Abstract
Objective
We aimed to perform a qualitative synthesis of evidence on the role of 68Ga-Pentixafor PET in atherosclerosis.
Methods
A systematic search of the PubMed and Embase databases for studies reporting the evaluation of atherosclerotic lesions by 68Ga-Pentixafor PET was performed with a search time frame from database creation to 2022-12-26. The diagnostic test evaluation tool QUADAS-2 was used to evaluate the quality of the included literature and to perform descriptive analyses of relevant outcome indicators.
Results
A total of 6 studies with 280 patients were included. One study reported only imaging outcome metrics, while the other five studies reported imaging outcome metrics and clinical correlation metrics. For imaging outcomes, three studies reported imaging results for 68Ga-Pentixafor PET only, and the other three studies reported imaging results for comparative analysis of 68Ga-Pentixafor PET with 18F-FDG PET. For clinical correlation, three studies reported the correlation between tracer uptake and cardiovascular risk factors, one study reported the correlation between tracer uptake and plaque calcification, and one study reported the correlation between all three: tracer uptake, cardiovascular risk factors, and plaque calcification.
Conclusion
68Ga-Pentixafor PET has a good imaging effect on atherosclerotic lesions, and it is a promising imaging modality that may replace 18F-FDG PET for atherosclerosis imaging in the future. In patients with atherosclerosis, there is a clear clinical correlation between cardiovascular risk factors, tracer uptake, and plaque calcification.
Similar content being viewed by others
Introduction
Atherosclerosis is the pathological basis of cardiovascular disease. Unstable atherosclerotic plaque rupture, platelet aggregation and thrombosis lead to narrowing or occlusion of blood vessels, resulting in acute cardiovascular disease [1, 2], and it is one of the most common causes of death in the elderly. Because inflammation plays an important role in all stages of the atherosclerotic process [3], atherosclerosis is also considered to be a chronic inflammatory disease [4]. PET imaging can use biological processes to characterize high-risk features of atherosclerotic plaques that are prone to rupture. [18F]-fluorodeoxyglucose (18F-FDG) is the most commonly used radiotracer in vascular studies and can be used as a surrogate marker of plaque inflammation. However, the clinical application of 18F-FDG is somewhat limited. 18F-FDG can be taken up extensively by glucose-metabolizing cells. Structures such as the myocardium and neck can take up 18F-FDG in large amounts, which makes it difficult to accurately assess tracer uptake in the coronary arteries [5]. Therefore, the development of an alternative PET tracer with high specificity for arterial inflammation became necessary. Inflammatory cells overexpress the chemokine receptor type 4 (CXCR4), and 68Ga-Pentixafor is a novel PET tracer with high affinity and selectivity for CXCR4 [6]. Hyafil et al. reported 68Ga-Pentixafor a promising PET radiotracer that can be used to identify macrophage infiltration present in high-risk atherosclerotic plaques [7]. Therefore, we aimed to perform a qualitative synthesis of evidence on the role of 68Ga-Pentixafor PET in atherosclerosis.
Materials and methods
The study strictly followed the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analysis) guidelines, and the registration number on PROSPERO is CRD42023388079.
Search strategy
PubMed and Embase databases were searched with a search time frame of build to 2022-12-26. Due to the small amount of published literature on Pentixafor, a single search term “Pentixafor” was used for a more comprehensive search of the literature related to 68Ga-Pentixafor PET assessment of atherosclerotic lesions. The literature on 68Ga-Pentixafor PET assessment of atherosclerotic lesions was then screened one by one. A manual supplemental search was also performed for all references in the included literature.
Inclusion and exclusion criteria
Inclusion criteria
The literature was included in this study according to the principle of “PICOS”. (1) “Patients” with atherosclerosis; (2) 68Ga-Pentixafor PET as “intervention”; (3) 18F-FDG PET as a “comparator”; (4) Imaging results and clinical correlation as “outcomes” (Indicators of imaging results include site, amount, and the target-to-background ratios (TBR) of tracer uptake, agreement and correlation analysis of the two tracer uptakes); (5) Prospective or retrospective original research as “study type”.
Inclusion criteria
(1) Other types of publications, including conference abstracts, reviews, review articles, editorials and letters, etc.; (2) Articles with incomplete information and unable to extract valid data; (3) Literature with different research purposes; (4) Repeated publications.
Literature screening and data extraction
Two investigators independently screened the literature in the order of title, abstract, and full text, and independently extracted basic information about the included literature, including first author, year of publication, country, study type, disease population, age, sample size, and outcome indicators, according to a pre-designed data extraction form. If relevant data were missing in the included literature, the corresponding authors were contacted by e-mail to obtain the data. When 2 investigators disagreed, this was resolved by discussion or consultation with the corresponding authors of this article.
Quality evaluation
Two authors independently evaluated each study using the QUADAS-2 (Quality Assessment of Diagnostic Accuracy Studies) tool [8], and discrepancies were discussed and resolved by consensus with a third reviewer. The tool includes four domains: case selection, index testing, reference standard, process, and time. Each method was assessed according to the risk of bias, and the first three were also assessed according to questions of applicability. Each question is answered with “yes”, “no”, and “unclear”, and the degree of risk of bias can be judged as “low risk “, “high risk”, or “unclear risk”. Finally, the risk of bias for each included study was assessed using ReviewManager 5.4 software, and the risk of bias was plotted.
Statistical processing
The database was created with Microsoft Excel 2021 software, entered in pairs, and proofread. When combining data, if 2 or more papers reported the same outcome indicator, Meta-analysis was performed using STATA17.0. The odds ratio (OR) and its 95% confidence interval (CI) were used for statistical data, and the mean difference (MD) and its 95% CI were used for measurement data. Conversely, only descriptive analyses of outcome indicators were performed when Meta-analysis was not feasible due to the reporting of outcome indicators in a single paper or heterogeneity among study populations.
Results
Literature screening results
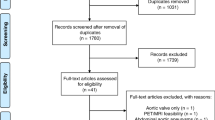
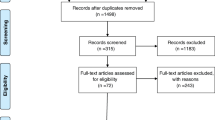
The first 478 papers were detected, including 150 papers in PubMed and 328 papers in Embase. After reading the titles and abstracts, 201 publications were removed, including conference abstract (n = 136), case (n = 20), editorial (n = 6), letter (n = 1), review (n = 31), note (n = 2) and 5 other publications. Excluding 56 papers with incompatible study subjects, including neuroendocrine tumours (n = 4), multiple myeloma (n = 13), lung cancer (n = 7), glioblastoma (n = 2), lymphoma (n = 18), myocardial infarction (n = 6), primary aldosteronism (n = 5), and Cushing’s syndrome (n = 1). Excluding 79 papers with different study purposes and duplicate publications. Further reading of the full text excluded 8 papers without clinical outcomes and was unable to extract valid data. After the screening process, 6 studies were finally included [9,10,11,12,13,14]. The flow of the included literatures is shown in Fig. 1.
Basic characteristics of included studies and quality evaluation results
Of the 6 included papers, 3 were prospective studies and 3 were retrospective studies. For the subject population, two studies were single subjects and four studies were non-single subjects. Subjects mainly include oncologic patients, patients with infection, etc. Cardiovascular risk factors included in the study included smoking, hypertension, dyslipidemia, diabetes, C-reactive protein (CRP) (≥ 3 mg/L), obesity, family history of cardiovascular disease and history of cardiovascular diseases. One study [10] reported only imaging outcome metrics and 5 studies [9, 11,12,13,14] reported imaging outcome metrics and clinical correlation metrics. Lawal et al. [10] reported imaging outcome indicators of atherosclerotic lesion uptake of 68Ga-Pentixafor and 18F-FDG; Lu et al. [9] reported imaging outcome indicators of lesion uptake of 68Ga-Pentixafor and 18F-FDG and correlation indicators of tracer uptake with cardiovascular risk factors; Kircher et al. [11] reported imaging outcome indicators of lesion uptake of 68Ga-Pentixafor and 18F-FDG and correlation indicators of tracer uptake with plaque calcification; Li et al. [12, 13] reported imaging outcome indicators of lesion uptake of 68Ga-Pentixafor and correlation indicators of tracer uptake with cardiovascular risk factors; Weiberg et al. [14] reported imaging outcome indicators of lesion uptake 68Ga-Pentixafor and also reported correlation indicators of tracer uptake with cardiovascular risk factors, correlation indicators of tracer uptake with plaque calcification and correlation indicators of cardiovascular risk factors with plaque calcification. The basic characteristics of the included studies are shown in Table 1.
Quality evaluation was performed with the QUADAS-2 tool. Risk of bias: for case selection, 5 studies [9,10,11, 13, 14] were medium risk, with the main risk arising from continuity or randomization of patient inclusion; for trials to be evaluated, 1 study [13] was high risk and 5 studies [9,10,11,12, 14] were medium risk, with the main risk arising from the implementation of blinding and the determination of thresholds; for gold standard 4 studies [10, 12,13,14] were medium risk, with the main risk arising from the implementation of the blinding method; for case, flow and progression, 2 studies [12, 13] were high risk and 1 study [14] was a medium risk, with the main risk arising from the completeness of the case inclusion analysis and the appropriate interval. All studies had a low risk of clinical applicability. The quality assessment of the included literature is shown in Fig. 2 (a) (b).
Systematic evaluation results
Limited by clinical heterogeneity with different reported outcome indicators, among other reasons, only descriptive analysis was performed in this study. (The comparison between 68Ga-Pentixafor and 18F-FDG is presented in Table 2. The abstracts of the included literatures are shown in Table 3).
Analysis of imaging results of tracer uptake in atherosclerotic lesions
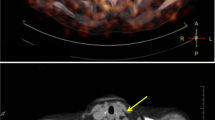
Lu et al. [9] retrospectively analyzed 19 patients with lymphoma, and in a lesion-based analysis, 68Ga-Pentixafor PET detected more lesions than 18F-FDG PET (88% vs. 48%, p < 0.001) and showed higher uptake than 18F-FDG PET (TBR: 1.90 ± vs. 1.63 ± 0.29, p < 0.001); 68Ga-Pentixafor uptake was also significantly higher than 18F-FDG in patient-based analysis (TBR: 1.85 ± 0.20 vs. 1.42 ± 0.19, p < 0.001). Lawal et al. [10] prospectively included 12 AIDS patients and performed 68Ga-Pentixafor PET and 18F-FDG PET imaging of the patients. For analysis of 18F-FDG PET imaging, TBR was elevated and statistically significant on delayed scans of both aorta (early: 1.76 ± 0.3, delayed: 2.76 ± 0.52, t: -5.738, p < 0.001) and carotid artery (early: 1.51 ± 0.38, delayed: 2.38 ± 0.66, t: -4.741, p = 0.001). significance. Correlation analysis of the two imaging modalities showed a positive correlation between the TBR of the early aorta (r = 0.344, p = 0.274), late aorta (r = 0.225,p = 0.483), early carotid artery (r = 0.123, p = 0.704) and late carotid artery (r = 0.295, p = 0.352), but neither reached statistical significance. Analysis of the agreement between the two imaging modalities showed good agreement between the two imaging modalities, and the degree of agreement was higher for early scans than for delayed scans. Kircher et al. [11] retrospectively analyzed a total of 652 lesions detected in 92 patients, and for each patient, the median number of positive lesions was 4 (0–13) for 68Ga-Pentixafor PET compared to 1 (0–10) for 18F-FDG PET, and the number of positive lesions for 68Ga-Pentixafor PET correlated moderately with 18F-FDG PET-positive lesion (r = 0.46, P < 0.0001); the mean TBR of 68Ga-Pentixafor PET was significantly higher than that of 18F-FDG PET (1.8 ± 0.5 vs. 1.4 ± 0.4, P < 0.01), and the 68Ga-Pentixafor PET and 18F-FDG PET TBR showed a weak positive correlation; based on patient analysis, individual mean TBR was significantly higher for 68Ga-Pentixafor PET than for 18F-FDG PET (1.8 ± 0.3 vs. 1.4 ± 0.3, P < 0.001 ), and there was a modest correlation (r = 0.36, P < 0.001). Li et al. [12] analyzed 72 patients and grouped them, showing that patients in group 1 (non-eccentric carotid atherosclerotic lesions, n = 27, TBRmax = 1.29 ± 0.21) had significantly lower 68Ga-Pentixafor uptake than those in group 2 (mild eccentric carotid atherosclerotic lesions, n = 67, TBRmax = 1.57 ± 0.27), group 3 (moderate eccentric atherosclerotic carotid lesions, n = 41, TBRmax = 1.64 ± 0.37) and group 4 (severe eccentric atherosclerotic carotid lesions, n = 19, TBRmax = 1.55 ± 0.26) (p < 0.05), whereas between groups 2, 3 and 4 68Ga-Pentixafor uptake were not statistically different. Li et al. [13] included 34 patients in the study and 68Ga-Pentixafor PET detected a total of 611 (TBRmax = 1.8 ± 0.4) lesions, with the descending aorta being the vessel segment with the highest number of lesions and strongest tracer uptake (n = 225, TBRmax = 1.9 ± 0.4), followed by the abdominal aorta (n = 168, TBRmax = 1.9 ± 0.4), aortic arch (n = 83, TBRmax = 1.8 ± 0.2), common carotid artery (n = 74, TBRmax = 1.7 ± 0.3) and ascending aorta (n = 61, TBRmax = 1.7 ± 0.2). Weiberg et al. [14] retrospectively analyzed a total of 1411 (TBR = 2.0 ± 0.5) lesions in 51 patients with the following uptake characteristics: right common carotid artery (n = 49, TBR = 1.7 ± 0.4), left common carotid artery (n = 55, TBR = 1.6 ± 0.4), thoracic aorta (n = 339, TBR = 1.9 ± 0.4), abdominal aorta (n = 369, TBR = 2.1 ± 0.6), right iliac artery (n = 115, TBR = 1.9 ± 0.4), left iliac artery (n = 115, TBR = 2.0 ± 0.5), right femoral artery (n = 180, TBR = 1.9 ± 0.5), and left femoral artery (n = 189, TBR = 2.1 ± 0.6).
Clinical correlation analysis of tracer uptake in atherosclerotic lesions
Lu et al. [9] retrospectively analyzed the relationship between tracer uptake and cardiovascular risk factors in 19 patients with lymphoma and showed that comparing the high-risk group (n = 9) with cardiovascular risk factors to the low-risk group (n = 10), TBR was significantly increased in active lesions of 68Ga-Pentixafor (2.02 ± 0.15 vs. 1.86 ± 0.10, p = 0.015), but this was not found for 18F-FDG (1.85 ± 0.10 vs. 1.80 ± 0.07, p = 0.149). Kircher et al. [11] analyzed the relationship between tracer uptake and plaque calcification in 92 patients and found an inverse relationship between the degree of plaque calcification and the intensity of uptake of both tracers (as measured by TBR), with non-calcified lesions (n = 467) showing the highest TBR values for both tracers (1.9 ± 0.4 and 1.5 ± 0.4, respectively), mildly calcified lesions (n = 99) showed higher TBR values for both (1.7 ± 0.4 and 1.3 ± 0.3, respectively, P < 0.01), while severely calcified lesions (n = 86) showed the lowest TBR values (1.4 ± 0.6 and 1.1 ± 0.4, respectively). TBR was higher in 68Ga-Pentixafor PET than in 18F-FDG PET when analyzing different subgroups of calcification. Li et al. [12] analyzed 72 patients and found a significant correlation between 68Ga-Pentixafor uptake (TBRmax) and the prevalence of hypertension (Pearson’s r = 0.27/ Pearson’s r r = 0.35, p < 0.05), and there was a significant correlation between the prevalence of type II diabetes mellitus (Pearson’s r = 0.27/ Pearson’s r r = 0.35, p < 0.05). Li et al. [13] analyzed the correlation between tracer intake and cardiovascular risk factors. The results showed that in patients with TBR > 1.7, patients with diabetes, hypercholesterolemia, and cardiovascular history accounted for 27.3%, 36.4%, and 36.4% respectively, while in patients with TBR ≤ 1.7, patients with diabetes, hypercholesterolemia and cardiovascular history only accounted for 0%, 8.3%, and 8.3%. (P < 0.05) This shows that when TBR > 1.7, the high-risk group of cardiovascular risk factors is more likely to appear. At the same time, by comparing and analyzing the TBR values of patients in a high-risk group and a low-risk group of cardiovascular risk factors, the results showed that the TBR values in the high-risk group were significantly higher than those in the low-risk group (1.9 ± 0.3 vs. 1.7 ± 0.2, p < 0.05). Weiberg et al. [14] retrospectively analyzed 51 patients and found significant correlations between the number of cardiovascular risk factors and the number of calcified plaques (r = 0.46, P = 0.0007), the number of lesions with tracer ingestion (r = 0.70, P < 0.0001) and TBR (r = 0.36, P = 0.009). Univariate regression analysis showed significant correlations between the number of lesions for tracer uptake and age at risk (r = 0.60, P < 0.0001), arterial hypertension (r = 0.56, P < 0.0001), hypercholesterolemia (r = 0.47, P = 0.0005), smoking history (r = 0.35, P = 0.01), and previous vascular events (r = 0.47, P = 0.0004); multiple regression analysis showed that age at risk (r = 0.50, P = 0.0003), arterial hypertension (r = 0.52, P = 0.0001), and smoking history (r = 0.36, P = 0.01) were all independently associated with atherosclerotic lesions. There was a statistically significant association between the number of lesions ingested with tracer and calcified plaque burden (r = 0.67, P < 0.0001), maximum plaque thickness (r = 0.56, P < 0.0001), and calcification score (r = 0.69, P < 0.0001), all of which described different aspects of the degree of arterial calcification. Also, there was a significant correlation between calcified plaque burden and age at risk (r = 0.51, P = 0.0001), arterial hypertension (r = 0.37, P = 0.008), and prior vascular events (r = 0.46, P = 0.0008); multiple regression analysis showed that calcified plaque burden was associated with age at risk (r = 0.49, P = 0.0003) and prior vascular events (r = 0.38, P = 0.008) were independently associated.
Discussion
Atherosclerosis is a chronic systemic disease in which inflammation is a dynamic trigger for progression [15,16,17]. Progressive systemic enlargement of atherosclerotic plaques leads to a range of debilitating cardiovascular diseases, including peripheral arterial disease, ischemic stroke, coronary artery disease, and acute myocardial infarction [18]. These diseases are the leading cause of morbidity and mortality in the United States and worldwide [19,20,21]. Conventional imaging examinations (including ultrasound, CT, and MRI angiography) have limited ability to assess the early stages of atherosclerosis [22, 23]. Therefore, molecular imaging offers an attractive opportunity to examine the pathological features of atherosclerotic disease at the microscopic level [24]. Studies have suggested that 68Ga-Pentixafor may be a potential imaging molecule for atherosclerosis, but the use of 68Ga-Pentixafor PET for imaging atherosclerotic lesions is not yet widely used in clinical practice due to the lack of evidence-based medical evidence.
To further clarify the role of 68Ga-Pentixafor PET in atherosclerotic, we aimed to perform a qualitative synthesis of evidence on the role of 68Ga-Pentixafor PET in atherosclerosis for the first time. The results of this study found that 68Ga-Pentixafor PET has better imaging results than 18F-FDG PET and can overcome some limitations of 18F-FDG PET imaging, and may be able to replace 18F-FDG PET for atherosclerosis imaging in the future. In addition, there is a clear clinical correlation between cardiovascular risk factors, tracer uptake and plaque calcification.
Specifically, a comparative analysis of imaging results between 68Ga-Pentixafor PET and 18F-FDG PET revealed good agreement and correlation between the two imaging modalities, and higher uptake of tracers (both in terms of quantity and intensity of uptake) by patients and lesions with 68Ga-Pentixafor PET compared to 18F-FDG PET. In addition, it was found that the uptake of tracers was higher in eccentric carotid atherosclerotic lesions than in non-eccentric carotid atherosclerotic lesions. In terms of clinical correlation, the uptake of tracers (both quantity and TBR) by patients and lesions increased with the number of cardiovascular risk factors in patients, the number of plaque calcifications increased with the number of cardiovascular risk factors, and the uptake of tracers by lesions decreased instead with the increased burden of plaque calcification, suggesting a positive correlation between cardiovascular risk factors and tracer uptake and plaque calcification; whereas a negative correlation existed between tracer uptake and the degree of plaque calcification.
Atherosclerosis is a global health problem. Although some progress has been made in understanding the complex underlying biology of atherosclerosis, we still need radioactive tracers targeting molecular changes in vulnerable plaques to identify vulnerable plaques and prevent adverse events. At present, the diagnostic localization of 68Ga-Pentixafor still needs to be improved. Bartlett et al. [25] summarized the efficacy of various radioactive tracers for PET imaging in plaque characterization and risk assessment. This study thought that further elucidating the potential biological mechanism of CXCR4 would help to improve the understanding of the clinical application of this radioactive tracer. In addition, an accurate estimate of the tracer uptake in vascular lesions is extremely challenging given the small size of the lesions compared to the spatial resolution of PET. Some studies [26, 27] have shown that vascular inflammation imaging with 18F-FDG PET requires optimized imaging conditions. The research results of Lawal et al. [28] have shown that vascular quantification can be improved by increasing the uptake of vascular tracers and improving the clearance of blood-pool background activity. Therefore, a set of standard imaging protocols and quantitative methods is very important for molecular imaging of vascular inflammation.Some limitations remain in this study. First, given the relative novelty of the Pentixafor tracer, only a few studies (n = 6) were available for review. Some of them were conducted by the same study group and possible overlap of patient data cannot be excluded exclusively based on the information reported in the manuscript. In addition, most studies included a different population of subjects. The TBR values defining positive atherosclerotic lesions in the included studies were not all the same. All of the above factors could be sources of heterogeneity in this study. Due to the limitations of clinical heterogeneity and different outcome indicators, only a descriptive analysis was performed in this study, and a large number of prospective randomized studies are needed in the future to further validate the clinical utility of 68Ga-Pentixafor PET application in atherosclerosis.
Conclusion
In this study, a systematic evaluation of 68Ga-Pentixafor PET for atherosclerosis imaging was performed. The results showed that 68Ga-Pentixafor PET has a good imaging effect on atherosclerotic lesions, and it is a promising imaging modality that may replace 18F-FDG PET for atherosclerosis imaging in the future. In patients with atherosclerosis, there is a clear clinical correlation between cardiovascular risk factors, tracer uptake, and plaque calcification.
Availability of data and materials
The dataset(s) supporting the conclusions of this article is(are) included within the article.
Abbreviations
- 18F-FDG:
-
[18F]-fluorodeoxyglucose
- CXCR4:
-
Chemokine receptor type 4
- TBR:
-
Target-to-background ratios
- OR:
-
Odds ratio
- CI:
-
Confidence interval
- MD:
-
Mean difference
- AIDS:
-
Acquired immune deficiency syndrome
- CRP:
-
C-reactive protein
References
Kanter JE, Kramer F, Barnhart S, Averill MM, Vivekanandan-Giri A, Vickery T, et al. Diabetes promotes an inflammatory macrophage phenotype and Atherosclerosis through acyl-CoA synthetase 1. Proc Natl Acad Sci U S A. 2012;109(12):E715–24.
Li JJ, Chen JL. Inflammation may be a bridge connecting Hypertension and Atherosclerosis. Med Hypotheses. 2005;64(5):925–9.
Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of Atherosclerosis. Nature. 2011;473(7347):317–25.
Ross R. Atherosclerosis–an inflammatory Disease. N Engl J Med. 1999;340(2):115–26.
Sriranjan RS, Tarkin JM, Evans NR, Le EP, Chowdhury MM, Rudd JH, et al. Atherosclerosis imaging using PET: insights and applications. Br J Pharmacol. 2021;178(11):2186–203.
Velikyan I. Prospective of 68Ga Radionuclide Contribution to the Development of Imaging Agents for Infection and inflammation. Contrast Media Mol Imaging. 2018;2018:9713691. Published 2018 Jan 4.
Hyafil F, Pelisek J, Laitinen I, Schottelius M, Mohring M, Döring Y, et al. Imaging the cytokine receptor CXCR4 in atherosclerotic plaques with the Radiotracer 68Ga-Pentixafor for PET. J Nucl Med. 2017;58(3):499–506.
Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155(8):529–36.
Lu X, Calabretta R, Wadsak W, Haug AR, Mayerhöfer M, Raderer M, et al. Imaging inflammation in Atherosclerosis with CXCR4-Directed [68Ga]PentixaFor PET/MRI-Compared with [18F]FDG PET/MRI. Life (Basel). 2022;12(7):1039. Published 2022 Jul 12.
Lawal IO, Popoola GO, Mahapane J, Kaufmann J, Davis C, Ndlovu H, et al. [68Ga]Ga-Pentixafor for PET Imaging of Vascular expression of CXCR-4 as a marker of arterial inflammation in HIV-Infected patients: a comparison with 18F[FDG] PET Imaging. Biomolecules. 2020;10(12):1629. Published 2020 Dec 3.
Kircher M, Tran-Gia J, Kemmer L, Zhang X, Schirbel A, Werner RA, et al. Imaging inflammation in Atherosclerosis with CXCR4-Directed 68Ga-Pentixafor PET/CT: correlation with 18F-FDG PET/CT. J Nucl Med. 2020;61(5):751–6.
Li X, Yu W, Wollenweber T, Lu X, Wei Y, Beitzke D, et al. [68Ga]Pentixafor PET/MR imaging of chemokine receptor 4 expression in the human carotid artery. Eur J Nucl Med Mol Imaging. 2019;46(8):1616–25.
Li X, Heber D, Leike T, Beitzke D, Lu X, Zhang X, et al. [68Ga]Pentixafor-PET/MRI for the detection of chemokine receptor 4 expression in atherosclerotic plaques. Eur J Nucl Med Mol Imaging. 2018;45(4):558–66.
Weiberg D, Thackeray JT, Daum G, Sohns JM, Kropf S, Wester HJ, et al. Clinical molecular imaging of chemokine receptor CXCR4 expression in atherosclerotic plaque using 68Ga-Pentixafor PET: correlation with Cardiovascular Risk factors and calcified plaque burden. J Nucl Med. 2018;59(2):266–72.
Libby P, Buring JE, Badimon L, Hansson GK, Deanfield J, Bittencourt MS, et al. Atherosclerosis Nat Rev Dis Primers. 2019;5(1):56. Published 2019 Aug 16.
Libby P, Hansson GK. From focal lipid storage to systemic inflammation: JACC Review topic of the Week. J Am Coll Cardiol. 2019;74(12):1594–607.
Libby P. Inflammation in Atherosclerosis. Nature. 2002;420(6917):868–74.
Wenger NK. Prevention of Cardiovascular Disease: highlights for the clinician of the 2013 American College of Cardiology/American Heart Association guidelines. Clin Cardiol. 2014;37(4):239–51.
Heidenreich PA, Trogdon JG, Khavjou OA, Butler J, Dracup K, Ezekowitz MD, et al. Forecasting the future of Cardiovascular Disease in the United States: a policy statement from the American Heart Association. Circulation. 2011;123(8):933–44.
Sidney S, Quesenberry CP, Jaffe MG, Sorel M, Nguyen-Huynh MN, Kushi LH, et al. Recent trends in Cardiovascular Mortality in the United States and Public Health goals. JAMA Cardiol. 2016;1(5):594–9.
Herrington W, Lacey B, Sherliker P, Armitage J, Lewington S. Epidemiology of Atherosclerosis and the potential to reduce the global burden of Atherothrombotic Disease. Circ Res. 2016;118(4):535–46.
Syed MB, Fletcher AJ, Forsythe RO, Kaczynski J, Newby DE, Dweck MR, et al. Emerging techniques in Atherosclerosis imaging. Br J Radiol. 2019;92(1103):20180309.
Takx RA, Partovi S, Ghoshhajra BB. Imaging of Atherosclerosis. Int J Cardiovasc Imaging. 2016;32(1):5–12.
Raynor WY, Borja AJ, Rojulpote C, Høilund-Carlsen PF, Alavi A. 18F-sodium fluoride: an emerging tracer to assess active vascular microcalcification. J Nucl Cardiol. 2021;28(6):2706–11.
Bartlett B, Ludewick HP, Lee S, Verma S, Francis RJ, Dwivedi G. Imaging inflammation in patients and animals: Focus on PET Imaging the vulnerable plaque. Cells. 2021;10(10):2573.
Huet P, Burg S, Le Guludec D, Hyafil F, Buvat I. Variability and uncertainty of 18F-FDG PET imaging protocols for assessing inflammation in Atherosclerosis: suggestions for improvement. J Nucl Med. 2015;56(4):552–9.
Bucerius J, Hyafil F, Verberne HJ, Slart RH, Lindner O, Sciagra R, et al. Position paper of the Cardiovascular Committee of the European Association of Nuclear Medicine (EANM) on PET imaging of Atherosclerosis. Eur J Nucl Med Mol Imaging. 2016;43(4):780–92.
Lawal IO, Mokoala KG, Popoola GO, Lengana T, Ankrah AO, Stoltz AC, et al. Impact of optimized PET imaging conditions on 18F-FDG uptake quantification in patients with apparently normal aortas. J Nucl Cardiol. 2021;28(4):1349–59.
Acknowledgements
Not applicable.
Funding
There is no funding source for this study.
Author information
Authors and Affiliations
Contributions
MW, JM, JZ, and CZ contributed to the conception and design of the study. MW organized the database. MW, LL, and JW performed the statistical analysis. MW wrote the first draft of the manuscript. JM, JZ, and LL wrote sections of the manuscript. All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, M., Zhang, J., Ma, J. et al. Imaging findings and clinical relevance of 68Ga-Pentixafor PET in atherosclerosis: a systematic review. BMC Med Imaging 23, 166 (2023). https://doi.org/10.1186/s12880-023-01134-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12880-023-01134-y