Abstract
Introduction
Evidence on the real-world effects of “Treat All” on attrition has not been systematically reviewed. We aimed to review existing literature to compare attrition 12 months after antiretroviral therapy (ART) initiation, before and after “Treat All” was implemented in Sub-Saharan Africa and describe predictors of attrition.
Methods
We searched Embase, Google Scholar, PubMed, and Web of Science in July 2020 and created alerts up to the end of June 2023. We also searched for preprints and conference abstracts. Two co-authors screened and selected the articles. Risk of bias was assessed using the modified Newcastle–Ottawa Scale. We extracted and tabulated data on study characteristics, attrition 12 months after ART initiation, and predictors of attrition. We calculated a pooled risk ratio for attrition using random-effects meta-analysis.
Results
Eight articles and one conference abstract (nine studies) out of 8179 screened records were included in the meta-analysis. The random-effects adjusted pooled risk ratio (RR) comparing attrition before and after “Treat All” 12 months after ART initiation was not significant [RR = 1.07 (95% Confidence interval (CI): 0.91–1.24)], with 92% heterogeneity (I2). Being a pregnant or breastfeeding woman, starting ART with advanced HIV, and starting ART within the same week were reported as risk factors for attrition both before and after “Treat All”.
Conclusions
We found no significant difference in attrition before and after “Treat All” one year after ART initiation. While “Treat All” is being implemented widely, differentiated approaches to enhance retention should be prioritised for those subgroups at risk of attrition.
PROSPERO number
Similar content being viewed by others
Introduction
At the end of 2020, there were an estimated 38 million people living with HIV (PLHIV) globally, and of these, 25 million (67%) were in Sub-Saharan Africa [1]. To control HIV-related mortality and HIV transmission, UNAIDS launched the 90–90-90 targets: 90% of PLHIV should know their status, 90% of people with confirmed HIV infection should receive antiretroviral therapy (ART), and 90% of those on ART should be virally suppressed by the end of 2020 [2]. The region of Eastern and Southern Africa made important progress toward these 90–90-90 targets, with an estimated 87% of PLHIV aware of their status, 72% of them receiving ART, and 65% of them having achieved viral load (VL) suppression by 2020. In Western and Central Africa, the 90–90-90 targets had reached 81%, 73%, 59%, respectively, at the end of 2020 [3]. While most countries (Burundi, Eswatini, Kenya, Malawi, Namibia, Lesotho Rwanda, Uganda, Zambia, and Zimbabwe) in the region are close to reaching the 90–90-90 targets, others. like Botswana, have already surpassed them [4]. Most countries now aim at achieving the next target: 95–95-95 [5, 6].
Since 2016, the World Health Organization (WHO) has recommended “Treat All”; ART for all PLHIV, regardless of their clinical or immunological status [7]. A majority of low- and middle-income countries, including those in Sub-Saharan Africa, have adopted this policy: 93% had rolled-out “Treat All” by the end of 2019, while another 2% planned roll-out before the end of 2021 [8]. This recommendation was informed by several clinical trials on “Treat All”, which included universal testing, activities enhancing linkage to HIV care, rapid ART start and patient-centred care [9, 10]. In these trial settings, outcomes improved across the cascade of care, resulting in population-level VL suppression and decreased HIV incidence and mortality. However, despite significant population-level gains, VL suppression was not uniform across all sub-populations, long-term retention in care was not the primary outcome and HIV elimination targets were not reached [9]. Furthermore, there is limited evidence on the real-world effect of “Treat All” across the cascade of care. Clinical trial data are not generalisable to the reality of national programmes, as they are implemented in a controlled environment, often relying on more resources than what is available in routine care. Moreover, patients with a poor clinical condition are often excluded from trial participation [10]. A common term to quantify those not retained on ART is attrition; a combination of death, lost-to-follow-up, and often those who stopped ART and were transferred out [11].
The International Prospective Register of Systematic Reviews (PROSPERO) registered one on-going review assessing strategies to improve linkage to care under “Treat All” [12]. To our knowledge, there are no on-going or published reviews comparing attrition before and after “Treat All” implementation under programmatic conditions in Sub-Saharan Africa. We therefore conducted a systematic review to compare attrition 12 months after ART initiation, before and after the implementation of “Treat All”. We also describe risk factors for attrition as reported by the included studies.
Methods
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines for protocol development and reporting of this review [13, 14]. The protocol was registered on PROSPERO (CRD42020191582) [15]. We used the term before Treat All” for patients who were initiated on ART based on existing guidelines i.e. immunological/clinical criteria or pregnancy/breastfeeding status at a time point prior to the launch of “Treat All”, and we used the term “Treat All” for those who were initiated on ART based solely on an HIV-positive status i.e. at a time point after implementation of the WHO “Treat All” guidelines [7].
Eligibility criteria
Table 1 shows the components of the search string for population, intervention, comparison outcomes and study designs (PICOs) used for this systematic review.
The WHO “Treat All” recommendation was published in 2016 [7]. We included peer-reviewed studies published since 2018, to allow enough time since the existence of the “Treat All” policy for guideline implementation, collection of programme data and publication. We only included articles which directly compared attrition among PLHIV at 12 months after ART initiation, with data drawn from the same study setting at two different time points, situated before and after “Treat All” implementation in that setting.
Search strategy
In July 2020, two co-authors (R.M. and T.G.) searched Embase, Google Scholar, Medline through PubMed, and Web of Science for relevant studies with pre-defined search terms (Additional file 1). We searched articles published in the three main academic languages used in Sub-Saharan Africa i.e., English, French and Portuguese. We used the same strings to create alerts in the databases and included additional publications until the end of June 2023. We hand-searched unpublished preprint manuscripts (medRxiv and bioRxiv) and conference abstracts from the following HIV conferences: Conference on Retroviruses and Opportunistic Infections (CROI), International AIDS Society (IAS), International Conference on AIDS and Sexually Transmitted Infections in Africa (ICASA) and International Francophone Conference on HIV, Hepatitis and Sexual Health (AfraVIH) by combining different terms of the PICOs criteria. We also searched for cited references in the articles for which we performed full-text screening.
Study selection and data extraction
After deduplication, unique results were imported into the Rayyan software and screened independently by two blinded co-authors (R.M. and T.G) in two phases (i.e. titles and abstracts, and full-text screening) [16]. Disagreements between researchers were resolved by discussion and consensus, and when necessary, arbitration by another pair of researchers (co-authors T.D. and L.L.). The two independent researchers (R.M. and T.G.) used a standardised tool for data extraction, collecting information on the study characteristics (e.g., study design, location, period, objectives), participant characteristics (e.g., inclusion/exclusion criteria, baseline characteristics), attrition on ART at 12 months, and predictors of these outcomes as shown in the original publication. We additionally extracted data on pre-ART attrition. When authors only reported outcomes on patients in care (without defining the denominator for ART patients), they were contacted by R.M. to retrieve data restricted to patients on ART.
Risk of bias assessment
We used a modified Newcastle–Ottawa Scale for cohort studies to assess the risk of bias, based on three parameters (selection, comparability and outcome) [17]. The maximum score a study could get was eight stars (a maximum of four stars was awarded for selection, two for comparability and two for outcome). The results from the Newcastle–Ottawa Scale were classified following the Agency for Healthcare Research and Quality standards of “good”, “fair” and “poor” quality [18].
Data synthesis
Study and participant characteristics from included studies were tabulated. As most authors reported outcomes as proportions among all PLHIV initiated on ART, we used risk ratios (RRs) to compare attrition at 12 months, before and after “Treat All”. Authors who reported attrition with survival data were contacted to retrieve data restricted to participants with a potential follow-up period of minimum 12 months on ART. Attrition was defined as a composite of death, LTFU and those who stopped ART among all those who initiated ART, including transfers in/out. If only the proportion retained was reported, attrition was defined as 100% minus the proportion retained. A pooled estimate was calculated for attrition, and a forest plot constructed with Stata/IC 16.1 (StataCorp, USA). An inverse variance random-effect model was used for the meta-analysis. Heterogeneity was evaluated using the I-squared statistic, with < 25%, 25–75% and > 75% respectively indicating low, moderate, and high heterogeneity. Predictors of the main outcomes were presented in structured tables.
Results
Selection of the included studies
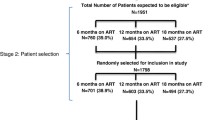
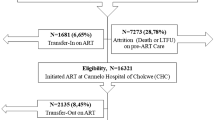
We identified 8179 records in our databases search and from alerts, and after removing the duplicates, 7971 articles were screened from which 27 [19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46] full-text articles were retrieved for eligibility assessment. From the 27 articles, eight articles [19, 20, 26, 30, 35, 39, 41, 43], and one conference abstract identified through hand search [46] were retained in the systematic review and meta-analysis (Fig. 1).
Risk of bias assessment for the included studies
The results of the quality assessment are shown in Additional file 2. Of the nine studies, six were scored as “good” [19, 20, 26, 30, 35, 43], one as “fair” [41] and two as “poor” [39, 46]. The study by Awoh et al. was scored poor because no multivariable analysis was conducted [39]. The study by Owona et al. was scored poor because this conference abstract showed limited detail in the methods section [46].
Characteristics of the studies included
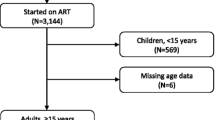
Of the nine studies included, three were from Zimbabwe [20, 26, 41], two from Cameroon [39, 46], one from Kenia [43], one from Malawi [35], one from South Africa [30], and one from the Democratic Republic of Congo [19] (Table 2). Of the nine studies, five included more than 20 health facilities. More than half (5/8) of the studies included both urban and rural facilities, while the others were mainly urban. All the included studies were retrospective cohort studies. Three studies included all patients starting ART [26, 41, 46], three included only adults [20, 30, 39], two included patients above 15 years old [43, 46], and one above 10 years old [35].
The baseline characteristics (age, sex, WHO stage and CD4 counts) of the study participants for the included studies were summarised by cohort (before or after “Treat All”) (Additional file 3). The median/mean age in years ranged from 31–40 years, and age was similar between before and after “Treat All” cohorts for each of the studies. The proportion of males ranged between 29 and 48% and was similar between the before and after “Treat All” cohorts for the individual studies. The proportion of males was lower compared to females in all studies. In most of the studies, the proportion of patients with stage III/IV was lower in the “Treat All” cohort. Half of the studies reported baseline CD4 counts. The mean CD4 counts (cells/µL) ranged between 194 and 369 before, and between 220 and 308 after “Treat All”, respectively. We contacted Owona et al. to retrieve more information on participant baseline characteristics, but this information was not provided [46].
Attrition after 12 months on ART before and after “Treat All” implementation
All studies reported attrition, retention or outcomes from which attrition could be derived 12 months after ART initiation. Three authors who reported attrition by survival analysis were contacted to provide data restricted to those with 12 months potential follow-up on ART. Tlhajoane et al. provided data for a few patients who had 12 months follow-up, while Makurumidze et al. and Mayasi et al. confirmed that none of the included participants was followed for less than 12 months [19, 20, 26]. None of the studies reported pre-ART attrition. Four articles reported a statistically significant higher 12-month attrition after “Treat All” compared to before [19, 26, 30, 46] (Additional file 4). Only one study, by Alhaj et al., reported a significantly higher 12-month retention after “Treat All” compared to before [35]. Data from the nine studies were included in the meta-analysis (Fig. 2).
When study data were reported as RRs, three studies had a statistically significant difference in attrition before and after “Treat All” [30, 35, 46]. The random-effects adjusted pooled RR comparing attrition before and after “Treat All” was not significant [RR = 1.07 (95% CI: 0.91–1.24)] and there was high between-study variation (heterogeneity measured with I2 = 92%). Several sensitivity analyses were performed, and the results were generally robust (Additional file 5).
Predictors of attrition before and after “Treat All” implementation
Five of the included studies investigated predictors of attrition on ART [19, 20, 26, 35, 43] (Table 3). All the studies reported predictors of attrition for the entire study population (including before and after “Treat All”). Four studies used attrition at any time point as an outcome, while Alhaj et al. used attrition 12 months after ART initiation [35]. Patients who started ART under “Treat All” as compared to before had a significantly higher hazard of attrition in two studies, [19, 26] a significantly lower hazard in one study [35], and no significant association was found in two studies [20, 43].
Demographic risk factors for attrition reported both before and after “Treat All” included being an adolescent or young adult, being male and being a pregnant or breastfeeding woman. Two studies reported advanced HIV disease (based on WHO stage III/IV) as a risk factor for attrition. Two studies reported initiation of ART within a week after diagnosis (versus later) as a risk factor for attrition. One study reported that, while pregnancy remained a risk factor for attrition under “Treat All”, the hazard of attrition decreased by 17% after, compared to before, “Treat All” [26].
Discussion
Our systematic review is the first assessment of potential changes in attrition since the programmatic scale-up of “Treat All” in Sub-Saharan Africa. We found no difference in ART attrition at 12 months before and after “Treat All” in a meta-analysis of nine studies. There was considerable heterogeneity between studies. The reported significant predictors for attrition included advanced HIV, pregnancy and starting ART in the week after diagnosis (versus later).
Our findings on the effect of “Treat All” on attrition are inconclusive. The systematic review did not show a significant difference in attrition at 12 months post ART initiation, when comparing before and after “Treat All” (random effects risk ratio, 1.07, 95% confidence interval: 0.91–1.24). Only nine studies were included in the systematic review. The heterogeneity between studies was very high, in terms of study populations and settings, and study design (including the time between the end of observation of the before “Treat All” and the start of observation of the “Treat All” cohort). The results of the meta-analysis are thus unlikely to reflect the true effect of “Treat All” on attrition. The obtained estimate of the effect of “Treat All” on attrition should therefore be interpreted with caution.
Studies from both low (Cameroun, Democratic Republic of Congo, Kenya) and high (Zimbabwe, South Africa, and Malawi) HIV prevalence settings were included in the systematic review. In low prevalence settings, especially in West-and Central Africa, ART services are often concentrated in urban settings. There is often a delay in adoption of new treatment guidelines in these settings, which can contribute to a poor performance of these programmes along the 90–90-90 HIV care cascades (Cameroon: 78%, 74%, unknown; Democratic Republic of Congo: 75%, 75%, unknown) [1]. Data missing for the 3rd 90 suggest that monitoring of these HIV programmes is suboptimal. On the other hand, high prevalence settings often have well-performing HIV programmes, with better HIV care 90–90-90 cascades (Malawi: 91%, 86%, 81%, South Africa: 92%, 72%, 66% & Zimbabwe: 93%, 93%, 82%) [1]. In Kenya, the well performing ART programme (HIV care 90–90-90 cascade: 96%, 86%, 81%) has even allowed prevalence to drop below the high prevalence threshold of 5% in 2020 [1]. The level of ART coverage before “Treat All” was implemented may also affect outcomes under “Treat All”. Countries with poor performing HIV care cascades, thus with low coverage of ART needs, will have relatively higher proportions of pre-ART patients with advanced HIV disease [19], at risk of attrition. When this group of patients suddenly becomes eligible for ART initiation under “Treat All”, a bulk of patients at risk of attrition will join the after “Treat All” cohort. This may contribute to a higher attrition in the beginning of “Treat All” implementation. In well performing programmes, with a high coverage of ART needs, we speculate that an opposite effect can be observed. Those retained in pre-ART care would have a high CD4 and would be the most motivated patients. The effect of enrolling them on ART under Treat All may have had an opposite effect compared to what happened in poorly functioning programmes. Moreover, the proportion of pregnant and breastfeeding women and TB patients (risk groups known for higher attrition) differed between studies. A study from Malawi, which was at the extreme end in our meta-analysis (attrition higher before “Treat All”), had a higher proportion of pregnant and breastfeeding women in the before “Treat All” cohort as compared to after “Treat All” [35]. A South African study, at the other extreme end (attrition lower before “Treat All”), excluded TB patients and pregnant women, which might have led to proportionally more exclusions from the before “Treat All” compared to the “Treat All” cohort [30]. The differences in the proportion of these known risk groups for higher attrition may help explain the direction of the individual study findings.
The time between the recruitment of two study populations i.e., between before “Treat All” and after “Treat All”, in the included studies was usually short (ranging between 1–9 months). It is thus unlikely that “Treat All” had reached full coverage in all settings when the “Treat All” observation period began. Especially in already malfunctioning programmes/clinics “Treat All” implementation may have been delayed. In settings where implementation was delayed, patients would have remained on pre-ART follow-up, thus would not be included in the “Treat All” cohort. Moreover, the definition of attrition differed significantly between studies. In some studies transfer out was not included in the definition [39] while in others it was included [46].
Despite our efforts to limit the studies included in our systematic review and meta-analysis to those with similar before and after study designs, the included studies used different statistical analysis approaches. Six of the studies presented a cross-sectional analysis of treatment outcomes at 12 months on ART, while the remainder used survival analysis [19, 26, 35]. Survival analysis takes into account time under observation [47]. The difference between the two groups is calculated over the entire period of follow-up (thus not restricted to the first 12 months) and follow-up time differs for each patient. At each point in time, the probability of survival is calculated for those who remained at risk (who have not yet experienced the event) [48]. In our meta-analysis analysis, we transformed survival data to a simple proportion for comparability and to be able to calculate a pooled estimate of attrition before and after “Treat All” at one year after starting ART. We restricted all study populations to those who started ART at least one year before the end of the study period. In the meta-analysis, we used the total number of patients initiated on ART as denominator, and the number with attrition (= dead, LTFU, stopped ART) as numerator. Using either a cross-sectional analysis or a survival analysis may result in different estimates of the effect of “Treat All” on attrition. Calculating individual and pooled estimates based on the number of events and patients at risk at different time points assumes that all follow-up is equal and complete to the relevant time-point. As a result, this method is overly simplistic and possibly unreliable [48]. The studies which used survival analysis and had their data transformed to calculate proportions showed discordant results in the meta-analysis when compared to the results of the survival analysis in the original studies [19, 26, 35]. When the difference between two groups is small, the conclusion may differ, depending on the methodological approach used. Advanced methods to handle survival data in meta-analyses could not be employed due to the smaller sample of studies that had time to event data [47,48,49].
The before-and-after design, which served as our inclusion criterion, is another reason why the findings of the systematic review and meta-analysis should be interpreted with caution. The primary utility of these studies lies in their ability to show ‘proof of concept’ for an intervention effect [50]. Given the potential for secular changes and other external variables to influence the results between pre- and post- “Treat All” implementation, it is difficult to draw conclusions on the isolated effect of “Treat All” [51, 52]. In reality, many factors independent of “Treat All” could have had an important effect on attrition, such as same day ART initiation, availability of more tolerable ART regimens, better access to monitoring of treatment failure, more decentralisation of care, and growing knowledge on adherence strategies [53, 54]. These factors were not adjusted for in our analysis.
Another issue that makes comparing before and after “Treat All” cohorts difficult is the difference in characteristics of patients starting ART during the two periods, which was influenced primarily by the different guidelines that were implemented during the two periods. Prior to “Treat All”, patients were started on ART based on clinical and immunological criteria (CD4 counts < 500 cells/µL or WHO Stage III/IV) and those not eligible had ART deferred and were followed-up under pre-ART care. Studies done in the before “Treat All” era showed a high level of attrition during the pre-ART period [55, 56]. Only those patients who survived and were retained in care until they were eligible for ART were finally initiated on ART. Patients retained during pre-ART follow-up and who were started on ART thus included those who were most motivated and had engaged with their health facility for a longer time. This selected group of patients were thus also more likely to be retained in care after starting ART [30, 34]. During “Treat All”, everyone diagnosed with HIV is eligible for ART initiation soon after diagnosis, with only a few exceptions receiving pre-ART care (those being treated for opportunistic infections and not ready for life-long treatment). We were not able to account for these differences in terms of characteristics of the study populations of the two periods. Our analysis did not include pre-ART attrition because this was not reported in any of the included studies. As a result, data on those who did not eventually initiate ART were not available, resulting in an underestimation of attrition prior to “Treat All” if all PLHIV were considered. Indeed, when including all patients in ART care before “Treat All”, Onoya et al. found a much lower attrition after “Treat All” was implemented (aHR 0.7, 95% CI: 0.5 to 1.0) [24].
Despite the differences in the effect of “Treat All” on attrition, predictors for attrition pointed in the same direction. Advanced HIV disease, pregnancy and breastfeeding and earlier ART initiation (within one week after HIV diagnosis, compared to later) were associated with attrition across before and after “Treat All” cohorts. Advanced HIV and rapid ART initiation as risk factors for attrition have been reported elsewhere in the post- “Treat all”- era [57]. One study found a reduction (but not annulation) of pregnancy and breastfeeding as a risk factor for attrition after “Treat All” [26]. Additional significant risk factors for attrition found in at least one study were young age, being male, having a lower weight, being divorced, not having data on adherence, not receiving cotrimoxazole and being treated in a small versus large facility.
Since the scale-up, “Treat All” has already had a huge impact on the control of the HIV epidemic. Some countries in Sub-Saharan Africa, where there is the highest HIV-burden, have already met the 90–90-90 targets [3]. Even if the outcomes of those on ART after “Treat All” would be worse, the absolute numbers of PLHIV who are on ART, retained and virologically suppressed are higher. Under “Treat All”, the denominator includes (almost) all PLHIV tested positive; only those not put on ART for clinical or psychosocial reasons may remain under-reported. Before “Treat All”, those ineligible for ART were not shown in clinical programme data, and rarely reported in studies [58]. However, they were also at risk of dying, or likely to be unsuppressed thus transmitting the virus. This translates to increased clinical benefits for a larger number of individuals and reduced transmission overall, which benefits society as a whole.
While “Treat All” should be implemented for both individual and societal benefits, still, potential individual harms in terms of attrition and VL suppression should be mitigated. Integration of ART services (with antenatal care, postnatal care, family planning, immunisation and growth monitoring), use of lay community health workers, such as mothers who have successfully passed through the prevention of mother-to-child programme, and family-centred approaches, should be considered as strategies to improve retention and virologic outcomes in pregnant and breastfeeding women [59]. The advanced HIV care package, which has shown to reduce mortality and morbidity among patients with advanced HIV disease, should be scaled up in low-resource settings [60, 61]. Youth-friendly services, flexible scheduling that considers schooling, and availability of peer caregivers have shown to be effective in retaining adolescents and young adults in care [62, 63]. For men, strategies such as flexible clinic hours to accommodate work and male-friendly services should be considered [64, 65]. However, the identified at-risk groups offer an opportunity to target interventions in resource-restricted settings where it may not be feasible to provide them at scale. Most of the above-mentioned interventions would improve services for everyone (e.g., flexible clinic hours would benefit women as well as men).
Our study has several limitations. Only nine studies were eligible for meta-analysis of attrition at 12 months ART. Because of the small study sample, we did not perform subgroup analysis, as reducing the sample size even more would limit generalisability of the results further. We used attrition after 12 months on ART as an outcome, which might be a short period to measure the true difference in attrition between before and after “Treat All”. All the studies were observational studies, prone to selection and measurement bias. None of the studies reported pre-ART attrition, which is particularly relevant before “Treat All”. Hence, we could not compare outcomes between participants with similar clinical and immunological profiles before and after “Treat All”. We included studies that used a temporal before and after design, i.e., that did measure outcomes in the same study sites at another period. The design has the known limitation of not accounting for the potential for secular changes and other external variables that can influence outcomes. Hence, it was difficult to draw definitive conclusions about Treat All's isolated effect. The differences in outcomes between original articles using survival data and the results of the meta-analysis with RRs shows the importance of the choice of analysis method, and a limitation of meta-analysis using aggregate data. For this meta-analysis, however, it is unlikely that time-adjusted data would have changed the results towards a higher attrition under “Treat All”, since the opposite result was found in time-adjusted analysis by Alhaj et al. [36].
Our study also has some strengths. The studies included cover different geographical regions of Sub-Saharan Africa, and low- and middle-income countries. We employed a rigorous review approach, with two reviewers performing article selection, extraction, and quality assessment in parallel, and a pair of researchers addressing areas of disagreement. We created alerts to get updates on recent articles which were published after our initial search and included yet unpublished conference abstracts in the screening process to address publication bias.
To better understand the effect of attrition on “Treat All” under programmatic conditions, long term implementation data are required. Efforts should be made to conduct future high-quality observational studies with well-defined and measured outcomes and sufficient follow-up time. Since patients starting ART under “Treat All” are not a homogeneous group, more studies should be conducted to assess how each of the subgroups is faring on outcomes. Our review was quantitative, and a qualitative review might assist to understand contextual differences and reasons for differences in attrition.
Conclusion
In our study, we found the effect of “Treat All” on attrition inconclusive. Considering the benefits of ART on individual health and its impact on HIV transmission, programmes should continue to invest in “Treat All” and optimise its implementation to sustain patient outcomes. Differentiated approaches to enhance retention should be prioritized for those subgroups at risk of poor outcomes.
Availability of data and materials
All the data which were used in the preparation of this systematic review are part of this publication. Any additional data requests can be sought from Dr Richard Makurumidze, University of Zimbabwe, Faculty of Medicine and Health Sciences. Email: rmakurumidze@medsch.uz.ac.zw.
Abbreviations
- HIV:
-
Human Immunodeficiency Virus
- AIDS:
-
Acquired Immunodeficiency Syndrome
- ART:
-
Antiretroviral therapy
- PLHIV:
-
People living with HIV
- WHO:
-
World Health Organization
- UNAIDS:
-
Joint United Nations Programme on HIV/AIDS
- VL:
-
Viral load
- LTFU:
-
Lost to follow up
- BTA:
-
Before "Treat All"
- TA:
-
"Treat All"
- CI:
-
Confidence interval
- aHR:
-
Adjusted hazard ratio
- RR:
-
Risk ratio
References
UNAIDS Data 2021. Available online: https://www.unaids.org/sites/default/files/media_asset/JC3032_AIDS_Data_book_2021_En.pdf. Accessed 16 Jan 2023.
UNAIDS. 90–90–90 An ambitious treatment target to help end the AIDS epidemic. Available online : http://www.unaids.org/sites/default/files/media_asset/90-90-90_en.pdf. Accessed 16 Jan 2023.
UNAIDS. Global AIDS Update 2021: Confronting Inequalities - Lessons for pandemic responses from 40 years of AIDS. Available online: https://www.unaids.org/sites/default/files/media_asset/2021-global-aids-update_en.pdf. Accessed 16 Jan 2023.
UNAIDS. Understanding fast-track: Accelerating action to end the AIDS epidemic by 2030. Available online: https://www.unaids.org/sites/default/files/media_asset/201506_JC2743_Understanding_%0AFastTrack_en.pdf. Accessed 16 Jan 2023.
UNAIDS Data 2020. Available online: https://www.unaids.org/sites/default/files/media_asset/2020_aids-data-book_en.pdf. Accessed 16 Jan 2023.
World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. Available online: https://apps.who.int/iris/bitstream/handle/10665/208825/9789241549684_eng.pdf?sequence=1. Accessed 16 Jan 2023.
World Health Organization.Treat All: Policy Adoption and Implementation Status in Countries HIV. Available online: https://apps.who.int/iris/bitstream/handle/10665/259532/WHO-HIV-2017.58-eng.pdf. Accessed 16 Jan 2023.
Perriat D, Balzer L, Hayes R, Lockman S, Walsh F, Ayles H, Floyd S, Havlir D, Kamya M, Lebelonyane R, et al. Comparative assessment of five trials of universal HIV testing and treatment in Sub-Saharan Africa. J Int AIDS Soc. 2018;21(1):e25048.
Havlir D, Lockman S, Ayles H, Larmarange J, Chamie G, Gaolathe T, Iwuji C, Fidler S, Kamya M, Floyd S, et al. What do the universal test and treat trials tell us about the path to HIV epidemic control? J Int AIDS Soc. 2020;23(2): e25455.
Pawson R. Pragmatic trials and implementation science: grounds for divorce? BMC Med Res Methodol. 2019;19(1):176.
Rosen S, Fox MP, Gill CJ. Patient retention in antiretroviral therapy programs in Sub-Saharan Africa: a systematic review. PLoS Med. 2007;4(10): e298.
Kelly N, Maokola W, Mudasiru O, McCoy SI. Interventions to improve linkage to HIV Care in the Era of “Treat All” in Sub-Saharan Africa: a systematic review. Curr HIV/AIDS Rep. 2019;16(4):292–303.
Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;350: g7647.
Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1.
Comparison of retention in care and virologic suppression among people living with HIV (PLHIV) who started antiretroviral therapy before and after the implementation of “Treat All” under programmatic conditions in Sub-Saharan Africa: protocol for a systematic review and metaanalysis. PROSPERO 2020 CRD42020191582. Available online : https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020191582. Accessed 16 Jan 2023.
Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):210.
Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available online : https://www.ohri.ca//programs/clinical_epidemiology/oxford.asp. Accessed 16 Jan 2023.
Assessing the Risk of Bias in Systematic Reviews of Health Care Interventions. Methods Guide for Comparative Effectiveness Reviews. AHRQ Publication No. 17(18)-EHC036-EF. https://doi.org/10.23970/AHRQEPCMETHGUIDE2. Available online : https://effectivehealthcare.ahrq.gov/sites/default/files/pdf/assessing-the-risk-of-bias-in-systematic-reviews-of-health-care-interventions-01_0.pdf. Accessed 16 Jan 2023.
Mayasi N, Situakibanza H, Mbula M, Longokolo M, Maes N, Bepouka B, Ossam JO, Moutschen M, Darcis G. Retention in care and predictors of attrition among HIV-infected patients who started antiretroviral therapy in Kinshasa, DRC, before and after the implementation of the ‘treat-all’ strategy. PLOS Global Public Health. 2022;2(3): e0000259.
Tlhajoane M, Dzamatira F, Kadzura N, Nyamukapa C, Eaton JW, Gregson S. Incidence and predictors of attrition among patients receiving ART in eastern Zimbabwe before, and after the introduction of universal ‘treat-all’ policies: a competing risk analysis. PLOS Global Public Health. 2021;1(10): e0000006.
Ross J, Ribakare M, Remera E, Murenzi G, Munyaneza A, Hoover DR, Shi Q, Nsanzimana S, Yotebieng M, Nash D, et al. High levels of viral load monitoring and viral suppression under Treat All in Rwanda – a cross-sectional study. J Int AIDS Soc. 2020;23(6): e25543.
Vu L, Burnett-Zieman B, Stoman L, Luu M, Mdala J, Granger K, Forsythe S, Zegeye A, Geibel S. Effects of the implementation of the HIV Treat all guidelines on key ART treatment outcomes in Namibia. PLoS ONE. 2021;15(12): e0243749.
Dorward J, Drain PK, Osman F, Sookrajh Y, Pillay M, Moodley P, Garrett N. Short communication: early antiretroviral therapy is associated with better viral suppression and less HIV drug resistance after implementation of universal treatment in South Africa. AIDS Res Hum Retroviruses. 2019;36(4):297–9.
Onoya D, Hendrickson C, Sineke T, Maskew M, Long L, Bor J, Fox MP. Attrition in HIV care following HIV diagnosis: a comparison of the pre-UTT and UTT eras in South Africa. J Int AIDS Soc. 2021;24(2): e25652.
Kamacooko O, Mayanja Y, Bagiire D, Namale G, Hansen CH, Seeley J. Predictors of lost to follow-up in a “test and treat” programme among adult women with high-risk sexual behavior in Kampala, Uganda. BMC Public Health. 2020;20(1):353.
Makurumidze R, Buyze J, Decroo T, Lynen L, de Rooij M, Mataranyika T, Sithole N, Takarinda KC, Apollo T, Hakim J, et al. Patient-mix, programmatic characteristics, retention and predictors of attrition among patients starting antiretroviral therapy (ART) before and after the implementation of HIV “Treat All” in Zimbabwe. PLoS ONE. 2020;15(10): e0240865.
Kerschberger B, Schomaker M, Jobanputra K, Kabore SM, Teck R, Mabhena E, Mthethwa-Hleza S, Rusch B, Ciglenecki I, Boulle A. HIV programmatic outcomes following implementation of the ‘Treat-All’ policy in a public sector setting in Eswatini: a prospective cohort study. J Int AIDS Soc. 2020;23(3): e25458.
Dorward J, Sookrajh Y, Gate K, Khubone T, Mtshaka N, Mlisana K, Ngobese H, Yende-Zuma N, Garrett N. HIV treatment outcomes among people with initiation CD4 counts >500 cells/µL after implementation of Treat All in South African public clinics: a retrospective cohort study. J Int AIDS Soc. 2020;23(4): e25479.
Ross J, Sinayobye JDA, Yotebieng M, Hoover DR, Shi Q, Ribakare M, Remera E, Bachhuber MA, Murenzi G, Sugira V, et al. Early outcomes after implementation of treat all in Rwanda: an interrupted time series study. J Int AIDS Soc. 2019;22(4):e25279.
Hirasen K, Fox M, Hendrickson C, Sineke T, Onoya D. HIV treatment outcomes among patients initiated on antiretroviral therapy pre and post-universal test and treat guidelines in South Africa. Ther Clin Risk Manag. 2020;16:169–80.
Kiwanuka J, Mukulu Waila J, Muhindo Kahungu M, Kitonsa J, Kiwanuka N. Determinants of loss to follow-up among HIV positive patients receiving antiretroviral therapy in a test and treat setting: a retrospective cohort study in Masaka, Uganda. PLoS ONE. 2020;15(4): e0217606.
Girum T, Yasin F, Wasie A, Shumbej T, Bekele F, Zeleke B. The effect of “universal test and treat” program on HIV treatment outcomes and patient survival among a cohort of adults taking antiretroviral treatment (ART) in low income settings of Gurage zone, South Ethiopia. AIDS Res Ther. 2020;17(1):19.
Arpadi S, Lamb M, Nzeyimana IN, Vandebriel G, Anyalechi G, Wong M, Smith R, Rivadeneira ED, Kayirangwa E, Malamba SS, et al. Better Outcomes Among HIV-Infected Rwandan Children 18–60 Months of Age After the Implementation of “Treat All.” J Acquir Immune Defic Syndr. 2019;80(3):e74.
Stafford KA, Odafe SF, Lo J, Ibrahim R, Ehoche A, Niyang M, Aliyu GG, Gobir B, Onotu D, Oladipo A, et al. Evaluation of the clinical outcomes of the test and treat strategy to implement treat all in Nigeria: results from the Nigeria multi-center ART Study. PLoS ONE. 2019;14(7): e0218555.
Alhaj M, Amberbir A, Singogo E, Banda V, van Lettow M, Matengeni A, Kawalazira G, Theu J, Jagriti MR, Chan AK, et al. Retention on antiretroviral therapy during universal test and treat implementation in Zomba district, Malawi: a retrospective cohort study. J Int AIDS Soc. 2019;22(2): e25239.
Opito R, Mpagi J, Bwayo D, Okello F, Mugisha K, Napyo A. Treatment outcome of the implementation of HIV test and treat policy at The AIDs Support Organization (TASO) Tororo clinic, Eastern Uganda: a retrospective cohort study. PLoS ONE. 2020;15(9): e0239087.
Kiwuwa-Muyingo S, Abongomera G, Mambule I, Senjovu D, Katabira E, Kityo C, Gibb DM, Ford D, Seeley J. Lessons for test and treat in an antiretroviral programme after decentralisation in Uganda: a retrospective analysis of outcomes in public healthcare facilities within the Lablite project. Int Health. 2020;12(5):429–43.
Khan S, Spiegelman D, Walsh F, Mazibuko S, Pasipamire M, Chai B, Reis R, Mlambo K, Delva W, Khumalo G, et al. Early access to antiretroviral therapy versus standard of care among HIV-positive participants in Eswatini in the public health sector: the MaxART stepped-wedge randomized controlled trial. J Int AIDS Soc. 2020;23(9): e25610.
Awoh RA, Ekane HG, Dzudie A, Thomas EO, Adedimeji A, Jules AN: Implications of the human immunodeficiency virus test and treat strategy on antiretroviral treatment uptake and retention outcomes in Cameroon. International Journal Of Community Medicine And Public Health; Vol 6, No 11 (2019): November 2019DO - 1018203/2394–6040ijcmph20195045 2019.
Kabogo J, Muniu E, Wamunyokoli F, Musoke R, Songok E. Evidence of reduced treatment adherence among HIV infected paediatric and adolescent populations in Nairobi at the onset of the UNAIDS Universal Test and Treat Program. BMC Res Notes. 2018;11(1):134.
Matare T, Shewade HD, Ncube RT, Masunda K, Mukeredzi I, Takarinda KC, Dzangare J, Gonese G, Khabo BB, Choto RC, et al. Anti-retroviral therapy after “Treat All” in Harare, Zimbabwe: What are the changes in uptake, time to initiation and retention? F1000Research. 2020;9:287.
Lippman SA, El Ayadi AM, Grignon JS, Puren A, Liegler T, Venter WDF, Ratlhagana MJ, Morris JL, Naidoo E, Agnew E, et al. Improvements in the South African HIV care cascade: findings on 90–90-90 targets from successive population-representative surveys in North West Province. J Int AIDS Soc. 2019;22(6): e25295.
Mwamuye IC, Karanja S, Msanzu JB, Adem A, Kerich M, Ngari M. Factors associated with poor outcomes among people living with HIV started on anti-retroviral therapy before and after implementation of “test and treat” program in Coastal Kenya. PLoS ONE. 2022;17(9): e0270653.
Kimanga DO, Oramisi VA, Hassan AS, Mugambi MK, Miruka FO, Muthoka KJ, Odhiambo JO, Yegon PK, Omoro GO, Mbaire C, et al. Uptake and effect of universal test-and-treat on twelve months retention and initial virologic suppression in routine HIV program in Kenya. PLoS ONE. 2022;17(11): e0277675.
Musengimana G, Umugisha JP, Habinshuti P, Anderson T, Mukesharurema G, Remera E, Ndahimana JD, Barnhart DA. Characteristics and clinical outcomes of patients presenting with advanced HIV disease in the “treat all” era: a retrospective cohort study from rural Rwanda. BMC Infect Dis. 2022;22(1):706.
Owona NFAN, Teka MTD, Anoubissi JDD, Mabongo D: Effet de la Stratégie « Tester et Traiter » sur la Rétention en Soins des Personnes Vivant avec le VIH Suivies dans les Services de Prise en Charge au Cameroun: Une Approche Comparative. In: International Conference on AIDS and Sexually Transmitted Infections in Africa (ICASA) 2019.
Fink SA, Brown RS Jr. Survival analysis. Gastroenterol Hepatol. 2006;2(5):380–3.
Claire L Vale JFTaLAS: Effects of adjusting for censoring on meta-analyses of time-to-event outcomes. International Epidemiological Association 2002.
Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16.
Sedgwick P. Before and after study designs. BMJ. 2014;349: g5074.
Bosk CL. The Zen of quality improvement: the waves and the tide form a unity. BMJ Qual Saf. 2016;25(5):297–8.
Pape UJ, Millett C, Lee JT, Car J, Majeed A. Disentangling secular trends and policy impacts in health studies: use of interrupted time series analysis. J R Soc Med. 2013;106(4):124–9.
Operational and Service Delivery Manual for the Prevention, Care and Treatment of HIV in Zimbabwe. Available online: [https://differentiatedservicedelivery.org/Portals/0/adam/Content/JAOEkYYIREyKQ6R637vBmA/File/Zimbabwe_OSDM_webrevised_2017.pdf. Accessed 16 Jan 2023.
World Health Organization. Consolidated Guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: Recommendations for a public health approach. Second Edition. Available online: https://apps.who.int/iris/bitstream/handle/10665/85321/9789241505727_eng.pdf. Accessed 16 Jan 2023.
Rosen S, Fox MP. Retention in HIV care between testing and treatment in Sub-Saharan Africa: a systematic review. PLoS Med. 2011;8(7): e1001056.
Plazy M, Orne-Gliemann J, Dabis F, Dray-Spira R. Retention in care prior to antiretroviral treatment eligibility in Sub-Saharan Africa: a systematic review of the literature. BMJ Open. 2015;5(6): e006927.
Bantie B, Abate MW, Nigat AB, Birlie TA, Dires T, Minuye T, Kerebeh G, Tiruneh CM, Moges N, Chanie ES, et al. Attrition rate and its predictors among adults receiving anti-retroviral therapy following the implementation of the “Universal Test and Treat strategy” at public health institutions in Northern Ethiopia. A retrospective follow-up study Heliyon. 2022;8(11): e11527.
Fox MP, Rosen S. A new cascade of HIV care for the era of “treat all.” PLoS Med. 2017;14(4): e1002268.
Vrazo AC, Firth J, Amzel A, Sedillo R, Ryan J, Phelps BR. Interventions to significantly improve service uptake and retention of HIV-positive pregnant women and HIV-exposed infants along the prevention of mother-to-child transmission continuum of care: systematic review. Tropical Med Int Health. 2018;23(2):136–48.
Mfinanga S, Chanda D, Kivuyo SL, Guinness L, Bottomley C, Simms V, Chijoka C, Masasi A, Kimaro G, Ngowi B, et al. Cryptococcal meningitis screening and community-based early adherence support in people with advanced HIV infection starting antiretroviral therapy in Tanzania and Zambia: an open-label, randomised controlled trial. Lancet. 2015;385(9983):2173–82.
Hakim J, Musiime V, Szubert AJ, Mallewa J, Siika A, Agutu C, Walker S, Pett SL, Bwakura-Dangarembizi M, Lugemwa A, et al. Enhanced Prophylaxis plus Antiretroviral Therapy for Advanced HIV Infection in Africa. N Engl J Med. 2017;377(3):233–45.
Zanoni BC, Sibaya T, Cairns C, Haberer JE. Barriers to retention in care are overcome by adolescent-friendly services for adolescents living with HIV in South Africa: a qualitative analysis. AIDS Behav. 2019;23(4):957–65.
Murray KR, Dulli LS, Ridgeway K, Dal Santo L. Darrow de Mora D, Olsen P, Silverstein H, McCarraher DR: improving retention in HIV care among adolescents and adults in low- and middle-income countries: a systematic review of the literature. PLoS ONE. 2017;12(9): e0184879.
Koole O, Tsui S, Wabwire-Mangen F, Kwesigabo G, Menten J, Mulenga M, Auld A, Agolory S, Mukadi YD, Colebunders R, et al. Retention and risk factors for attrition among adults in antiretroviral treatment programmes in Tanzania, Uganda and Zambia. Tropical Med Int Health. 2014;19(12):1397–410.
Brown LB, Getahun M, Ayieko J, Kwarisiima D, Owaraganise A, Atukunda M, Olilo W, Clark T, Bukusi EA, Cohen CR, et al. Factors predictive of successful retention in care among HIV-infected men in a universal test-and-treat setting in Uganda and Kenya: a mixed methods analysis. PLoS ONE. 2019;14(1): e0210126.
Acknowledgements
The authors want to thank the following organisations the University of Zimbabwe, Faculty of Medicine and Health Sciences, Department of Primary Health Care Sciences and the Institute of Tropical Medicine (ITM), Department of Clinical Sciences, Antwerp, Belgium for the support during the study. The authors are grateful to authors and funders of the scientific work used in this systematic review.
Funding
Richard Makurumidze receives a PhD scholarship grant from the Institute of Tropical Medicine, funded by the Belgian Development Cooperation. The funder had no role in the design of the study; collection, analysis and interpretation of data; and in the writing of the manuscript.
Author information
Authors and Affiliations
Contributions
R.M. and T.G. developed the initial search strategy, were involved in title and abstract screening, extracted the data and received support from all the other authors during the process. T.D. and L.L. verified the eligibility of the included studies, verified extracted data and resolved discrepancies at each step of the review. T.G. performed the analysis. R.M. wrote the initial draft of the manuscript. All authors critically reviewed and commented on the drafts and approved the final version of the manuscript.
Disclaimer
The views and opinions expressed in this article are solely those of the authors in their private capacity and do not necessarily reflect the views of the organizations they are affiliated to.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Search strategy (PubMed) for the systematic review to compare retention and viral suppression before and after HIV “Treat All” implementation in Sub-Saharan Africa
Additional file 2.
Results Newcastle-Ottawa Scale assessment for cohort studies.
Additional file 3.
Baseline characteristics of participants in studies measuring retention at 12 months before and after "Treat All" in Sub-Saharan Africa
Additional file 4.
Reported attrition at 12 months for patients initiating ART before and after "Treat All" implementation in Sub-Saharan Africa
Additional file 5.
Sensitivity analysis for meta-analysis on attrition 12 months after ART initiation before and after "Treat All" in Sub-Saharan Africa
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Makurumidze, R., Decroo, T., Jacobs, B.K.M. et al. Attrition one year after starting antiretroviral therapy before and after the programmatic implementation of HIV “Treat All” in Sub-Saharan Africa: a systematic review and meta-analysis. BMC Infect Dis 23, 558 (2023). https://doi.org/10.1186/s12879-023-08551-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-023-08551-y