD and variables in the Cox proportional hazard model were performed.
Results
Among 15,166 participants in the CNSR-III, 12,603 participants participated in biomarker substudy, and 11,261 participants were with blood sample sent to the central laboratory. There were 827 patients excluded due to without sCr or sCysC data on admission, and 5,308 patients were excluded without sCr or sCysC data at 3-month. 61 patients were lost to follow-up. Eventually, 5,065 patients were included in this study, shown in Fig. 1. The study population was from 171 hospitals in 25 provinces and 4 municipalities across China. The baseline clinical characteristics of patients on admission were presented in Table 1. Demographic data showed that 59.9% of patients were older than 60 years, 68.2% were male, and 67.9% had the NIHSS score less than 5 on admission. There was 6.4% of patients with eGFRsCr < 60 mL/min/1.73m2, 14.6% with eGFRsCysC < 60 mL/min/1.73m2, and 8.4% with eGFRsCr+sCysC < 60 mL/min/1.73m2 on admission, respectively (Table 1). The median value of sCr was 69.0 (58.0–80.0) umol/L on admission, and 68.0 (58.0–80.0) umol/L at 3-month. The median value of sCysC was 0.94 (0.83–1.07) mg/L on admission, and 1.01 (0.89–1.18) mg/L at 3-month. sCr levels at 3-month decreased by 1.4% of the value on admission, and sCysC increased by 7.4%. Demographic data about excluded patients were shown in Supplemental Material (Table S1, available as online supplementary material).
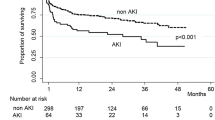
Of the 5,065 patients, AKD was diagnosed in 198 (3.9%), 337 (6.7%), 500 (9.9%) and 314 (6.2%) patients based on sCr, eGFRsCr, eGFRsCysC, and eGFRsCr+sCysC, respectively (Table 1). AKD patients, compared to non-AKD patients, were more likely to have a history of diabetes and hypertension, had higher NIHSS score and higher percentage of eGFR < 60 mL/min/1.73m2 on admission (Table 1). Since 3 months after patient enrolment, 71 (1.4%) deaths, and 184 (3.6%) stroke recurrence, have occurred within the subsequent 9-month follow-up. The specific causes of death for the 71 patients were as follows: ischemic stroke, 7 patients; hemorrhagic stroke, 12 patients; acute myocardial infarction and other cardiovascular death, 7 patients; non-vascular death and undetermined cause, 45 patients. 491 (9.7%) post stroke disability occurred at 1-year. All of the proportional hazard assumptions were met (P value greater than 0.05), and the statistical analysis results were listed in Table S2 (available as online supplementary material). As shown in Table 2, AKD, defined as sCr or eGFRsCr-based criteria, significantly increased the risk of all-cause mortality even adjusted by the confounders. AKD diagnosed by eGFRsCysC or eGFRsCr+sCysC-based criteria had no significant impact on the adjusted HR of primary outcome. Similarly, sCr or eGFRsCr-based, but not eGFRsCysC or eGFRsCr+sCysC-based AKD, remarkably increased the risk of post stroke disability. Notably, AKD, no matter which criteria was used, was not associated with the risk of stroke recurrence in the adjusted model. No obvious collinearities between variables in Cox proportional hazard model were found (all variance inflation factors far less than 10), as shown in Table S3 (available as online supplementary material). The Kaplan–Meier estimates of probability of the primary and secondary outcomes were shown in Fig. 2 and Fig. 3.
Kaplan–Meier estimates of probability of all-cause death by the development of AKD. (A) sCr-based criteria, (B) eGFRsCr-based criteria (C) eGFRsCysC-based criteria, and (D) eGFRsCr+sCysC-based criteria. X-coordinate from 3-month to 1-year. AKD, acute kidney disease; sCr, serum creatinine; sCysC, serum cystatin C; eGFR, estimated glomerular filtration rate
Kaplan–Meier estimates of probability of stroke recurrence by the development of AKD. (A) sCr-based criteria, (B) eGFRsCr-based criteria (C) eGFRsCysC-based criteria, and (D) eGFRsCr+sCysC-based criteria. X-coordinate from 3-month to 1-year. AKD, acute kidney disease; sCr, serum creatinine; sCysC, serum cystatin C; eGFR, estimated glomerular filtration rate
Discussion
This is the first study, to our knowledge, to investigate the impact of AKD on the clinical outcomes of ischemic stroke. The results showed that AKD, defined by sCr or eGFRsCr-based criteria, significantly increased the risk of all-cause death and post stroke disability during a 1-year follow-up after ischemic stroke.
AKI is an abrupt decrease in kidney function occurring over 7 days or less, whereas CKD is defined by the persistence of kidney disease for a period of ≥ 3 months. In 2012, the KDIGO AKI workgroup proposed the term AKD to define any acute kidney diseases and disorders lasting for a period of < 3 months, that encompasses both AKI and any newly recognized kidney disease that does not meet the current definitions for AKI or CKD [1]. In this study, we used similar methodology to AKI studies to modify AKD criteria developed by KDIGO in 2012, as increase or decrease in sCr > 50% or eGFR ≥ 35% of the admission level at 3 months post admission [1, 10, 11]. Previous studies have demonstrated the concomitant renal dysfunction in patients with stroke, either AKI or CKD, were associated with morbidity and mortality [4,5,6,7]. However, the impact of AKD on the clinical outcomes after stroke has not been investigated. In the study, the risk of all-cause mortality was 2.67 folds in those with AKD defined by sCr criteria compared to those without AKD. Development of AKD also increased the risk of post stroke disability (adjusted OR 1.60, 95% CI: 1.04–2.44), suggesting that AKD occurred within 3 months post stroke may negatively impact the outcome of stroke. Given that sCr is accessible and inexpensive, the present study supports to use sCr to detect AKD after stroke.
Cystatin C alone or in combination with creatinine was reported to strengthen the association between the eGFR and the risks of death and end-stage renal disease in general-population or CKD [13]. While in the present study, no association was observed between either eGFR-based AKD involving sCysC (eGFRsCysC or eGFRsCr+sCysC) and the 1-year clinical outcomes. Cystatin C, as an inhibitor of cysteine proteases, plays an important role in the pathogenesis of atherosclerosis [14]. The sCysC concentrations was reported to be significantly higher in patients with acute ischemic stroke than in the control group, and sCysC was independently associated with acute ischemic stroke [15]. On the other hand, increased cystatin C was suggested to be involved in endogenous neuroprotection, and exogenous cystatin C was found to exerte neuroprotective effects by reducing infarct volume in the animal stroke model [16, 17]. In the present study, the sCysC values increased by 7.4% at 3-month compared with admission, in contrast to sCr which was relatively stable (down 1.4%). Whether sCysC up-regulation represents a neuroprotective compensatory response, warrants further investigation. We hypothesized that, factors associated with ischemic stroke, may influence sCysC levels at 3 months and therefore weaken the relationship between eGFRsCysC or eGFRsCr+sCysC-based AKD and clinical outcomes.
There were some limitations. Firstly, two-thirds of patients were excluded due to without blood sample or missing sCr or sCysC data. The excluded patients were those with higher NIHSS scores on admission, thus the prevalence of AKD in stroke population may be underestimated. Secondly, as the CNSR-III was carried out in 2015, the updated AKD definition by the Acute Disease Quality Initiative 16 Workgroup in 2017 was not applied in the study [18], and AKI could not be defined due to the lack of the renal function data during 7 days. Thirdly, we had incomplete data on assessment of muscle mass and other clinical conditions that might affect sCr or sCysC levels independently of GFR. More in-depth evaluation in the clinical practice was warranted.
Conclusions
In summary, AKD, defined by sCr or eGFRsCr criteria, was independently associated with 1-year all-cause mortality and stroke-induced disability in patients with acute ischemia stroke. Monitoring the change in sCr on a regular basis after attack of stroke may help to identify patients with AKD who are at high risk of adverse outcomes of stroke.
Availability of data and materials
Anonymized data are available to researchers on request for reproducing the results or replicating the procedures by contacting the corresponding author.
Abbreviations
- AKD:
-
Acute kidney disease
- CNSR-III:
-
The Third China National Stroke Registry-III;
- KDIGO:
-
Kidney Disease Improving Global Outcomes
- AKI:
-
Acute Kidney Injury
- CKD:
-
Chronic kidney disease
- sCr:
-
Serum creatinine
- sCysC:
-
Serum cystatin C
- eGFR:
-
Estimated glomerular filtration rate
- TIA:
-
Transient ischemic attack
- NIHSS:
-
National Institutes of Health Stroke Scale
- mRS:
-
Modified Ranking Scale
- BMI:
-
Body mass index
- TOAST:
-
Trial of Org 10,172 in Acute Stroke Treatment
- LAA:
-
Large-artery atherosclerosis
- SAO:
-
Small-artery occlusion
- CE:
-
Cardioembolism
- ACEI/ARBs:
-
Angiotensin converting enzyme inhibitors/angiotensin receptor blockers
- CKD-EPI:
-
Chronic Kidney Disease Epidemiology Collaboration
- OR:
-
Odds ratios
- HR:
-
Hazard ratio
- CI:
-
Confidence intervals
- SD:
-
Standard deviation
- IQR:
-
Interquartile range
References
National Kidney Foundation: Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2:1–138.
Chu R, Li C, Wang S, Zou W, Liu G, Yang L. Assessment of KDIGO definitions in patients with histopathologic evidence of acute renal disease. Clin J Am Soc Nephrol. 2014;9:1175–82.
Yahalom G, Schwartz R, Schwammenthal Y, Merzeliak O, Toashi M, Orion D, et al. Chronic kidney disease and clinical outcome in patients with acute stroke. Stroke. 2009;40:1296–303.
Mizuguchi KA, Huang CC, Shempp I, Wang J, Shekar P, Frendl G. Predicting kidney disease progression in patients with acute kidney injury after cardiac surgery. J Thorac Cardiovasc Surg. 2018;155:2455–63.
Tsagalis G, Akrivos T, Alevizaki M, Manios E, Theodorakis M, Laggouranis A, et al. Long-term prognosis of acute kidney injury after first acute stroke. Clin J Am Soc Nephrol. 2009;4:616–22.
Tsagalis G, Akrivos T, Alevizaki M, Manios E, Stamatellopoulos K, Laggouranis A, et al. Renal dysfunction in acute stroke: an independent predictor of long-term all combined vascular events and overall mortality. Nephrol Dial Transplant. 2009;24:194–200.
Covic A, Schiller A, Mardare NG, Petrica L, Petrica M, Mihaescu A, et al. The impact of acute kidney injury on short-term survival in an Eastern European population with stroke. Nephrol Dial Transplant. 2008;23:2228–34.
Wang Y, Jing J, Meng X, Pan Y, Wang Y, Zhao X, et al. The Third China National Stroke Registry (CNSR-III) for patients with acute ischaemic stroke or transient ischaemic attack: design, rationale and baseline patient characteristics. Stroke Vasc Neurol. 2019;4:158–64.
Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41.
Mehta RL, Burdmann EA, Cerdá J, Feehally J, Finkelstein F, García-García G, et al. Recognition and management of acute kidney injury in the International Society of Nephrology 0by25 Global Snapshot: a multinational cross-sectional study. Lancet. 2016;387:2017–25.
Yang L, Xing G, Wang L, Wu Y, Li S, Xu G, et al. Acute kidney injury in China: a cross-sectional survey. Lancet. 2015;386:1465–71.
Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367:20–9.
Shlipak MG, Matsushita K, Ärnlöv J, Inker LA, Katz R, Polkinghorne KR, et al. Cystatin C versus creatinine in determining risk based on kidney function. N Engl J Med. 2013;369:932–43.
Loew M, Hoffmann MM, Koenig W, Brenner H, Rothenbacher D. Genotype and plasma concentration of cystatin C in patients with coronary heart disease and risk for secondary cardiovascular events. Arterioscler Thromb Vasc Biol. 2005;25:1470–4.
Wang Y, Li W, Yang J, Zhang M, Tian C, Ma M, et al. Association between Cystatin C and the risk of ischemic stroke: a systematic review and meta-analysis. J Mol Neurosci. 2019;69:444–9.
D’Adamio L. Role of cystatin C in neuroprotection and its therapeutic implications. Am J Pathol. 2010;177:2163–5.
Fang Z, Deng J, Wu Z, Dong B, Wang S, Chen X, et al. Cystatin C is a crucial endogenous protective determinant against stroke. Stroke. 2017;48:436–44.
Chawla LS, Bellomo R, Bihorac A, Goldstein SL, Siew ED, Bagshaw SM, et al. Acute kidney disease and renal recovery: consensus report of the Acute Disease Quality Initiative (ADQI) 16 Workgroup. Nat Rev Nephrol. 2017;13:241–57.
Acknowledgements
Not applicable.
Funding
This study was supported by grants from the Ministry of Science and Technology of the People’s Republic of China (2018YFC1311700 and 2018YFC1311706), and grants from Beijing Municipal Commission of Health and Family Planning (No.2016–1-2041).
Author information
Authors and Affiliations
Contributions
Yilun Zhou and Dongxue Wang were responsible for conception and design of the study, and drafting the article; Yuesong Pan, and Xianglong Xiang analyzed and interpretated data; Hao Li, Yu Wu, Yang Luo, Xuewei Xie, Xianwei Wang, Xia Meng, Hong Wang, Jinxi Lin, Yong Huo, Kunihiro Matsushita, and Jing Chen provided intellectual content of critical importance to the work described; Fan Fan Hou, and Yongjun Wang, are both corresponding authors who final approval of the version to be published. The author(s) read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The CNSR-III study was performed in accordance with the Declaration of Helsinki, and was approved by ethics committee at Beijing Tiantan Hospital (IRB approval number: KY2015-001–01) and all participating centres [8]. Written informed consent was got from patient or legally authorized representative (primarily spouse, parents, adult children, otherwise indicated).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests relevant to the manuscript.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhou, Y., Wang, D., Li, H. et al. Association of acute kidney disease with the prognosis of ischemic stroke in the Third China National Stroke Registry. BMC Nephrol 23, 188 (2022). https://doi.org/10.1186/s12882-022-02817-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12882-022-02817-4