Abstract
Background
COVID-19 has been a public health emergency of international concern (PHEIC) for a lengthy period of time. The novel coronavirus is primarily spread via aerosols at a short distance, with infected individuals releasing large amounts of aerosols when speaking and coughing. However, there is an open question regarding whether mouthwash could effectively reduce virus transmission during the COVID-19 pandemic and support the prevention of infection among medical workers.
Methods
Cochrane Library, PubMed, Web of Science, and Embase databases were systematically searched from the inception of each database to January 12, 2023 for currently available randomized clinical trials (RCTs) on the effect of mouthwash on novel coronavirus load in the oral cavity in COVID-19 patients. The treatment group received mouthwash for rinsing the mouth, while the control group received a placebo or distilled water for COVID-19 patients. The primary outcomes were CT value and viral load. Odds ratios (ORs) were estimated using a random-effects model. Subgroup and sensitivity analyses were performed to minimize the bias and the impact of heterogeneity.
Results
Thirteen RCTs were included. Seven studies reported the intervention effect of mouthwash on the CT value of novel coronavirus. The analysis results showed that the mouthwash group had a positive impact on the CT value of novel coronavirus [ SMD = 0.35, 95% CI (0.21, 0.50)] compared with the control group. In addition, subgroup analysis showed a significant positive effect of mouthwash on CT values in the treatment group compared with the control group, with chlorhexidine (CHX) [SMD = 0.33, 95% CI (0.10, 0.56)], povidone-iodine (PVP-I) [SMD = 0.61, 95% CI (0.23, 0.99)], or hydrogen peroxide (HP) [SMD = 1.04, 95% CI (0.30, 1.78)] as an ingredient of the mouthwash. Six studies reported the intervention effect of mouthwash on the viral load, 263 cases in the treatment group and 164 cases in the control group. The analysis results showed that there was no statistical difference between the mouthwash group and the control group in the viral load of novel coronavirus [SMD = -0.06, 95% CI (-0.18, 0.05)]. In the subgroup analysis by measurement time, there were statistically significant differences between the mouthwash and control groups for CT values [SMD = 0.52, 95% CI (0.31, 0.72)] and viral load [SMD = − 0.32, 95% CI (− 0.56, − 0.07)] within 30 min of gargling.
Conclusions
In summary, mouthwash has some efficacy in reducing the viral load of novel coronavirus, especially within 30 min after rinsing the mouth. Mouthwash containing CHX, PVP-I and HP all had significant positive effects on CT values, and PVP-I-containing mouthwash may be a promising option to control novel coronavirus infections and relieve virus-related symptoms. However, studies on the dose and frequency of use of mouthwash for infection control are still lacking, which may limit the clinical application of mouthwash.
Trial registration
Protocol registration: The protocol was registered at PROSPERO (CRD42023401961).
Similar content being viewed by others
Introduction
Since December 31, 2019, the outbreak of Coronavirus Disease 2019 (COVID-19) caused by the 2019 novel coronavirus ((2019-nCoV)) has seriously threatened public health [1]. As of May 31, 2022, the Institute for Health Metrics and Evaluation (IHME) reported 6.9 million deaths due to COVID-19, with an estimated 17.2 million deaths [2]. At the same time, the increasing prevalence of coronavirus reinfection and long-term COVID-19 have weakened millions of people, and the number continues to increase [3].
Despite efforts to contain the virus, it continues to mutate, and as recently as January 30, 2023, the Director-General of the World Health Organization announced that COVID-19 had been a public health emergency of international concern (PHEIC) over a lengthy period of time [4]. Therefore, it is crucial to prepare for potential future outbreaks with effective public infection control measures.
The novel coronavirus is primarily spread via aerosols at a short distance, with infected individuals releasing large amounts of aerosols when speaking and coughing [2]. The microorganism found in dental bioaerosols are mainly attributed to patients' nasopharyngeal secretions, saliva, blood, and dental unit waterlines [5]. With this in mind, the US Occupational Safety and Health Administration (OSHA 2020) listed the dental department as one of the occupations with the highest risk of SARS-CoV-2 transmission, indicating that healthcare professionals in the department of stomatology and otolaryngology should take measures to prevent infection when treating patients with the novel coronavirus.
Previously, preprocedural mouth rinsing has been applied before routine oral treatment as an important method for healthcare professionals to reduce contamination. Chlorhexidine (CHX) is recommended as the gold standard of mouthrinse for chemical control of supragingival biofilm. Among antiviral molecules contained in mouthwashes, hydrogen peroxide (HP), β-cyclodextrin, flavonoids, essential oils, cetylpyridinium chloride (CPC) or povidone-iodine (PVP-I) could be useful in the fight against SARS-CoV-2 [6,7,8]. In the early stage of the COVID-19 pandemic, many international guidelines and articles recommended the use of mouthwashes containing hydrogen peroxide (H2O2) and povidone-iodine (PVP-I) against SARS-CoV-2 [9, 10]. CPC is recommended for rinsing the mouth by the National Dental Center of Singapore [11]. To date, the conclusions of studies on the effect of mouthwashes on SARS-CoV-2 viral load remain inconsistent.
The aim of this study was to investigate whether mouthwash could effectively reduce virus transmission during the COVID-19 pandemic and provide evidence to support the prevention of infection among medical workers, through the collection of randomized controlled trial studies (RCTs).
Materials and methods
Protocol and registration
This meta-analysis is performed based on Cochrane Handbook for the Systematic Review of Interventions (for details, see http://training.cochrane.org/handbook) and the Preferred Reporting Items for Systematic Review and Meta-Analyses [12]. This study protocol was approved in PROSPERO (registration number: CRD42023401961).
Eligibility criteria
Inclusion criteria
Inclusion criteria followed the PICOS strategy.
P (Population): The study population consisted of adult patients who were diagnosed with COVID-19 and not allergic to mouthwash ingredients.
I (Intervention): The interventions were performed with an experimental antiseptic mouthwash.
C (Comparison): The comparisons included placebo or distilled water.
O (Outcome): The outcomes assessed were cycle threshold (CT) values of polymerase chain reaction (PCR) assay or values of viral load as copies/ml.
S (Study design): The study design belonged to randomized controlled trials (RCTs).
Exclusion criteria
Exclusion criteria followed the PICOS strategy.
P (Population): Studies in which the study population had other diseases were excluded.
I (Intervention): Studies in which mouthwash was not used as the interventions or used in conjunction with other treatments were excluded.
C (Comparison): Studies in which the comparison used other mouthwashes or mouthwash duration as controls were excluded.
O (Outcome): Studies that did not assess outcomes of interest or with incomplete data were excluded.
S (Study design): Non-randomized controlled trials, reviews, case reports, animal experiments, in vitro studies, and observational study designs were excluded. Articles without full texts were also excluded.
Search strategy
The Cochrane Library, PubMed, Web of Science, and Embase databases were searched for studies on the effect of mouthwash on novel coronavirus load in oral cavity. The search period was from inception of each database to January 12, 2023. The search strategy of a combination of subject terms and free words was used, and the search terms included "mouthwash", "mouthrinse", "COVID-19" and "SARS-CoV-2". Specific search strategies are presented in Table S1.
Literature screening and data extraction
Two researchers searched the literature in strict accordance with the inclusion and exclusion criteria, and Endnote X9 was used to manage all literature. The retrieved literature was imported into Endnote X9. After duplicate publications were excluded, the preliminarily eligible studies were screened out based on titles or abstracts, and their full texts were downloaded. After the full texts were read, the original studies that met this systematic review were screened out. Literature data were extracted and cross-checked, and units of measurement were unified. Disagreements, if any, were resolved by discussion with a third researcher. The extracted data mainly included the first author, publication year, country, type of study, sample size and age distribution of the treatment group and control group, mouthwash ingredients, follow-up time, and outcome measures.
Risk of bias assessment for the included studies
The assessment work was performed by two researchers separately and the results were cross-checked. Cochrane Handbook for Systematic Reviews of Interventions version 6.3, Chapter 8: To assess the risk of bias in a randomized trial, the Cochrane risk-of-bias tool for randomized trials (RoB 2) was adopted for quality evaluation of the included studies, and the results were cross-validated. The assessment items include seven aspects: generation of random sequences (selection bias), allocation concealment (selection bias), blinding of investigators and subjects (implementation bias), blinding evaluation for study outcomes (measurement bias), integrity of outcome data (follow-up bias), selective reporting of study results (reporting bias), and other sources (other biases). The Review Manager (RevMan) Version 5.4, and [Computer program. The Cochrane Collaboration, 2020.] software were used to draw the risk of bias graph and summary figure.
Statistical methods
Stata 15.0 software was used for statistical analysis of the included studies, including heterogeneity test, publication bias analysis, and sensitivity analysis. Continuous variables were pooled using standard mean difference (SMD) and the 95% confidence interval (CI) was calculated, while binary variables were pooled using relative risk (RR) and 95% confidence interval (CI) was calculated. Q statistic and I2 test were used to evaluate heterogeneity. P > 0.1 and I2 ≤ 50% indicated acceptable heterogeneity among studies, and the fixed effects model was adopted for meta-analysis; P ≤ 0.1 or I2 > 50% indicated greater heterogeneity among studies, and the random effects model was selected for meta-analysis. The "metabias" command was used to detect publication bias of the included studies, and for all results, P < 0.05 was considered statistically significant. When there is a publication bias exist, a funnel plot was further analyzed using the trim-and-fill method by entering the code of 'metatrim _ES _selogES, funnel'.
Results
Literature search results
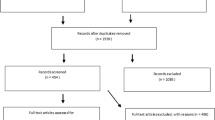
A total of 892 publications were obtained. The retrieved studies were imported into EndNote X9, and 179 duplicated articles were eliminated. A total of 651 irrelevant articles were eliminated by reading the titles and abstracts, 49 articles that did not meet the criteria were eliminated by reading the full texts, and 13 articles were finally included in the present study [11, 13,14,15,16,17,18,19,20,21,22,23,24]. Literature screening process and results are shown in Fig. 1.
Basic characteristics of the included literature
Thirteen articles were included [11, 13,14,15,16,17,18,19,20,21,22,23,24], involving 832 subjects, with 523 in the treatment group and 309 in the control group. All literature reporting related intervention indicators were English publications. The basic characteristics of the included literature are shown in Table 1.
Risk of bias assessment
The Cochrane risk-of-bias tool was used to assess the quality of the 13 included RCTs, which were at low or unknown risk in each scoring item, including the generation of random sequence, blinding, allocation concealment, integrity of outcome data, and selective reporting of study results. Assessment results are shown in Fig. 2
Meta-analysis results
Meta-analysis results of CT value
Seven studies [11, 16,17,18, 21, 23, 24] reported the intervention effect of mouthwash on the CT value of novel coronavirus, with 260 cases in the treatment group and 145 cases in the control group. The fixed effects model (I2 = 10.3%, P = 0.316) was used to pool the effect size, and the analysis results showed a positive effect in terms of CT value of novel coronavirus [SMD = 0.35, 95% CI (0.21, 0.50)] in the mouthwash group compared with the control group as shown in Fig. 3.
Subgroup analyses were performed based on measurement time and different mouthwash ingredients. Subgroup analysis by measurement time revealed that mouthwash showed a significant positive effect on CT values within 30 min [SMD = 0.52, 95% CI (0.31, 0.72)] and after six hours [SMD = 1.48, 95% CI (0.34, 2.62)] compared with the control group, while there was no statistical difference between the treatment group and the control group within the periods of 30 min-60 min [SMD = 0.16, 95% CI (− 0.14, 0.46)] and 2 h–3 h [SMD = 0.12, 95% CI (− 0.19, 0.42)] as shown in Fig. 4.
Subgroup analysis by mouthwash ingredients showed a significant positive effect on CT values in the treatment group compared with the control group with CHX [SMD = 0.33, 95% CI (0.10, 0.56)], PVP-I [SMD = 0.61, 95% CI (0.23, 0.99)], or HP [SMD = 1.04, 95% CI (0.30, 1.78)] as an ingredient of the mouthwash, and indicated no statistically significant difference between the treatment group and the control group with HCIO [SMD = 0.09, 95% CI (-0.53, 0.71)], CPC + Zn [SMD = 0.61, 95% CI (-0.11, 1.39)], HP + CHX [SMD = 0.29, 95% CI (-0.31, 0.88)] and CPC [SMD = 0.15, 95% CI (-0.15, 0.45)] as an ingredient of the mouthwash as shown in Fig. 5.
Meta-analysis results of viral load
Six studies [13,14,15, 19, 20, 22] reported the intervention effect of mouthwash on viral load, with 263 cases in the treatment group and 164 cases in the control group. The fixed effect model (I2 = 0.0%, P = 0.632) was used to pool the effect size, and the analysis results showed that there was no statistical difference in viral load value of novel coronavirus between mouthwash group and the control group [SMD = -0.06, 95% CI (-0.18, 0.05)] as shown in Fig. 6. Subgroup analysis were performed based on measurement time and different mouthwash ingredients.
Subgroup analysis by measurement time showed significantly lower viral load values within 30 min [SMD = − 0.32, 95% CI (− 0.56, − 0.07)] in the mouthwash group compared with the control group, while there was no statistical difference between the treatment group and the control group within the periods of 30 min-60 min [SMD = 0.09, 95% CI (− 0.12, 0.31)], 60 min-240 min [SMD = -0.07, 95% CI (-0.27, 0.14)] and on day seven [SMD = 0.01, 95% CI (− 0.28, 0.31)] as shown in Fig. 7.
Subgroup analysis by mouthwash ingredients showed no statistical differences between treatment group and control group, and the results were as follows: PVP-I [SMD = -0.03, 95% CI (-0.38, 0.33)], CPC [SMD = -0.01, 95% CI (-0.22, 0.20)], H2O2 [SMD = -0.25, 95% CI (-0.58,0.08)], HOCI [SMD = 0.06, 95% CI (-0.64, 0.77)], BAC [SMD = -0.39, 95% CI (-1.06.0.27)], CHX [SMD = 0.18, 95% CI (-0.20, 0.56)], β-cyclodextrin and citrox [SMD = -0.11, 95% CI (-0.32, 0.10)] as shown in Fig. 8.
Sensitivity analysis and publication bias
There were no sensitivity problems for each of the included indicators (Figure S1, S2). A funnel plot was used to visually display publication bias, and Egger's test was used to analyze the funnel plot of the included studies (Figure S3, S4). A value of P > 0.05 in Egger's test indicated no publication bias existed. For the indicators of the included literature in this study, there was bias in the CT values (p = 0.000), and no publication bias in the viral loads (Table 2). The indicators with publication bias were further analyzed with the trim-and-fill method, and the funnel plot became symmetrical after adding 10 studies to the model (Figure S5), with a pooled effect size of 0.244 (0.106, 0.383) (Table 3).
Discussion
The oral cavity is the second most complex microbiota of the human body, and most of these microorganisms are inseparable from human health. In 2019, 2019-nCoV, which caused the global respiratory infectious disease pandemic (worldwide), have been detected in the oral cavity of patients diagnosed with novel coronavirus. Studies have shown that [25] angiotensin converting enzyme 2 (ACE2), the main host cell receptor of coronavirus, is highly expressed in oral mucosa, especially the tongue epithelial cells. This also explains why high viral loads in saliva and throat swab samples from the vast majority of infected individuals are feasible for detecting novel coronavirus. Therefore, Huang et al. (2021) investigated the oral viral load in COVID-19 infected patients and concluded that the oral cavity may be one of the important routes for SARS-CoV-2 transmission [26]. A study by Da Silva Santos et al. revealed that the use of mouthwash, in addition to standard care, reduced viral load in the oral cavity, thereby reducing the length of hospital stay [27]. The Centers for Disease Control and Prevention (CDC) has advocated the benefits of preprocedural mouthwashes in reducing airborne pathogens of all types before clinical procedures [28]. Studies by Bernardo da Fonseca Orcina [29] and Marcelo Lupion Poleti et al. [30] indicated that mouthwashes containing antimicrobial phthalocyanine derivative (APD) had been found to have a positive impact on the relief of early clinical symptoms of patients with COVID-19, such as sore throat, cough, and mouth ulcers.
Prior to this, Cavalcante-Leao BL et al. included the results of two in vitro trials in a review that attempted to verify the efficacy of mouthwashes in reducing viral load. The results showed that the mouthwash containing PVP-I solution with a concentration of 1% (without dilution) and one of 7% (diluted at 1:30) examined in this systematic review had a killing effect on bacteria and viruses [31]. A review by Hernandez-Vasquez A et al., according to the present systematic review, indicated that the effect of mouthwash on SARS-CoV-2 viral load in the saliva of COVID-19 patients remained uncertain. Evidence from well-designed RCTs is required for further and more objective evaluation of this effect [32]. Majdy Idrees et al. [33]performed a meta-analysis of in vitro and in vivo experiments about the effect of mouthwash and nasal spray on viral load reduction, respectively, and concluded that a variety of active ingredients in mouthwash have confirmed therapeutic effects on SARS-CoV-2, but the duration of action of each active ingredient in vivo was not clear.
Therefore, questions remained regarding the use of mouthwash in SARS-CoV-2 patients before receiving clinical treatment.
Most studies have adopted CT values and viral loads to assess the posttreatment efficacy of COVID-19. In response to the SARS-CoV-2 pandemic, the CDC 2019 novel Coronavirus (2019-nCoV) Real-Time PCR Diagnostic Panel was approved by U.S. Food and Drug Administration and adopted by the NIH Clinical Center, herein referred to as the SARS-CoV-2 RT–PCR assay. Coronaviruses have a number of molecular targets within their positive-sense, single-stranded RNA genome that can be used for PCR assays. These include genes encoding structural proteins, including envelope glycoproteins spike (S), envelope (E), transmembrane (M), helicase (Hel), and nucleocapsid (N). In addition to the genes that encode structural proteins, there are species-specific accessory genes that are required for viral replication, including RNA-dependent RNA polymerase (RdRp), hemagglutinin-esterase (HE), and open reading frame 1a (ORF1a) and ORF1b [34]. Although SARS-CoV-2 RT-PCR is the gold standard for viral load estimation, this assay is semi-quantitative. Therefore, some studies have used reverse transcription-polymerase chain reaction (RT-PCR) to quantify viral load. Virus copies were normalized by mL of saliva. In this regard, it should be noted that viral loads of over 106 copies/ml were required for infectivity studies [35]. In view of the difficulties in culturing SARS-CoV-2 virus from clinical specimens, the current use of viral RNA load as a substitute remains reasonable [36].
This meta-analysis, which combined the results of 13 RCTs, provided strong evidence for the effectiveness of mouthwash in reducing novel coronavirus load. In the current study, CT value and viral load were the outcome measures for analyzing the effect of mouthwash on novel coronavirus. According to subgroup analysis by measurement time, it was concluded that the use of mouthwash was effective within 30 min in reducing viral load compared with routine oral care with placebo. According to subgroup analysis of the mouthwash ingredients, PVP-I-containing mouthwashes significantly elevated the CT value, which was consistent with the results of existing in vitro experimental findings, and SARS-CoV-2 virus could be completely inactivated with PVP-I-containing oral disinfectant in vitro [37]. PVP-I is composed of iodine and the water-soluble polymer polyvinylpyrrolidone. PVP-I has antimicrobial activity when it dissociates and releases iodine. The action of mouth rinses containing PVP-I against SARS-CoV-2 is due to the sensitivity of the virus to oxidation [38]. CHX is a cationic surfactant and synthetic biguanide with broad-spectrum antimicrobial activity, and is widely used as an antiseptic formulation in dental practice [39]. The results of this study showed that CHX-containing mouthwash was second only to PVP-I-containing mouthwash in reducing viral load. According to studies by Y Hanna Huang [40] et al., chlorhexidine was highly effective in preventing SARS-CoV-2 infection for some medical workers, with no infections among medical workers during the use of chlorhexidine, while the prevalence of novel coronavirus in medical workers from general hospitals approached 50% during the same period. Besides, Matheus Dos Santos Fernandez et al. systematically reviewed the killing effect of CHX on some virus strains, and the results showed that CHX had a good inactivation effect on herpes simplex virus-1 and influenza virus A. However, CHX is less effective in the elimination of influenza virus A compared with povidone-iodine [41]. HP is a broad-spectrum antibacterial agent, and is especially effective against coronavirus and influenza viruses [42]. This was also confirmed in a subgroup analysis of CT values presented in this review. CPC, BAC, β-cyclodextrin and citrox, as widely used antimicrobials, did not show statistically significant differences in the subgroup analysis of decreasing viral load of COVID-19, and more clinical trials are needed to demonstrate this finding.
In terms of safety, no adverse reactions were mentioned in the included 13 articles, and no relevant systematic reviews have reported any increase in the risk of oral disease caused by mouthwash. However, it should be noted that PVP-I is contraindicated in patients with thyroid disease, who are allergic to iodine and radioiodine therapy, or during pregnancy [43].
Compared with previous systematic reviews, this study is a meta-analysis based entirely on in vivo experiments. While discussing the effects of different ingredients of mouthwash on SARS-CoV-2 viral load, we also explored the effects of different mouthwash duration on SARS-CoV-2 viral load. This provides a positive reference for the clinical prevention of SARS-CoV-2. However, this study has some limitations in that some of the included studies had short and various follow-up durations, and high-quality studies with longer follow-up durations are still needed. Therefore, any conclusions from pooled outcome measures and their interpretations should be treated with caution. More RCTs of large-scale, high-quality, and large-sample may be needed in the future to validate the efficacy of various mouthwash ingredients in relieving SARS-CoV-2 symptoms after infection.
Conclusions
In summary, mouthwash has some efficacy in reducing the viral load of novel coronavirus, especially within 30 min after rinsing the mouth. Mouthwash containing CHX, PVP-I and HP all had significant positive effects on CT values, and PVP-I-containing mouthwash may be a promising option to control novel coronavirus infections and relieve virus-related symptoms. However, studies on the dose and frequency of use of mouthwash for infection control are still lacking, which may limit the clinical application of mouthwash.
Availability of data and materials
All data generated or analyzed during this study are included in this published article (and its Supplementary Information files).
References
Wu JT, Leung K, Leung GM. Nowcasting and forecasting the potential domestic and international spread of the 2019-nCoV outbreak originating in Wuhan, China: a modelling study. Lancet (London, England). 2020;395(10225):689–97.
Sachs JD, Karim SSA, Aknin L, Allen J, Brosbøl K, Colombo F, Barron GC, Espinosa MF, Gaspar V, Gaviria A, et al. The Lancet Commission on lessons for the future from the COVID-19 pandemic. Lancet (London, England). 2022;400(10359):1224–80.
Davis HE, McCorkell L, Vogel JM, Topol EJ. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol. 2023;21(3):133–46.
McVernon J, Liberman J. WHO keeps covid-19 a public health emergency of international concern. BMJ (Clinical Research Ed). 2023;380:504.
Zemouri C, Volgenant CMC, Buijs MJ, Crielaard W, Rosema NAM, Brandt BW, Laheij A, De Soet JJ. Dental aerosols: microbial composition and spatial distribution. J Oral Microbiol. 2020;12(1):1762040.
Carrouel F, Gonçalves LS, Conte MP, Campus G, Fisher J, Fraticelli L, Gadea-Deschamps E, Ottolenghi L, Bourgeois D. Antiviral activity of reagents in mouth rinses against SARS-CoV-2. J Dent Res. 2021;100(2):124–32.
Chen MH, Chang PC. The effectiveness of mouthwash against SARS-CoV-2 infection: A review of scientific and clinical evidence. J Formosan Med Assoc =Taiwan Yi Zhi. 2022;121(5):879–85.
Garcia-Sanchez A, Peña-Cardelles JF, Salgado-Peralvo AO, Robles F, Ordonez-Fernandez E, Ruiz S, Végh D. Virucidal activity of different mouthwashes against the salivary load of SARS-CoV-2: a narrative review. Healthc (Basel, Switzerland). 2022;10(3):469.
Peng X, Xu X, Li Y, Cheng L, Zhou X, Ren B. Transmission routes of 2019-nCoV and controls in dental practice. Int J Oral Sci. 2020;12(1):9.
Ather A, Patel B, Ruparel NB, Diogenes A, Hargreaves KM. Coronavirus Disease 19 (COVID-19): Implications for clinical dental care. J Endodontics. 2020;46(5):584–95.
Seneviratne CJ, Balan P, Ko KKK, Udawatte NS, Lai D, Ng DHL, Venkatachalam I, Lim KS, Ling ML, Oon L, et al. Efficacy of commercial mouth-rinses on SARS-CoV-2 viral load in saliva: randomized control trial in Singapore. Infection. 2021;49(2):305–11.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ (Clin Res Ed). 2009;339: b2700.
Alemany A, Perez-Zsolt D, Raïch-Regué D, Muñoz-Basagoiti J, Ouchi D, Laporte-Villar C, Baro B, Henríquez N, Prat N, Gianinetto MO, et al. Cetylpyridinium chloride mouthwash to reduce shedding of infectious SARS-CoV-2: a double-blind randomized clinical trial. J Dent Res. 2022;101(12):1450–6.
Alzahrani MM, Bamashmous S, Alkharobi H, Alghamdi A, Alharbi RH, Hassan AM, Darwish M, Bukhari A, Mahmoud AB, Alfaleh MA, et al. Mouth rinses efficacy on salivary SARS-CoV-2 viral load: a randomized clinical trial. J Med Virol. 2023;95(1): e28412.
Carrouel F, Valette M, Gadea E, Esparcieux A, Illes G, Langlois ME, Perrier H, Dussart C, Tramini P, Ribaud M, et al. Use of an antiviral mouthwash as a barrier measure in the SARS-CoV-2 transmission in adults with asymptomatic to mild COVID-19: a multicentre, randomized, double-blind controlled trial. Clin Microbiol Infect. 2021;27(10):1494–501.
Costa DD, Brites C, Vaz SN, de Santana DS, Dos Santos JN, Cury PR. Chlorhexidine mouthwash reduces the salivary viral load of SARS-CoV-2: a randomized clinical trial. Oral Dis. 2022;28(Suppl 2):2500–8.
Eduardo FP, Corrêa L, Heller D, Daep CA, Benitez C, Malheiros Z, Stewart B, Ryan M, Machado CM, Hamerschlak N, et al. Salivary SARS-CoV-2 load reduction with mouthwash use: a randomized pilot clinical trial. Heliyon. 2021;7(6): e07346.
Elzein R, Abdel-Sater F, Fakhreddine S, Hanna PA, Feghali R, Hamad H, Ayoub F. In vivo evaluation of the virucidal efficacy of chlorhexidine and povidone-iodine mouthwashes against salivary SARS-CoV-2. A randomized-controlled clinical trial. J Evid-Based Dental Pract. 2021;21(3):101584.
Ferrer MD, Barrueco ÁS, Martinez-Beneyto Y, Mateos-Moreno MV, Ausina-Márquez V, García-Vázquez E, Puche-Torres M, Giner MJF, González AC, Coello JMS, et al. Clinical evaluation of antiseptic mouth rinses to reduce salivary load of SARS-CoV-2. Sci Rep. 2021;11(1):24392.
Meister TL, Gottsauner JM, Schmidt B, Heinen N, Todt D, Audebert F, Buder F, Lang H, Gessner A, Steinmann E, et al. Mouthrinses against SARS-CoV-2 - High antiviral effectivity by membrane disruption in vitro translates to mild effects in a randomized placebo-controlled clinical trial. Virus Res. 2022;316: 198791.
Natto ZS, Bakhrebah MA, Afeef M, Al-Harbi S, Nassar MS, Alhetheel AF, Ashi H. The short-term effect of different chlorhexidine forms versus povidone iodine mouth rinse in minimizing the oral SARS-CoV-2 viral load: An open label randomized controlled clinical trial study. Medicine. 2022;101(30): e28925.
Sánchez Barrueco Á, Mateos-Moreno MV, Martínez-Beneyto Y, García-Vázquez E, Campos González A, Zapardiel Ferrero J, Bogoya Castaño A, Alcalá Rueda I, Villacampa Aubá JM, Cenjor Español C, et al. Effect of oral antiseptics in reducing SARS-CoV-2 infectivity: evidence from a randomized double-blind clinical trial. Emerg Microbes Infect. 2022;11(1):1833–42.
Sevinç Gül SN, Dilsiz A, Sağlık İ, Aydın NN. Effect of oral antiseptics on the viral load of SARS-CoV-2: a randomized controlled trial. Dental Med Prob. 2022;59(3):357–63.
Tarragó-Gil R, Gil-Mosteo MJ, Aza-Pascual-Salcedo M, Alvarez MJL, Ainaga RR, Gimeno NL, Viñuales RF, Fernández YM, Marco JM, Bolsa EA, et al. Randomized clinical trial to assess the impact of oral intervention with cetylpyridinium chloride to reduce salivary SARS-CoV-2 viral load. J Clin Periodontol. 2023;50(3):288–94.
Xu H, Zhong L, Deng J, Peng J, Dan H, Zeng X, Li T, Chen Q. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 2020;12(1):8.
Huang N, Pérez P, Kato T, Mikami Y, Okuda K, Gilmore RC, Conde CD, Gasmi B, Stein S, Beach M, et al. SARS-CoV-2 infection of the oral cavity and saliva. Nat Med. 2021;27(5):892–903.
da Silva Santos PS, da Fonseca OB, Machado RRG, Vilhena FV, da Costa Alves LM, Zangrando MSR, de Oliveira RC, Soares MQS, Simão ANC, Pietro E, et al. Beneficial effects of a mouthwash containing an antiviral phthalocyanine derivative on the length of hospital stay for COVID-19: randomised trial. Sci Rep. 2021;11(1):19937.
Kohn WG, Collins AS, Cleveland JL, Harte JA, Eklund KJ, Malvitz DM. Guidelines for infection control in dental health-care settings–2003. MMWR Recommend Rep. 2003;52(Rr-17):1–61.
da Fonseca Orcina B, Vilhena FV, de Cardoso Oliveira R, da Costa Marques Alves L, Araki K, Toma SH, Ragghianti Zangrando MS, da Silva Santos PS. A Phthalocyanine derivate mouthwash to gargling/rinsing as an option to reduce clinical symptoms of COVID-19: case series. Clin Cosmetic Invest Dentistry. 2021;13:47–50.
Poleti ML, Gregório D, Bistaffa AGI, Fernandes KBP, Vilhena FV, Santos P, Simão ANC, Lozovoy MAB, Tatibana BT, Fernandes TMF. use of mouthwash and dentifrice containing an antimicrobial phthalocyanine derivative for the reduction of clinical symptoms of covid-19: a randomized triple-blind clinical trial. J Evid Based Dent Pract. 2022;22(4): 101777.
Cavalcante-Leão BL, de Araujo CM, Basso IB, Schroder AG, Guariza-Filho O, Ravazzi GC, Gonçalves FM, Zeigelboim BS, Santos RS, Stechman-Neto J. Is there scientific evidence of the mouthwashes effectiveness in reducing viral load in Covid-19? A systematic review. J Clin Exp Dent. 2021;13(2):e179–89.
Hernández-Vásquez A, Barrenechea-Pulache A, Comandé D, Azañedo D. Mouthrinses and SARS-CoV-2 viral load in saliva: a living systematic review. Evid Based Dent. 2022:1–7. https://doi.org/10.1038/s41432-022-0253-z.
Idrees M, McGowan B, Fawzy A, Abuderman AA, Balasubramaniam R, Kujan O. Efficacy of Mouth Rinses and Nasal Spray in the Inactivation of SARS-CoV-2: A Systematic Review and Meta-Analysis of In Vitro and In Vivo Studies. Int J Environ Res Public Health. 2022;19(19):12148.
Tang YW, Schmitz JE, Persing DH, Stratton CW. Laboratory diagnosis of COVID-19: current issues and challenges. J Clin Microbiol. 2020;58(6):e00512.
Wölfel R, Corman VM, Guggemos W, Seilmaier M, Zange S, Müller MA, Niemeyer D, Jones TC, Vollmar P, Rothe C, et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581(7809):465–9.
Joynt GM, Wu WK. Understanding COVID-19: what does viral RNA load really mean? Lancet Infect Dis. 2020;20(6):635–6.
Bidra AS, Pelletier JS, Westover JB, Frank S, Brown SM, Tessema B. Comparison of In Vitro Inactivation of SARS CoV-2 with Hydrogen Peroxide and Povidone-Iodine Oral Antiseptic Rinses. J Prosthodontics. 2020;29(7):599–603.
Pattanshetty S, Narayana A, Radhakrishnan R. Povidone-iodine gargle as a prophylactic intervention to interrupt the transmission of SARS-CoV-2. Oral Dis. 2021;27(3):752–3.
Arteagoitia I, Rodriguez Andrés C, Ramos E. Does chlorhexidine reduce bacteremia following tooth extraction? A systematic review and meta-analysis. PLoS ONE. 2018;13(4): e0195592.
Huang YH, Huang JT. Use of chlorhexidine to eradicate oropharyngeal SARS-CoV-2 in COVID-19 patients. J Med Virol. 2021;93(7):4370–3.
Fernandez MDS, Guedes MIF, Langa GPJ, Rösing CK, Cavagni J, Muniz F. Virucidal efficacy of chlorhexidine: a systematic review. Odontology. 2022;110(2):376–92.
Dev Kumar G, Mishra A, Dunn L, Townsend A, Oguadinma IC, Bright KR, Gerba CP. Biocides and novel antimicrobial agents for the mitigation of coronaviruses. Front Microbiol. 2020;11:1351.
Gray PE, Katelaris CH, Lipson D. Recurrent anaphylaxis caused by topical povidone-iodine (Betadine). J Paediatr Child Health. 2013;49(6):506–7.
Acknowledgements
Not applicable.
Funding
This work was supported by a grant from the Key Research and Development Program in Hebei Province of China (No.22377741D).
Author information
Authors and Affiliations
Contributions
Conceptualization: Qing Dong, Jianqi Gu; Methodology: Nan Meng, Mingrui Zhang, Hong Duo; Formal analysis and investigation: Nan Meng, Mingrui Zhang, Hong Duo, Yuanbo Yang; Writing—original draft preparation: Mingrui Zhang, Nan Meng; Writing—review and editing: Qing Dong, Jianqi Gu; Funding acquisition: Qing Dong, Yuanbo Yang; Resources: Qing Dong, Yuanbo Yang; Supervision: Qing Dong, Jianqi Gu.
And all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All analyses were based on previous published studies, thus no ethical approval and patient consent are required.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure S1.
Sensitivity analysis of CT values.
Additional file 2: Figure S2.
Sensitivity analysis of viral loads.
Additional file 3: Figure S3.
Funnel plot of CT values.
Additional file 4: Figure S4.
Funnel plot of viral loads.
Additional file 5: Figure S5.
Filled funnel plot of CT values.
Additional file 6: Table S1.
Search strategy.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhang, M., Meng, N., Duo, H. et al. Efficacy of mouthwash on reducing salivary SARS-CoV-2 viral load and clinical symptoms: a systematic review and meta-analysis. BMC Infect Dis 23, 678 (2023). https://doi.org/10.1186/s12879-023-08669-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-023-08669-z