Abstract
Background
HBP, a novel biomarker released from neutrophils, may induce inflammatory responses and exacerbate vascular permeability, representing the pathophysiological characteristics of sepsis and septic shock. However, it remains uncertain whether the combination of HBP with other biomarkers yields enhanced diagnostic capacity for sepsis. We hypothesized that measurements included IL-6·IL-8·HBP, IL-6·IL-8·HBP/ALB and HBP/ALB which based on HBP will improve its diagnostic efficacy and even better than the traditional infection biomarkers.
Methods
Between July 2021 and June 2022, we carried out a comprehensive, multi-center, observational cohort study spanning six leading tertiary hospitals located in Heilongjiang Province, China. Patients were stratified into three categories based on the severity of infection: non-sepsis, sepsis, and septic shock. We collected clinical and laboratory data, along with infection and inflammation biomarkers, for analysis.
Results
A total of 195 patients were enrolled. Among the three groups, patients with septic shock (n = 75, 38.5%) had significantly higher baseline levels of HBP, WBC, Lac, CRP, PCT, IL-6, IL-8, and IL-10 compared to non-sepsis patients (n = 43, 22.0%) and sepsis patients (n = 77, 39.5%), with statistically significant differences (p < 0.05) observed for all parameters. When compared to SOFA score and traditional markers of CRP, PCT, IL-6 and IL-8, the combined indexes of IL-6·IL-8·HBP and IL-6·IL-8·HBP/ALB demonstrated significantly improved diagnostic performance for sepsis and septic shock (AUC 0.911 and 0.902 respectively, p < 0.001).
Conclusions
The combined measurements of IL-6·IL-8·HBP and IL-6·IL-8·HBP/ALB can augment the diagnostic capacity of HBP for sepsis, and offer reliable early supplementary indicators to traditional biomarkers for assessing disease severity in patients with infection.
Similar content being viewed by others
Introduction
Patients with infection that progress to sepsis or septic shock have higher mortality than those who do not [1, 2]. Numerous biomarkers and clinical scores, such as C-reactive protein (CRP), white blood cell (WBC) counts, and procalcitonin (PCT), have been reported and extensively utilized to identify high-risk patients and optimize treatment to improve their outcomes [3,4,5]. However, most of them exhibit good sensitivity, but sometimes lack the specificity to predict the worsening of infections [6,7,8]. More importantly, they cannot represent the nature and the characterization of sepsis, which include uncontrolled inflammation and increased vascular permeability, leading to hypotension and organ dysfunction. Thus, rapid and efficient predictive markers are needed for risk stratification in infected patients.
Heparin-binding protein (HBP) is stored in secretory vesicles and azurophilic granules of neutrophils (NEUT) [9], and is rapidly released from activated NEUT in response to stimulation by various bacterial products and cytokines/chemokines stimulation [10]. HBP, known for its multiple biological functions, not only possesses microbicidal properties but also induces inflammatory responses through its chemotactic effect on immune cells and contributes to increased vascular leakage [11, 12]. which is associated with the development of organ injuries in sepsis. Higher HBP concentrations in plasma are found to be closely correlated with more severe illness and poorer prognosis in septic patients [13]. In recent years, HBP has been substantiated as a valuable diagnostic and prognostic marker for sepsis [14, 15].
The prompt and early diagnosis of sepsis and septic shock continues to pose a challenge in China, largely due to the relative scarcity of critical care resources and extended duration of stay prior to ICU admission. These factors potentially exacerbate the severity of illnesses and elevate mortality rates [1]. As point-of-care testing (POCT) has evolved, a plethora of biomarkers have become increasingly accessible for the swift assessment of infection and inflammation over the past decade. These include HBP, CRP, Serum Amyloid A (SAA), PCT, and various cytokines/chemokines markers [16,17,18]. In our study, we focused on the integration of the HBP with other biomarkers to enhance the diagnostic accuracy of sepsis and septic shock. This approach was taken to mitigate the limitations inherent in relying solely on any single biomarker. By combining multiple biomarkers, we aimed to achieve a more comprehensive and reliable diagnostic tool for sepsis and septic shock, which are critical for improving patient outcomes. We posited that the amalgamation of HBP with cytokine markers and albumin (ALB) could potentially augment the diagnostic efficacy of HBP, surpassing that of existing infection biomarkers in patients with sepsis.
Methods
Study population
We conducted a multi-center observational cohort study between July 2021 and June 2022 at the intensive care unit (ICU) of six tertiary hospitals in Heilongjiang Province. There were two centers in Harbin (the Second Affiliated Hospital of Harbin Medical University, the Fourth Affiliated Hospital of Harbin Medical University), two centers in Mudanjiang (Hongqi Hospital affiliated to Mudanjiang Medical University and The Second People’s Hospital of Mudanjiang), one center in Daqing (The People’s Hospital of Daqing), and one center in Qiqihar (the First Hospital of Qiqihar). The study was registered in the Chinese clinical trial registry (ChiCTR2100047008) and approved by the ethics committee of the Second Affiliated Hospital of Harbin Medical University (KY2020-015). Adult patients (≥ 18 years) who were admitted with suspicion of sepsis, as judged by an ICU senior attending physician, were enrolled and followed up for 72 h in our study. Suspected sepsis was defined as the presence of infection combined with a qSOFA score ≥ 2 [2]. Exclusion criteria were as follows: (1) Participants with hematological diseases, autoimmune diseases, immune deficiency diseases (such as cancers under chemotherapy or radiation, HIV infections, patients taking steroids or immunosuppressants); (2) pregnancy; (3) lactation (Lac), ALB or HBP measurement failed. Based on their infection status at the time of ICU admission, patients were divided into three distinct categories: the non-sepsis group, the sepsis group, and the septic shock group. Sepsis and septic shock diagnosis was based on the third international consensus guideline (Sepsis-3) [2].
Data extraction
During the study period, the following clinical variables and laboratory results were collected: demographic characteristics, comorbidities, infection sites, microbiology, mechanical ventilation conditions, renal replacement therapy (RRT) conditions and clinical outcomes; Daily laboratory measurements include WBC, NEUT, hemoglobin (HGB), platelet (PLT), ALB, aspartate aminotransferase (AST), alanine aminotransferase (ALT), creatinine (Crea), PCT, CRP. Baseline laboratory test results were established based on the first day of patient enrollment. The severity of illness was determined by Acute Physiology and Chronic Health Evaluation II (APACHE II) score and Sequential Organ Failure Assessment (SOFA) score.
Assessment of laboratory parameters
Blood samples were collected in EDTA-K2 anticoagulant vacuum tubes and analyzed using the Sysmex XN-9000 Hematology Analyzer with corresponding reagents, adhering strictly to the manufacturer’s instructions. The test encompassed key hematological indices: WBC, NEUT, HGB, PLT. The levels of ALB, AST, ALT, Crea, PCT and CRP were measured utilizing the Roche Modular DPP automated biochemical analyzer. The lower limits of detection were as follows: WBC 0.41 × 109/L, HGB 6.93 g/L, PLT 9.28 × 109/L, ALB 3 g/L, AST 5 U/L, ALT 5 U/L, Crea 15 µmol/L, PCT 0.02 ng/mL and CRP 0.15 mg/L.
Determination of HBP levels in plasma
Blood samples for HBP analysis were collected into EDTA tubes after enrollment immediately and the following two days. After centrifuging at 3500 rpm for 10 min, the plasma was separated and stored for further test. HBP was measured by a fluorescence dry quantitative immunoassay using the Jet-iStar 3000 analyzer (JoinStar, Hangzhou, China). The lower limits of detection were as follows: HBP 5.9 ng/ml.
Measurement of cytokines and chemokines
Blood samples were also tested by luminex assay using the iMatrix100 (JoinStar, Hangzhou, China) for cytokines and chemokine, including TNF-α, IL-1β, IL-4, IL-6, IL-8 and IL-10 according to the manufacturers’ instructions. The lower limits of detection were as follows: TNF-α 1.5 pg/mL, IL-1β 1.5 pg/mL, IL-4 1.5 pg/mL, IL-6 1.5 pg/mL, IL-8 1.5 pg/mL and IL-10 1.5 pg/mL.
Statistical analysis
The categorical variables were expressed as absolute numbers and proportions and compared using Chi-square tests or Fisher’s exact test. Continuous variables that conformed to a normal distribution were presented as mean ± SD and comparisons among multiple groups are performed using one-way analysis of variance. Continuous variables that did not conform to a normal distribution were expressed as median and interquartile range (IQR) and comparisons among multiple groups are conducted using the Kruskal-Wallis H test. The optimal cut-off values for different inflammatory indices were calculated by selecting the number corresponding to the maximum Youden index according to sensitivity and specificity, and the receiver operating characteristic curve (ROC) was used to determine the differentiation of individual inflammatory indices as well as the integrated inflammatory model for sepsis. Our primary outcome was to assess the diagnostic accuracy of various biomarkers and integrated diagnostic models for sepsis, which was accomplished through the estimation of the area under the curve (AUC) of the ROC curves. Variables with P < 0.05 were first screened by univariate logistic regression analysis and then included in multivariate logistic regression analysis to screen out risk factors for death. Data were analyzed using SPSS 25.0 (IBM, Armonk, New York, USA), and P values < 0.05 was considered statistically significant. Graphs were plotted using GraphPad Prism (version: 9.4).
Results
Patients
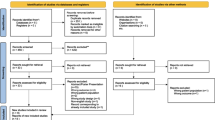
From July 2021 to June 2022, 233 patients were recruited, of whom 5 patients with ICU readmission, 18 patients with incomplete blood test data, and 15 patients who admitted to the ICU for less than 72 h were excluded (Fig. 1). Our final analysis included 195 patients in total, comprising 43 non-sepsis patients, 77 sepsis patients, and 75 septic shock patients. The demographic details of the patients are encapsulated in Table 1.
The average age of the participants was 62.16 ± 13.72 years, and males constituted 58.97% (115) of the sample. Notably, patients with septic shock demonstrated significantly higher APACHE II and SOFA scores, as well as a 28-day mortality rate, compared to those with non-sepsis and sepsis (p < 0.05). Furthermore, the proportion of patients undergoing mechanical ventilation was significantly higher in the sepsis group compared to the non-sepsis and septic shock groups. The dose of noradrenaline administered to the septic shock group was significantly higher compared to both the non-sepsis group and the sepsis group. Nevertheless, there were no significant differences observed among the three groups concerning the use of RRT.
Significant inter-group baseline differences in HBP, HBP/ALB, CRP, PCT, IL-6, and IL-10
Table 2 presents a comparison of the baseline characteristics, including Laboratory Tests, Inflammatory Marker Levels, and ALB Supplementation Dosage across the three groups. Patients with septic shock had significantly elevated serum levels of HBP, WBC, Lac, CRP, PCT, Crea, and inflammatory cytokines IL-6, IL-8, IL-10 compared to non-sepsis and sepsis patients (p < 0.05). The lowest serum concentrations of ALB and PLT were observed in septic shock patients. Remarkably, the HBP/ALB ratio was significantly higher in the septic shock group compared to the non-sepsis and sepsis groups (p < 0.05). Meanwhile, compared to non-sepsis patients, sepsis patients exhibited significantly elevated levels of HBP, CRP, PCT, IL-1β, IL-6, IL-10, and TNF-α (p < 0.05), along with a higher HBP/ALB ratio. Furthermore, we noted distinctions in the doses of ALB administered among the three groups, yet these differences lacked statistical significance.
Trends of decreased HBP, Lac, IL-6, IL-8, and IL-10 in septic shock group
Figure 2 illustrates the trends in the concentrations of infection biomarkers and cytokines over the first three days. Serum concentrations of HBP, Lac, IL-6, IL-8, and IL-10 significantly decreased in septic shock patients after three days (p < 0.05). Such substantial decreases were not evident in non-sepsis and sepsis patients. Despite a gradual decreasing trend, the SOFA score and WBC levels remained highest in septic shock patients after three days, compared to the other two groups (p < 0.05). Furthermore, the HBP/ALB ratio in the septic shock group also declined, aligning closely with that of the non-sepsis and sepsis groups. ALB levels increased in all three groups.
Comparisons of infection biomarkers among the three groups during 3 d. Serum levels of HBP (A), ALB (B), Lac (C), CRP (D), WBC (E), PCT (F), IL-6 (G), IL-8 (H), IL-10 (I) and SOFA score (J), HBP/ALB ratio (K) were displayed. P < 0.05 * vs. Non-sepsis group in the same day; # vs. Sepsis group in the same day; † vs. D2 in the same group; φ vs. D3 in the same group; respectively. HBP, heparin-binding protein; Lac, lactate; CRP, C-reactive protein; WBC, white blood cells; PCT, procalcitonin; IL, interleukin; SOFA, sequential organ failure assessment; ALB, albumin
Combined models optimize diagnostic performance for sepsis and septic shock
We performed a multivariate logistic regression and ROC curve analysis to identify independent and combined parameters associated with the diagnosis of sepsis and septic shock (refer to Table 3; Fig. 3). The diagnostic efficacy of the SOFA score and traditional markers such as Lac, WBC, CRP, PCT, IL-6, and IL-8 for sepsis and septic shock were found to be inferior to both HBP alone and the combined markers based on HBP. The respective AUCs for Lac, WBC, CRP, PCT, IL-6, and IL-8 were 0.542, 0.619, 0.734, 0.790, 0.819, and 0.663. In comparison to traditional markers and the SOFA score, the indices IL-6·IL-8·HBP and IL-6·IL-8·HBP/ALB were the most effective in diagnosing sepsis and septic shock, with AUCs of 0.911 and 0.902 respectively (p < 0.001). The diagnostic efficacy of the IL-6·IL-8·HBP combination was significantly higher than that of either HBP or HBP/ALB alone (p < 0.001). However, no significant difference was observed between IL-6·IL-8·HBP and IL-6·IL-8·HBP/ALB.
Positive correlation of HBP and its related markers with clinical scores
Correlations between laboratory values and clinical scores of all septic patients are shown in Table 4. Crea (r = 0.322, p < 0.01), WBC (r = 0.289, p < 0.01), IL-10 (r = 0.176, p < 0.05) were positively correlated with APACHE II score. Meanwhile, HBP and its related indicators had no correlation with AII score. For the SOFA score, PCT (r = 0.353, p < 0.01) exhibits the highest correlation among infection biomarkers, and both HBP and its related indicators show positive correlations with it.
APACHE II score: the sole independent risk factor for 28-day mortality in sepsis and septic shock patients
Table 5 reports the results of the univariate and multivariate analysis of patient characteristics and laboratory parameters associated with the 28-day mortality rate. A high APACHE II score emerged as the sole independent risk factor for death at 28 days, whereas the other parameters showed no significant differences.
Discussion
In our study, we examined the vascular leakage marker ALB and the inflammatory markers IL-6/IL-8, which we found to be effective supplements for improving the precision of HBP in evaluating sepsis patients. We determined that IL-6·IL-8·HBP and IL-6·IL-8·HBP/ALB could serve as early critical predictors in the ICU for assessing disease severity in infected patients. These indices demonstrated superior performance compared to HBP alone or other traditional infection biomarkers.
Sepsis and septic shock are characterized by an unregulated immune response, intense inflammation, and organ dysfunction. HBP, a novel biomarker, has been identified as a significant contributor to the pathogenesis of sepsis. It can exacerbate inflammation and enhance vascular permeability, resulting in endothelial impairment, organ dysfunction, and microcirculatory disturbances, phenomena commonly observed in sepsis and septic shock [17]. We observed a significant increase in HBP levels in septic patients, particularly in those with septic shock, followed by a noticeable decline after 3 days. Furthermore, inflammation was markedly more severe in septic shock patients at baseline, as indicated by higher WBC counts and elevated cytokine levels.
Numerous studies have noted that, following a bacterial infection, HBP is released from NEUT significantly earlier than the production of IL-8, IL-6, TNF-α, PCT and CRP [17, 19], and demonstrated superior performance in predicting and diagnosing sepsis compared to WBC, CRP, PCT and Lac [13, 15, 17, 20, 21]. Simultaneously, neither PCT nor CRP adequately captures the dynamic pathophysiological changes of sepsis, which can swiftly progress from systemic inflammation to acute organ failure. In contrast, HBP is integrally implicated in the pathological mechanisms underlying sepsis-associated organ dysfunction, specifically through its role in augmenting capillary permeability [9, 11, 12]. This heightened involvement in the disease process may account for HBP’s superior performance as a diagnostic biomarker compared to CRP, PCT, or Lac, particularly for discerning instances of evolving organ dysfunction.
Preliminary research has suggested that, at a threshold concentration of 28.1 ng/mL, HBP demonstrates a diagnostic sensitivity and specificity for sepsis of 84.9% and 78.3%, respectively [20]. In our study, we found that HBP had the best discriminative capability to distinguish sepsis from systemic infection, when compared with PCT, CRP or Lac. Notably, in our study, we observed that initial HBP levels after admission to the ICU demonstrated the highest specificity of 96.4% and a lower sensitivity of 63.2% at a cut-off value of 62.6 ng/ml. This value was higher than the similar cut-off values of 15–30 ng/mL reported in previous studies conducted with less severely ill patients in the emergency department [15, 22]. On the other hand, the correlation between HBP and its related indicators with the SOFA score appears less pronounced in comparison to that of PCT. This observation may plausibly be linked to the fact that all patients in our study cohort underwent pre-ICU interventions, which potentially masked the early stages of sepsis progression and, as a result, diminished the opportunity to discern the initial fluctuations in HBP levels.
Reduced levels of serum ALB are often observed in inflammatory diseases and have been correlated with increased capillary leakage [23]. Numerous studies have demonstrated that serum ALB serves as a powerful predictive marker for outcomes in patients with sepsis [24, 25]. A recent study indicated that a HBP/ALB ratio greater than 3.05, but not serum ALB alone, could identify septic patients at an increased risk for acute kidney injury (AKI) [26]. Additionally, ALB has been shown to inhibit the endothelial permeability induced by HBP in cases of septic AKI [27]. This suggests a strong association between ALB, HBP, and the severity of sepsis. We observed that the HBP/ALB ratio was significantly higher on D1 in septic patients, particularly those in septic shock. In subsequent periods, a significant decline in the HBP/ALB ratio was observed. This reduction was primarily attributed directly to the decrease in HBP levels itself. Additionally, changes in the HBP/ALB ratio were also partly associated with an elevation in ALB levels, which may in turn have been influenced by the administration of ALB.
IL-6 and IL-8 are central mediators in the development of HBP-mediated inflammatory responses during sepsis [17]. In our study, the levels of IL-6 and IL-8 were significantly elevated in septic patients, particularly those in septic shock. Furthermore, the diagnostic accuracy of HBP was significantly improved when combined with IL-6/IL-8, achieving the highest AUC of 0.911. Although the addition of ALB did not further enhance the diagnostic performance of the IL-6·IL-8·HBP combination, the IL-6·IL-8·HBP/ALB set still exhibited superior diagnostic capability compared to conventional biomarkers. It’s worth noting that the levels of IL-6, IL-8, IL-10, and Lac in the septic shock group significantly decreased and approximated the levels observed in the other two groups after 72 h.
Recent studies suggest that HBP or its dynamic changes may have potential prognostic implications for critically ill patients [28,29,30]. However, in our study, we found no significant associations between HBP and 28-day mortality, nor were there any significant associations with other biomarkers. These findings align with results from some previous studies [20, 31]. This discrepancy may be attributed to an underestimation of biomarker levels, potentially resulting from early extensive fluid resuscitation and vascular leakage that often occur during sepsis [31]. Meanwhile, HBP may play a protective role by regulating inflammation and host immune responses during sepsis [17]. Furthermore, the timing of measurement [28], the dynamics changes of itself [29], and the specific context of organ dysfunction [30] appear to be critical factors influencing the predictive power of HBP.
In contrast to static numerical outcomes, the sequential variations in diagnostic indices offer a more nuanced reflection of disease progression. POCT, by furnishing patients in intensive care units with real-time data upon any clinical shift, expedites the diagnostic process that would otherwise be delayed by conventional central laboratory turnaround times. This prompt accessibility to information significantly enhances the capacity of healthcare professionals to formulate immediate treatment strategies, thereby enhancing patient outcomes and prognosis [32, 33]. In the case of septic patients, real-time POCT of cytokines and chemokines could aid clinicians in easily identifying sepsis subtypes based on the inflammatory response, thereby guiding the selection of optimal therapeutic options. Given that the concentrations of HBP and inflammatory markers are currently readily available via POCT, it is recommended to obtain these immediate results for assessing severity and optimizing management in patients with infections.
Previous studies have indicated that levels of IL-6 and CRP can serve as observational indicators for the need of mechanical ventilation in COVID-19 patients [34]. In our study, we noted variations in the numbers of patients receiving mechanical ventilation across different groups, aligning with the notion that progression of sepsis exacerbates organ dysfunction. However, following a subgroup analysis distinguishing patients by the requirement for mechanical ventilation, we did not observe significant intergroup differences in several biomarkers. We speculate that this may be attributed to the majority of our study population necessitating immediate mechanical ventilation upon ICU admission, thereby bypassing the early observation window. This could have obscured potential differences that might otherwise be evident in the earlier stages of disease progression.
The current study has several limitations. For some patients, the infecting pathogens were not identified, and the correlation between HBP levels and pathogenic species was not examined. Furthermore, many patients had received treatment prior to their admission to the ICU, but the exact impact of these differing treatments on HBP levels remains unclear. Additionally, we interestingly found that the proportion of patients receiving mechanical ventilation was higher in the sepsis group compared to the septic shock group. This may be attributable to the fact that patients in the sepsis group primarily had diagnoses related to pulmonary diseases, which necessitated increased respiratory support. Finally, while our study involved various types of infections, future prospective studies with larger populations and different sepsis subtypes are necessary to confirm our results. Additionally, the clinical significance of these markers should be evaluated longitudinally during the course of sepsis.
Conclusions
In summary, our study demonstrated that the combined indices of IL-6, IL-8, and HBP, as well as IL-6, IL-8, HBP/ALB, outperformed HBP alone and other traditional infection biomarkers in diagnosing sepsis and septic shock. These findings may offer new insights into the clinical application of HBP and enhance the diagnosis and assessment of inflammation diseases mediated by HBP.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AKI:
-
Acute kidney injury
- ALB:
-
Albumin
- ALT:
-
Alanine aminotransferase
- APACHE II:
-
Acute Physiology and Chronic Health Evaluation II
- AST:
-
Aspartate aminotransferase
- AUC:
-
Area under the curve
- Crea:
-
Creatinine
- CRP:
-
C-reactive protein
- HBP:
-
Heparin-binding protein
- HGB:
-
Hemoglobin
- ICU:
-
Intensive care unit
- IQR:
-
Interquartile range
- Lac:
-
Lactation
- NEUT:
-
Neutrophils
- PCT:
-
Procalcitonin
- PLT:
-
Platelet
- POCT:
-
Point-of-care testing
- ROC:
-
Receiver operating characteristic curve
- RRT:
-
Renal replacement therapy
- SAA:
-
Serum Amyloid A
- Sepsis-3:
-
The third international consensus guideline
- SOFA:
-
Sequential Organ Failure Assessment
- WBC:
-
White blood cell
References
Xie J, Wang H, Kang Y, et al. The epidemiology of Sepsis in Chinese ICUs: a National Cross-sectional Survey. Crit Care Med. 2020;48(3):e209–18.
Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus definitions for Sepsis and septic shock (Sepsis-3). JAMA. 2016;315(8):801–10.
Raith EP, Udy AA, Bailey M, et al. Prognostic accuracy of the SOFA score, SIRS Criteria, and qSOFA score for In-Hospital mortality among adults with suspected infection admitted to the Intensive Care Unit. JAMA. 2017;317(3):290–300.
Pierrakos C, Velissaris D, Bisdorff M, Marshall JC, Vincent JL. Biomarkers of sepsis: time for a reappraisal. Crit Care. 2020;24(1):287.
Tan M, Lu Y, Jiang H, Zhang L. The diagnostic accuracy of procalcitonin and C-reactive protein for sepsis: a systematic review and meta-analysis. J Cell Biochem. 2019;120(4):5852–9.
Pan YP, Fang YP, Xu YH, Wang ZX, Shen JL. The diagnostic value of Procalcitonin Versus other biomarkers in prediction of bloodstream infection. Clin Lab. 2017;63(2):277–85.
Ljungstrom L, Pernestig AK, Jacobsson G, Andersson R, Usener B, Tilevik D. Diagnostic accuracy of procalcitonin, neutrophil-lymphocyte count ratio, C-reactive protein, and lactate in patients with suspected bacterial sepsis. PLoS ONE. 2017;12(7):e0181704.
van Oers JAH, de Jong E, Kemperman H, Girbes ARJ, de Lange DW. Diagnostic accuracy of Procalcitonin and C-reactive protein is insufficient to Predict Proven infection: a retrospective cohort study in critically ill patients fulfilling the Sepsis-3 criteria. J Appl Lab Med. 2020;5(1):62–72.
Tapper H, Karlsson A, Morgelin M, Flodgaard H, Herwald H. Secretion of heparin-binding protein from human neutrophils is determined by its localization in azurophilic granules and secretory vesicles. Blood. 2002;99(5):1785–93.
Herwald H, Cramer H, Morgelin M, Russell W, Sollenberg U, Norrby-Teglund A, Flodgaard H, Lindbom L, Bjorck L. M protein, a classical bacterial virulence determinant, forms complexes with fibrinogen that induce vascular leakage. Cell. 2004;116(3):367–79.
Linder A, Soehnlein O, Akesson P. Roles of heparin-binding protein in bacterial infections. J Innate Immun. 2010;2(5):431–8.
Gautam N, Olofsson AM, Herwald H, Iversen LF, Lundgren-Akerlund E, Hedqvist P, Arfors KE, Flodgaard H, Lindbom L. Heparin-binding protein (HBP/CAP37): a missing link in neutrophil-evoked alteration of vascular permeability. Nat Med. 2001;7(10):1123–7.
Linder A, Christensson B, Herwald H, Bjorck L, Akesson P. Heparin-binding protein: an early marker of circulatory failure in sepsis. Clin Infect Dis. 2009;49(7):1044–50.
Katsaros K, Renieris G, Safarika A, et al. Heparin binding protein for the early diagnosis and prognosis of Sepsis in the Emergency Department: the prompt Multicenter Study. Shock. 2022;57(4):518–25.
Kahn F, Tverring J, Mellhammar L, et al. Heparin-Binding Protein as a Prognostic Biomarker of Sepsis and Disease Severity at the Emergency Department. Shock. 2019;52(6):e135–45.
Pfafflin A, Schleicher E. Inflammation markers in point-of-care testing (POCT). Anal Bioanal Chem. 2009;393(5):1473–80.
Fisher J, Linder A. Heparin-binding protein: a key player in the pathophysiology of organ dysfunction in sepsis. J Intern Med. 2017;281(6):562–74.
Chao J, Cui S, Liu C, et al. Detection of early cytokine storm in patients with septic shock after abdominal surgery. J Transl Int Med. 2020;8(2):91–8.
Yang Y, Liu G, He Q, Shen J, Xu L, Zhu P, Zhao M. A Promising Candidate: Heparin-Binding Protein Steps onto the Stage of Sepsis Prediction. J Immunol Res 2019, 2019:7515346.
Zhou Y, Liu Z, Huang J, Li G, Li F, Cheng Y, Xie X, Zhang J. Usefulness of the heparin-binding protein level to diagnose sepsis and septic shock according to Sepsis-3 compared with procalcitonin and C reactive protein: a prospective cohort study in China. BMJ Open. 2019;9(4):e026527.
Wu YL, Yo CH, Hsu WT, Qian F, Wu BS, Dou QL, Lee CC. Accuracy of heparin-binding protein in diagnosing Sepsis: a systematic review and Meta-analysis. Crit Care Med. 2021;49(1):e80–90.
Linder A, Arnold R, Boyd JH, et al. Heparin-binding protein measurement improves the prediction of severe infection with organ dysfunction in the Emergency Department. Crit Care Med. 2015;43(11):2378–86.
Duran-Bedolla J, Montes de Oca-Sandoval MA, Saldana-Navor V, Villalobos-Silva JA, Rodriguez MC, Rivas-Arancibia S. Sepsis, mitochondrial failure and multiple organ dysfunction. Clin Invest Med. 2014;37(2):E58–69.
Kendall H, Abreu E, Cheng AL. Serum Albumin Trend is a predictor of mortality in ICU patients with Sepsis. Biol Res Nurs. 2019;21(3):237–44.
Arnau-Barres I, Guerri-Fernandez R, Luque S, Sorli L, Vazquez O, Miralles R. Serum albumin is a strong predictor of sepsis outcome in elderly patients. Eur J Clin Microbiol Infect Dis. 2019;38(4):743–6.
Fisher J, Linder A, Bentzer P, Boyd J, Kong HJ, Lee T, Walley KR, Russell JA. Is heparin-binding protein inhibition a mechanism of Albumin’s efficacy in human septic shock? Crit Care Med. 2018;46(5):e364–74.
Fisher J, Russell JA, Bentzer P, Parsons D, Secchia S, Morgelin M, Walley KR, Boyd JH, Linder A. Heparin-Binding Protein (HBP): A Causative Marker and Potential Target for Heparin Treatment of Human Sepsis-Induced Acute Kidney Injury. Shock 2017, 48(3):313–320.
Han X, Dou Q, Zhu Y, Ling P, Shen YH, Liu J, Zhang Z, Zhou Y, Fan M, Huang SS, Lee CC. Heparin-binding protein-enhanced quick SOFA score improves mortality prediction in sepsis patients. Front Med (Lausanne). 2022;9:926798.
Xue H, Yu F. Changes in Heparin-Binding Protein, Procalcitonin, and C-Reactive protein within the first 72 hours predict 28-Day mortality in patients admitted to the Intensive Care Unit with septic shock. Med Sci Monit. 2023;29:e938538.
Kyriazopoulou E, Dalekos GN, Metallidis S, Poulakou G, Papanikolaou IC, Tzavara V, Argyraki K, Alexiou Z, Panagopoulos P, Samarkos M, Chrysos G, Tseliou A, Milionis H, Sympardi S, Vasishta A, Giamarellos-Bourboulis EJ. HEPARIN-BINDING PROTEIN LEVELS PREDICT UNFAVORABLE OUTCOME IN COVID-19 PNEUMONIA: A POST HOC ANALYSIS OF THE SAVE TRIAL. Shock. 2024;61(3):395–9.
Chew MS, Linder A, Santen S, Ersson A, Herwald H, Thorlacius H. Increased plasma levels of heparin-binding protein in patients with shock: a prospective, cohort study. Inflamm Res. 2012;61(4):375–9.
Léguillier T, Jouffroy R, Boisson M, Boussaroque A, Chenevier-Gobeaux C, Chaabouni T, Vivien B, Nivet-Antoine V, Beaudeux JL. Lactate POCT in mobile intensive care units for septic patients? A comparison of capillary blood method versus venous blood and plasma-based reference methods. Clin Biochem. 2018;55:9–14.
Lee EJ, Shin SD, Song KJ, Kim SC, Cho JS, Lee SC, Park JO, Cha WC. A point-of-care chemistry test for reduction of turnaround and clinical decision time. Am J Emerg Med. 2011;29(5):489–95.
Herold T, Jurinovic V, Arnreich C, Lipworth BJ, Hellmuth JC, von Bergwelt-Baildon M, Klein M, Weinberger T. Elevated levels of IL-6 and CRP predict the need for mechanical ventilation in COVID-19. J Allergy Clin Immunol. 2020;146(1):128–e1364.
Acknowledgements
Not applicable.
Funding
This work was supported by the National Key Research and Development Program of China (2021YFC2501800); The Outstanding Youth Project of Heilongjiang Natural Science Foundation (Nos. JQ2021H002); The Postdoctoral Scientific Research Developmental Fund of Heilongjiang Province (Nos. LBH-Q20037 and LBH-Q21147); Key R&D Plan Project in Heilongjiang Province (No. GY2023ZB0075); Latitudinal project (070500020243); Youth Medical Research Special Fund (Z-2018-35-1902).
Author information
Authors and Affiliations
Contributions
JZ, HW conducted the study and drafted the manuscript, LF, SL, JW, YG, FX, JG and SB contributed to clinical data acquisition, LF, SJ and JW contributed to data analysis and interpretation. ZY, YL and WL guided study design and revised the manuscript, CG and MZ contributed to measurements of blood samples. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study protocol was approved by the Ethics Committee of the Second Affiliated Hospital of Harbin Medical University (KY2020-015), and signed informed consent forms were collected from all study subjects or their families.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it.The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Feng, L., Liu, S., Wang, J. et al. The performance of a combination of heparin-binding protein with other biomarkers for sepsis diagnosis: an observational cohort study. BMC Infect Dis 24, 755 (2024). https://doi.org/10.1186/s12879-024-09666-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-024-09666-6