Abstract
Background
Nocardia is an ubiquitous soil organism. As an opportunistic pathogen, inhalation and skin inoculation are the most common routes of infection. Lungs and skin are the most frequent sites of nocardiosis. Testis is a highly unusual location for nocardiosis.
Case presentation
We report the case of an immunocompromised 75-year-old-man admitted for fever of unknown origin. He presented with skin lesions after gardening and was first suspected of Mediterranean spotted fever, but he did not respond to doxycycline. Then, physical examination revealed new left scrotal swelling that was compatible with a diagnosis of epididymo-orchitis. The patient’s condition did not improve despite empirical antibiotic treatment with the onset of necrotic scrotal abscesses requiring surgery. Nocardia brasiliensis yielded from the removed testis culture. High-dose trimethoprim-sulfamethoxazole and ceftriaxone were started. Multiple micro-abscesses were found in the brain and spinal cord on imaging studies. After 6 weeks of dual antibiotic therapy for disseminated nocardiosis, slight regression of the brain abscesses was observed. The patient was discharged after a 6-month course of antibiotics and remained relapse-free at that time of writing these lines. Trimethoprim-sulfamethoxazole alone is meant to be pursued for 6 months thereafter. We undertook a literature review on previously reported cases of genitourinary and urological nocardiosis; to date, only 36 cases have been published with predominately involvement of kidney, prostate and testis.
Conclusions
To the best of our knowledge, this is the first case of Nocardia brasiliensis simultaneously infecting skin, testis, brain and spinal cord in an immunocompromised patient. Knowledge on uncommon forms of nocardiosis remains scarce. This case report highlights the difficulty of diagnosing atypical nocardiosis and the importance of prompt bacteriological sampling in case of empirical antibiotics failure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Members of the genus Nocardia are aerobic, Gram-positive, beaded, and partially acid-fast bacilli with the microscopic appearance of branching hyphae, belonging to the Corynebacterineae suborder. They are ubiquitous soil organisms. As an opportunistic pathogen, inhalation and skin inoculation are the most common routes of infection. Lungs and skin are the most frequent sites of nocardiosis. Testis is a highly unusual location for nocardiosis. We herein report the case of an immunocompromised patient with fever of unknown origin unmasking disseminated nocardiosis involving testis, brain and spinal cord. We have included a literature review on previous case reports of genitourinary and urological nocardiosis.
Case presentation
A 75-year-old man was admitted for fever of unknown origin. He had previously been diagnosed with polymyalgia rheumatica, for which a treatment with methylprednisolone 16 mg once a day (OD) was begun 4 months before admission. Methotrexate 10 mg weekly had been introduced 2 months before his admission. He had a past history of acquired amegacaryocytic thrombocytopenia that had been treated with cyclosporin more than 10 years ago.
After his two-month vacation in South of France, where he had been gardening without wearing gloves, he developed a fever above 39 °C with complaints of sore throat. Amoxicillin-clavulanic acid was started after the patient was seen in the emergency room of another hospital.
As the fever persisted, he presented to the emergency room of our institution. An atypical papular skin rash with a necrotic lesion on the back of the left hand, combined with his recent vacation location, prompted an initial suspicion of Mediterranean spotted fever (Fig. 1).
Therefore, doxycycline 100 mg BID was introduced as an empirical treatment. Antibiotics were continued for 7 days. Meanwhile, a first serologic test for Rickettsia conorii turned negative. Fever and malaise persisted for more than two weeks in total and, after a week of ineffective doxycycline, antibiotics were discontinued.
After 2 weeks of pyrexia, the patient was hospitalized in our internal medicine department. He initially complained primarily about fatigue. He had no arthralgia. Nobody close to him was sick and there was no history of animal exposure. On admission, his vital signs were as follows: body temperature 37,8 °C; pulse rate 66/min; and blood pressure 110/60 mmHg. A physical examination revealed virtual disappearance of the skin lesions and a new aortic heart murmur. First-line laboratory analyses showed an elevation of the C-reactive protein level (CRP) at 45,0 mg/L (normal value < 5,0 mg/L). Transesophageal echocardiography did not show any evidence of infective endocarditis. A chest, abdomen and brain computed tomography (CT) was unremarkable. Methotrexate was discontinued on admission.
No obvious sign of vasculitis was noted on CT brain angiography. Serologic testing for Brucella, Rickettsia conorii and R. mooseri, Coxiella burnetii, Bartonella henselae, Borrelia burgdorferi, Treponema pallidum and human immunodeficiency virus proved negative. Cytomegalovirus and Epstein-Barr virus serologies were compatible with past infection. Rheumatoid factor and antinuclear antibodies turned negative. Repeated blood cultures remained sterile even after prolonged incubation of 7 days.
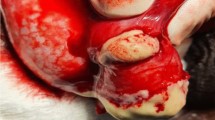
After 3 weeks of unexplained intermittent pyrexia, the patient was diagnosed with fever of unknown origin. Then, he mentioned a new scrotal swelling and left epididymo-orchitis was confirmed by ultrasound. Urinalysis was normal with no pyuria. Levofloxacin 500 mg OD was started empirically. In spite of a decrease in pain, swelling and CRP level, testis induration persisted. Scrotal abscesses appeared after one week of antibiotic therapy and despite increased levofloxacin doses, they evolved to necrosis. Left orchiectomy was performed. Clindamycin 600 mg TDS and ceftriaxone 2 g OD were started empirically. The dose of methylprednisolone was progressively reduced to a nadir of 2 mg OD.
Nocardia brasiliensis yielded from the testicular biopsy culture. High dose intravenous trimethoprim-sulfamethoxazole (TMP-SMX) (20 mg TMP/kg/day) was started on the 20th day of hospitalization. Ceftriaxone was increased to 2 g BID to treat potential brain involvement. Gadolinium contrast-enhanced magnetic resonance imaging (MRI) of the brain subsequently revealed multiple micro-abscesses mostly in the nucleus nuclei, dura-mater enhancement in the spinal bulb and ventriculitis (Fig. 2). Spinal cord MRI showed a “ring-enhancement” in right posterolateral area of the spinal cord in D12-L1, which was consistent with a 2 mm-abscess (Fig. 3).
A F-18 fluorodeoxyglucose positron emission tomography/CT was performed to assess the extent of invasive nocardiosis but was unremarkable.
Multi-susceptibility was confirmed and dual parenteral antibiotic therapy with TMP-SMX and ceftriaxone was pursued for 6 weeks.
Histopathologically, signs of inflammation were observed in the testicular biopsy, as well as filamentous branching bacilli. Direct Gram staining showed the typical gram-positive, beaded, filamentous bacilli (Fig. 4).
The colonies displayed also partial acid-fastness with the modified form of auramine-rhodamine stain (Fig. 5).
After 72 h incubation in 5% CO2 at 37 °C on sheep blood agar, colonies grew, appearing as chalky white cotton balls because of the presence of abundant aerial filaments (Fig. 6).
Nocardia brasiliensis was identified by matrix-associated laser desorption ionization-time of flight mass spectrometry (MALDI-TOF Biotyper Sirius IVD version 4.2.100; Bruker Daltonics, Bremen, Germany) with a reliable score value (2.06). To confirm the germ identification, 16S rRNA gene sequencing using universal primers (27F: 5’AGAGTTTGATCMTGGCTCAG3’ and 1492R: 5’TACGGYTACCTTGTTACGACTT3’; NF1: 5’TWACACATGCAAGTCGARCG3’ and NF2: 5’CCAACATCTCACGACACGAG3’) was performed on cultured colonies. Its yielded sequence (1025 bp) had 99.90% homology with N. brasiliensis strain DSM AUSMDU00024985 (GenBank accession no.: CP046171.1) by using the NCBI database and the EZBiocloud.
The antimicrobial susceptibility testing was performed by minimum inhibitory concentration (MIC) using E-test gradient strips (BioMérieux, Marcy l’Etoile, France) and interpreted following Clinical and Laboratory Standards Institute (CLSI) M62-ED1:2018 guidelines for Nocardia (Table 1).
Outcome
Clinical evolution was marked by a slow improvement of the patient’s general condition. Fever gradually decreased in intensity and frequency. Control brain and spinal cord MRI’s were obtained shortly before the end of the 6 weeks of parenteral bitherapy (Figs. 7 and 8): a slight regression of the brain micro-abscesses was observed, and they appeared to be less enhanced by gadolinium, while the spinal cord lesion remained stable.
High dose TMP-SMX was then switched from parenteral to oral route and pursued as a monotherapy. At the time of writing these lines, the patient has just been discharged from the rehabilitation department after almost 6 months of treatment. Another control brain MRI showed further reduction of the abscesses. Clinically, the patient still has some walking impairment that requires physiotherapy, with slow but constant improvement. TMP-SMX is meant to be pursued for 6 months thereafter.
Discussion
Nocardiosis most commonly affects immunocompromised patients but may occur in immunocompetent hosts [1,2,3,4]. Disseminated nocardiosis is defined as the involvement of at least two non-contiguous organs and/or demonstration of bloodstream infection [1, 2, 4, 5]. The most frequently infected sites are the lungs, brain and skin [3, 6, 7]. Fever at presentation is inconstant [1,2,3,4]. Diagnosing nocardiosis only by skin inspection is difficult because Nocardia lesions are not specific. They may appear as papules, pustules, nodules and skin infiltration [1, 5, 6]. In our case, with the patient’s epidemiological context, the skin lesions were considered suspicious of a rickettsial disease at the time of presentation. Nocardia skin inoculation while gardening seems to be the pathway to infection in our patient.
Nocardia infects the central nervous system (CNS) in one-third of all cases and it usually manifests as brain abscess while meningitis is rare [5, 8]. Multiple abscesses are seen in 50–80% of the patients [6]. It has been very rarely reported with N. brasiliensis. Patients sometimes present with headache, nausea, vomiting, seizure or alteration in consciousness [1, 7]. CNS invasion may nevertheless be asymptomatic and missing the diagnosis of CNS nocardiosis may cause treatment delay and failure. It has been suggested to perform systematic brain MRI to all patients with a diagnosis of nocardiosis [5, 6]. In our case, lumbar puncture was discussed but not performed because of thrombocytopenia (less than 50 000 platelets/µL), reactions to previous platelet transfusions and clinical improvement with antibiotics. Besides, Nocardia is only exceptionally identified in cerebral spinal fluid culture [8]. Although invasive nocardiosis is generally considered to occur through hematogenous dissemination, identifying Nocardia species on blood culture is very uncommon [2, 5].
Infections of the urinary and urological systems are usually caused by species of the family Enterobacteriaceae; Nocardia infection is extremely rare [4, 8, 9]. We searched PubMed for English-language reports of genitourinary and urological nocardiosis from 1970 to 2022. We found 36 complete cases previously published in the literature [9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41] (Table 2). All except four cases (nr 17, 27, 29 and 31) involved immunocompromised hosts, mostly transplant patients and patients on corticosteroids. Kidneys, prostate and testes were the most commonly infected organs. N. asteroides was the most frequent pathogen. Implication of N. brasiliensis causing urological and genitourinary infection seems rarer but all cases reported skin involvement. It has been described that this strain is more prevalent in cutaneous infections [1, 9]. In our case, N. brasiliensis is thought to have spread hematogenously from the skin to testicular, cerebral and spinal cord sites.
The diagnosis of nocardiosis requires the identification of Nocardia in a bacteriological sample. Nocardia can be isolated by culture from different samples such as sputum, bronchoalveolar lavage fluid, abscess fluid and blood [4, 7]. Because of the slow-growing nature of Nocardia, isolates can take up to 2 weeks to grow on routine culture media used in clinical laboratories, making them difficult to identify [7]. Nevertheless, identification of Nocardia species is important because antimicrobial susceptibility varies among species [1, 2, 4,5,6,7].
Optimal management for nocardiosis has not been established because of the lack of comparative controlled studies, due to the rarity of the cases. TMP-SMX remains the first-choice agent due to good responses as observed since 1950s and because of its good penetration in the CNS [1, 5, 6]. The main adverse reactions to high-dose TMP-SMX therapy are myelosuppression, hepatotoxicity, renal insufficiency and allergic reaction. Linezolid is a good alternative for disseminated and CNS nocardiosis, but its toxicity includes a high risk of myelosuppression and peripheral neuropathy [1]. Initial multidrug therapy is recommended for most forms of nocardiosis (except limited skin infection). Therapeutic changes should be based on initial therapy, susceptibility results and individual specificities. Treatment duration is generally extended to minimize the risk of disease relapse [6]. Nocardia infections may recur because of the slow replication of the pathogen and its intracellular presence [7]. Immunodeficient hosts and/or patients with CNS nocardiosis should receive at least 12 months of antimicrobial therapy (initially intravenous therapy for 4–6 weeks followed by oral agent for 6–12 months) [1, 6, 38]. Neurosurgical drainage should be considered in case of large brain abscess not responding to antimicrobial therapy. Patients with surgical and antibiotics therapy had lower mortality [6].
Conclusion
To the best of our knowledge, this is the first case of N. brasiliensis simultaneously infecting skin, testis, brain and spinal cord in an immunocompromised patient. Our case highlights the difficulty of nocardiosis diagnosis due to complex clinical manifestations. Even though pulmonary, neurological and dermatological involvement are commonly described, the two latter forms may have, as in our patient, tricky clinical presentations and the disease may spread to virtually any organ such as testis.
Knowledge on atypical forms of nocardiosis remains scarce. With our case, we aim to both raise clinician’s awareness and add our experience to the handful of cases described in the literature.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- CNS:
-
Central Nervous System
- CRP:
-
C-reactive Protein
- CT:
-
Computed Tomography
- MIC:
-
Minimum Inhibitory Concentration
- MRI:
-
Magnetic Resonance Imaging
- OD:
-
Once a Day
- TMP-SMX:
-
Trimethoprim-sulfamethoxazole
References
Wilson JW. Nocardiosis: updates and clinical overview. Mayo Clin Proc. 2012;87(4):403–7.
Williams E, Jenney AW, Spelman DW. Nocardia bacteremia: a single-center retrospective review and a systematic review of the literature. Int J Infect Dis. 2020;92:197–207.
Steinbrink J, Leavens J, Kauffman CA, Miceli MH. Manifestations and outcomes of Nocardia infections: comparison of immunocompromised and nonimmunocompromised adult patients. Medicine. 2018;97(40):e12436.
Haussaire D, Fournier PE, Djiguiba K, Moal V, Legris T, Purgus R, et al. Nocardiosis in the south of France over a 10-years period, 2004–2014. Int J Infect Dis. 2017;57:13–20.
Lerner P, Nocardiosis. Clin Infect Dis. 1996;22(6):891–903.
Margalit I, Lebeaux D, Tishler O, Goldberg E, Bishara J, Yahav D, et al. How do I manage nocardiosis? Clin Microbiol Infect. 2021;27(4):550–8.
Duggal SD, Chugh TD, Nocardiosis. A neglected disease. Med Princ Pract. 2020;29(6):514–23.
Meena DS, Kumar D, Bohra GK, Midha N, Garg MK. Clinical characteristics and treatment outcome of central nervous system nocardiosis: a systematic review of reported cases. Med Princ Pract. 2022;31(4):333–41.
Sakamaki I, Ueno A, Kawasuji H, Miyajima Y, Kawago K, Hishikawa Y, et al. Prostate abscess caused by Nocardia Farcina. IDCases. 2019;18:e00640.
Mahvi TA. Disseminated nocardiosis caused by Nocardia brasiliensis: first case report in the United States. Arch Dermatol. 1964;89(3):426–31.
Young LS, Armstrong D, Blevins A. Nocardia asteroides infection complicating neoplastic disease. Am J Med. 1971;50(3):356–67.
Diamond RD, Bennett JE. Disseminated Nocardia brasiliensis infection. Arch Intern Med. 1973;131(5):735–6.
Geelhoed GW, Myers GH. Nocardiosis of the testis. J Urol. 1974;111(6):791–3.
Strong D, Hodges C. Disseminated nocardiosis presenting as testicular abscess. Urology. 1976;7(1):57–9.
Wheeler JS, Culkin DJ, O’Connell J, Winters G. Nocardia epididymo-orchitis in an immunosuppressed patient. J Urol. 1986;136(6):1314–5.
Parmentier L, Salmon-Ceron D, Boiron P, Paul G, Guez T, Dupont B, et al. Pneumopathy and kidney abscess due to Nocardia farcinica in an HIV-infected patient. AIDS. 1992;6(8):891–3.
Shohaib S. Nocardial psoas and perinephric abscess in a renal transplant treated by surgery and antibiotics. Nephrol Dial Transpl. 1994;9(8):1209–10.
López E, Ferrero M, Lumbreras C, Gimeno C, González-Pinto I, Palengue E. A case of testicular nocardiosis and literature review. Eur J Clin Microbiol Infect Dis. 1994;13(4):310–3.
Diego Miralles G. Disseminated Nocardia farcinica infection in an AIDS patient. Eur J Clin Microbiol Infect Dis. 1994;13(6):497–500.
Salahuddin F, Sen P, Chechko S. Urinary tract infection with an unusual pathogen Nocardia asteroides. J Urol. 1996;155(2):654–5.
Frangié C, Morel D, Sassoust G, Pariente JL, Grenier N, Lacut JY, et al. A rare infection in a renal transplant recipient. Nephrol Dial Transpl. 2001;16(6):1285–7.
Benes J, Viechova J, Picha D, Horova B, Zatloukal P. Disseminated Nocardia asteroides infection in an immunocompetent woman following an arm injury. Infection. 2003;31(2):112–4.
Qu L, Strollo DC, Bond G, Kusne S. Nocardia prostatitis in a small intestine transplant recipient. Transpl Infect Dis. 2003;5(2):94–7.
Routh JC, Lischer GH, Leibovich BC. Epididymo-orchitis and testicular abscess due to Nocardia asteroides complex. Urology. 2005;65(3):591.
Severo CB, Oliveira FDM, Cunha L, Cantarelli V, Severo LC. Nocardia farcinica: diagnosis by thyroid abscess culture. Rev Inst Med Trop Sao Paulo. 2005;47(6):355–8.
Kepkep K, TunCay YA, YIgItbasI R. Nocardial tubo-ovarian abscess in a pregnant woman: a rare case report. Aust N Z J Obstet Gynaecol. 2006;46(4):363–5.
Gallo A, Bettoni G, Trezzi G, Lalinga V, Frigerio L. Primary vulvar nocardiosis. Obstet Gynecol. 2006;108(3):728–30.
Dehghani M, Davarpanah MA. Epididymo-orchitis and central nervous system nocardiosis in a bone marrow transplant recipient for acute lymphoblastic leukemia. Exp Clin Transpl. 2009;7(4):264–6.
Tanioka K, Tabu H, Uemura K, Matsumoto R, Takahashi R, Nagao M, et al. Disseminated Nocardia farcinica infection in a patient with myasthenia gravis successfully treated by linezolid: a case report and literature review. J Infect Chemoter. 2012;18(3):390–4.
De Montmollin E, Corcos O, Noussair L, Leflon-Guibout V, Belmatoug N, Joly F, et al. Retroperitoneal abscesses due to Nocardia farcinica: report of two cases in patients with malnutrition. Infection. 2012;40(1):93–6.
Yamaguchi H, Sekimoto E, Shirakami A, Shibata H, Ozaki S, Shigekiyo T, et al. Testicular nocardiosis accompanied by cutaneous lesions in an immunocompetent man. Intern Med. 2013;52(1):129–33.
Poisnel E, Roseau JB, Landais C, Rodriguez-Nava V, Bussy E, Gaillard T. Nocardia veterana: disseminated infection with urinary tract infection. Braz J Infect Dis. 2015;19(2):216–9.
Eren E, Ulu-Kilic A, Atalay A, Demiraslan H, Parkan O, Koc N. Report of an immunocompetent case with disseminated infection due to Nocardia otitidiscaviarum: identification by 16S rRNA gene sequencing. Infez Med. 2016;24(1):71–6.
Scorey H, Daniel S. Nocardia farcinica bacteraemia presenting as a prostate abscess. IDCases. 2016;5:24–6.
Bonifaz A, Espinosa-Díaz S, Argáez J, Hernández-Castro R, Xicohtencatl-Cortes J, Tirado-Sánchez A. Actinomycetoma due to Nocardia brasiliensis with extension to the ovaries. Eur J Obstet Gynecol Reprod Biol. 2017;211:224–5.
Biswas Roy S, Ross MD, Patil PD, Trepeta R, Bremner RM, Panchabhai TS. Primary Nocardia infection causing a fluorodeoxyglucose-avid right renal Mass in a redo lung transplant recipient. Case Rep Transpl. 2018;2018:1–5.
Marques C, Ribeiro M, Bonccoraglio MT, Braga J, Pereira AF, Ogando A. Disseminated nocardiosis: the complexity of the diagnosis. J Med Cases. 2021;12(5):205–8.
Pan L, Wang XH, Meng FQ, Su XM, Li Y, Xu MT, et al. Membranous nephropathy complicated with disseminated Nocardia farcinica infection: a case report and literature review. Infect Drug Resist. 2021;14:4157–66.
Shen TA, Hsu YH, Ding DC. Xanthogranulomatous inflammation caused by K. pneumonia and nocardiosis mimicking a uterine tumor and invading the ureter and colon: a case report and review of the literature. Taiwan J Obstet Gynecol. 2022;61(5):889–95.
Torres OH, Domingo P, Pericas R, Boiron P, Montiel JA, Vázquez G. Infection caused by Nocardia farcinica: case report and review. Eur J Clin Microbiol Infect Dis. 2000;19(3):205–12.
Van Luin A, Manson WL, Van Der Molen L, Van Der Heide JJH, Van Son WJ. An intrarenal abscess as presenting symptom of an infection with Nocardia farcinica in a patient after renal transplantation. Transpl Infect Dis. 2008;10(3):214–7.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
S.I. performed in-hospital clinical follow-up, wrote the original article, performed literature review and included the comments and corrections of the other authors; S.L. guided antibiotic treatment according to literature, provided clinical follow-up and edited the manuscript; MH performed microbiological analysis and edited the manuscript; JV supervised in-hospital clinical follow-up and edited the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Written informed consent was obtained from the patient for the publication of this case report and any accompanying images.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Inthasot, S., Leemans, S., Hing, M. et al. Fever of unknown origin revealing testicular nocardiosis: a case report and literature review. BMC Infect Dis 24, 614 (2024). https://doi.org/10.1186/s12879-024-09521-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-024-09521-8