Abstract
HIV remains a major cause of morbidity and mortality for people living in many low-income countries. With an HIV prevalence of 12.4% among people aged over 15 years, Mozambique was ranked in 2019 as one of eight countries with the highest HIV rates in the world. We analyzed routinely collected data from electronical medical records in HIV-infected patients aged 15 years or older and enrolled at Carmelo Hospital of Chokwe in Chokwe from 2002 to 2019. Attrition was defined as individuals who were either reported dead or lost to follow-up (LTFU) (≥ 90 days since the last clinic visit with missed medical pick-up after 3 days of failed calls). Kaplan–Meier survival curves and Cox regression analyses were used to model the incidence and predictors of time to attrition. From January 2002 to December 2019, 16,321 patients were enrolled on antiretroviral therapy (ART): 59.2% were women, and 37.9% were aged 25–34 years old. At the time of the analysis, 7279 (44.6%) were active and on ART. Overall, the 16,321 adults on ART contributed a total of 72,987 person-years of observation. The overall attrition rate was 9.46 per 100 person-years. Cox regression showed a higher risk of attrition in those following an inpatient regimen (hazard ratio [HR] 3.18, 95% confidence interval [CI] 2.89–3.50; p < 0.001), having CD4 counts under 50 cells/µL (HR 1.91, 95% CI 1.63–2.24, p < 0.001), receiving anti-TB treatment within 90 days of ART initiation (HR 6.53, 95% CI 5.72–7.45; p < 0.001), classified as WHO clinical stage III (HR 3.75, 95% CI 3.21–4.37; p < 0.001), and having Kaposi’s sarcoma (HR 1.99, 95% CI 1.65–2.39, p < 0.001). Kaplan–Meier analysis showed that patients with CD4 counts of less than 50 cells/µL on ART initiation had a 40% lower chance of survival at 18 years. Low CD4 cell counts, ART initiation as an inpatient, WHO clinical stage III, and anti-tuberculosis treatment within 90 days of ART initiation were strongly associated with attrition. Strengthening HIV testing and ART treatment, improving the diagnosis of tuberculosis before ART initiation, and guaranteed psychosocial support systems are the best tools to reduce patient attrition after starting ART.
Similar content being viewed by others
Introduction
Mozambique is a low-income country in southern Africa with among the highest HIV prevalence in the world, estimated at 12.6% among adults (15–49 years old)1,2. In 2020, there were an estimated 2.1 million people living with HIV (PLWHIV) in the country3, up from the 1.5 million who had been diagnosed in 20104. Likewise, the number of people on antiretroviral therapy (ART) nearly quadrupled from 2012 to 2017 (309,000–1.2 million)5,6. Therefore, significant progress in the fight against HIV/AIDS has been made with the support of programs and bodies such as the Global Fund, the President’s Emergency Plan for AIDS Relief (PEPFAR) and the Mozambican Ministry of Health. However, though Mozambique achieved treatment coverage of more than 55% by the end of 20183, this is far from the 95% recommended by UNAIDS7.
The attrition rate (resulting from mortality or loss to follow-up [LTFU]) remains one of the key challenges to the success of the Mozambican national ART program. Data from 94 health facilities from all the Mozambican provinces revealed that the overall mortality rate was 3.4 deaths per 100 person-years, and 12.9 deaths per 100 person-years in the first 90 days. Attrition rates were 19.8 and 57.2 losses per 100 person-years, respectively8. Data from multi-country ART programs in 350 health facilities in four other Eastern African countries (Ethiopia, Kenya, Mozambique, and Tanzania) from 2005 to 2014 reported attrition rates of 26.2% at 12 months, 34.0% at 24 months, and 40.1% at 36 months after starting ART9. Other data from a large multi-country program in 198 HIV clinics in Kenya (69 clinics), Mozambique (33 clinics), Rwanda (44 clinics) and Tanzania (52 clinics) revealed that the sex-related differences in the relationship between age, mortality and LTFU applied to both younger patients on ART as well as to older patients aged 50–59 years, and that women on ART appear to have disproportionate risk of death as they age compared to men10.
Known predictors of attrition include low initial CD4 T-cell lymphocyte counts, low baseline body mass index (BMI), history of hospitalization, advanced WHO clinical staging, older age at HIV diagnosis, and being a man11,12,13. Other studies from the region show anemia and decreased red blood cell counts and platelets as predictors of death during the first year of ART treatment14.
To continuously evaluate the success of ART programs in Southern Africa, data on attrition, one of the key WHO reportable indicators, should be periodically reported. Until now, there has been a paucity of data to describe attrition from ART care in Chokwe District, Mozambique. This study aimed to describe the incidence and predictors of attrition (death and loss to follow-up) in adults initiating ART in a rural HIV clinic of Carmelo Hospital Chókwè (CHC), Chokwe District, Mozambique.
Methods
Study design and population
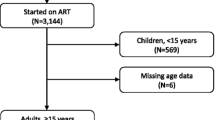
This retrospective cohort study included data for all HIV-infected individuals aged 15 years or older who initiated ART at the CHC between January 2002 and December 2019. Individuals who were referred to the CHC after initiating ART in a center other than CHC were excluded from the analysis (n = 1681) (Fig. 1).
Setting
CHC is a state-owned reference hospital in the district of Chókwè, a large rural district in Gaza Province in southern Mozambique, administered by Catholic missionaries (the Daughters of Charity, Saint Vincent de Paul) since 1993. Chókwè is predominantly rural, with a surrounding catchment population of approximately 200,000 inhabitants. Agriculture is the sole source of income for roughly 80% of the population, and approximately 40% of the population migrates seasonally to South Africa, seeking work in the mines. According to the last national HIV prevalence study in 2015, Gaza Province had the highest provincial HIV prevalence in the country at 24.4%2.
Definitions
We classified the ART outcomes as retention or attrition. Retention (encompassing adherence) was defined as documented active and alive HIV-infected adults who were still on ART (assessed at intervals longer than 6 months post-initiation). Attrition was defined as death and LTFU.
Our reported mortality rate represents the number of documented deaths during ART per 100 person-years of observed therapy. The attrition rate represents the number of patients lost through attrition (death, LTFU) per 100 person-years. Patients were considered LTFU if they did not show up for their consultation in the 90 days preceding data abstraction, with missed treatment pick-up after 3 days of failed calls, unless the medical record stated that the patient had died, stopped ART, or was transferred out. The date of LTFU was recorded as the date of the last recorded visit, or 1 day after ART initiation if the patients never returned after ART initiation. To enable comparison with published literature, we report 1-year attrition15,16. Patients transferred to other facilities during the 18 treatment years were excluded from the 18-year attrition proportion. Transfers were censored in time-to-event analyses at the date of transfer. To facilitate survival analysis, we assumed that individuals who initiated ART but never returned over the 18 years of follow-up contributed 1 day of follow-up. Individuals remaining in care were censored at the end of the 18-year study period. Individuals who were either reported dead, LTFU or had transferred to other health care facilities were censored at their last clinic visit date. To allow comparison with other ART programs in low-resource settings, the first CD4 count was used to estimate the treatment outcome17,18.
CD4 counts were determined using the FlowCount PLG CD4 assay and analyzed with Beckman Coulter Epics XL-MCL Flow Cytometers (both Beckman Coulter, Inc., Johannesburg, South Africa). Blood hemoglobin was determined with an automated hematology analyzer, Sysmex XT2000i (Sysmex Europe, Inc., Hamburg, Germany) or HemoCue photometer assay (HemoCue AB, Helsingborg, Sweden).
Similar to other cohort studies in resource-constrained settings16, adherence to ART was estimated by measuring timeliness of patient visits to scheduled medicine pick-up appointments at clinic-based pharmacies19,20 during the first 6 months of ART.
Categories used to classify exposure to anti-tuberculosis treatment (ATT) were: 1) completed ATT before ART initiation: 2) initiated ATT < 90 days after ART initiation; and 3) unexposed to ATT at ART initiation.
Data collection
Data collection was performed using DREAM software (Diseases Relief through Excellence and Advanced Means), version 6.1.0.757, and electronic medical record (EMRs), designed according to criteria of excellence to manage the prevention and treatment centers of the DREAM Program of Sant'Egidio Community, located in 11 African countries, including Mozambique. Since 2002, the Catholic missionary organizations Daughters of Charity Saint Vincent de Paul (http://www.daughtersips.org/hivaids) and the Community of Sant'Egidio (http://www.dream.santegidio.org) have a collaboration agreement that allows the Daughters of Charity health facilities to use the DREAM software to manage their HIV-infected patients. Carmelo Hospital of Chokwe is a health facility administered by the Daughters of Charity and uses the DREAM Dataset as main the main EMR system. It works through Microsoft Access and JavaScript and is supervised by the hospital's local IT department. All offices work electronically and use the database for routine consultations, so all patient information is automatically stored on a common server. The ART database collects and stores long-term EMRs, sociodemographic data, clinical histories, laboratory tests (hematology, biochemistry), identification codes, scheduling of new appointments, and pharmaceutical stocks.
Recorded data from eligible ART participants for the study were collected and anonymized to remove identifying details: each patient was assigned an alphanumeric code (ID), and the anonymous data were included in the data sheet.
To convert data from Microsoft Access to SPSS, the Access data were first exported to a Microsoft Excel spreadsheet, and in turn the spreadsheet data were imported to SPSS for subsequent data analysis.
For each patient, we included the following data: gender, age (age band and median), point of entry (inpatient or outpatient), CD4 count at baseline, previous ART exposure, previous ATT (ATT prior to ART initiation, ATT within 90 days of ART initiation, and no exposure to ATT), WHO clinical stage of HIV disease, Kaposi’s sarcoma diagnosis, ART therapy, and final ART outcome.
Data analysis
Statistical analysis was performed using IBM SPSS Statistics Software version 25 (IBM Corporation, International Business Machines Corp, Release 2017, https://www.ibm.com/legal/copytrade, USA). Patients’ baseline characteristics, as described above, were compared according to outcomes.
Time-to-event methods were used to investigate predictors of attrition. Time was measured from ART initiation to death or LTFU. Patients still alive at the date of data collection were considered active in ART. Patients who did not come to the clinic for at least 90 days after their last scheduled appointment were considered LTFU18. Attrition was calculated by summing the number of patients who experienced the event (deaths, LTFU) during a particular period divided by the total years of follow-up during this period. Multivariable analysis was performed by Cox regression models. The proportional hazard assumption was assessed with log survival curves based on Schoenfeld residuals. We used Kaplan–Meier survival estimates to calculate the cumulative incidence of attrition. The cumulative incidence of mortality and LTFU were calculated using competing risk analysis.
The Mozambican National Bioethics Committee for Health (Comité Nacional de Bioética para a Saúde, 25/CNBS/2019) approved this analysis. Analysis was performed on de-identified, aggregated patient level data, and no individual informed consent was obtained. The need for written informed consent was explicitly waived, and the research was performed in accordance with the Declaration of Helsinki.
Ethics approval and consent to participate
The Mozambican National Bioethics Committee for Health (Comité Nacional de Bioética para a Saúde, 25/CNBS/2019), approved this analysis (25/CNBS/2019). Analysis was performed on de-identified, aggregated patient level data, and the need for written informed consent was explicitly waived.
Consent for publication
We performed analysis on routine administrative data; consent for publication is not applicable.
Results
ART treatment outcomes
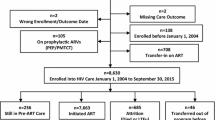
A total of 25,275 HIV-infected adults aged 15 years or older were enrolled in HIV care from 1 January 2002 to 31 December 2019. Of these, 16,321 (64.6%) initiated ART at CHC during the 18-year study period (Fig. 1).
Of the total sample, 2135 (13.1%) were listed as transferred to another facility, while 7279 (44.6%) were active on ART during the study period (Fig. 2).
Median age at enrollment was 35 years (interquartile range [IQR] 15–93); 9657 (59.2%) were women, and 6190 (37.9%) were aged 25–34 years. Median follow-up was 9 years (IQR, 1.0–18.0 years). The vast majority (n = 15,454, 94.7%) were classified as ART-naive (Table 1).
Outpatient consultations were the main source of entry (n = 13,890, 85.1%). At baseline, about a third of the patients (n = 5495, 33.7%) were classified as having WHO clinical stage IV disease. The median CD4 cell count was 192 cells/µL (IQR 0–2654), and nearly a third (n = 4665; 28.6%) of new enrollees had a CD4 count of 200–349 cells/µL. A similar proportion (n = 4667; 28.6%) were in TB treatment within the first 90 days of ART initiation, and 299 (1.8%) patients had Kaposi’s sarcoma (KS). A third (n = 5511; 33.8%) were under the AZT + 3TC + NVP regimen.
Of the 9042 (55.4%) patients with an unfavorable HIV treatment outcome, 2776 (17.0%) died, and 4131 (25.3%) were LTFU (Fig. 2). The proportion of deaths was significantly higher in adults aged 25–34 years (n = 982; 35.4%), those receiving ATT within 90 days of ART initiation (n = 2072; 97.4%), patients with CD4 counts of less than 50 cells/µL (n = 1220; 43.9%), men (n = 1448; 52.2%), those with WHO clinical stage IV of HIV disease (n = 2417; 87.1%), and people starting ART as inpatients (n = 1578; 56.8%);
Patient characteristics by gender, age, and point of entry
Women comprised 59.2% (n = 9657) of the sample initiating ART at CHC; 40.2% (n = 3883) of the women were aged 25–34 years-old, 88.9% (n = 8589) presented to CHC to initiate outpatient the ART regimen; 32.6% (n = 3145) had CD4 counts of 200–349 cells/µL, and 32.8% (n = 3168) presented with WHO clinical stage I disease (p < 0.0001) (Table 2).
Patient characteristics by CD4 count, WHO clinical stage, and ATT exposure
Of the total sample, 16.6% (n = 2713) of the patients initiated ART with CD4 counts of less than 50 cells/µL. Over half of these (n = 1474, 54.3%) were men, 39.6% (n = 1071) were aged 25–34 years, 39.6% (n = 1075) presented to CHC to initiate ART as inpatients; 64.7% (n = 1654) started ATT within 90 days of ART initiation, and 66.9% (n = 1814) presented with WHO clinical stage I disease (Table 3).
Cox proportional hazards model for attrition
Among the 2776 PLWHIV who died, 1448 (52.2%) were men, 982 (35.4%) were aged 25–34 years, 1578 (56.65%) started ART as inpatients, 1220 (43.9%) had CD4 counts of less than 50 cells/µL, 2072 (97.4%) were on ATT within 90 days of ART initiation, 2627 (84.6%) were ART-naïve, 133 (4.8%) had Kaposi’s sarcoma, 2417 (87.1%) presented with WHO stage IV disease, and 638 (23.0%) initiated ART on D4T (stavudine 30 mg) + 3TC (lamivudine 150 mg) + NVP (nevirapine 200 mg) (Table 1).
Overall, the 16,321 adults on ART contributed a total of 72,987 person-years to the study data. The overall attrition rate was 9.46 per 100 person-years. According to the multivariable Cox regression model, women were at lower risk of attrition than men (hazard ratio [HR] 0.930, 95% confidence interval [CI] 0.869–0.996; p = 0.039). Patients aged 35–44 years old were at lower risk of attrition compared to those aged 55 years or older (HR 0.83, 95% CI 0.74–0.94, p = 0.002).
Those who initiated ART as inpatients carried a three-fold higher risk of attrition compared to outpatients (HR 3.18, 95% CI 2.89–3.50, p < 0.001). Receiving ATT within 90 days of ART initiation was associated with a six-fold higher risk of attrition compared to those who were not on ATT (HR 6.53, 95% CI 5.72–7.45, p < 0.001). Compared to patients with WHO clinical stage IV disease, every other stage was associated with three times higher risk of attrition (stage I: HR 3.52, 95% CI 2.99–4.13, p < 0.001; stage II: HR 3.46, 95% CI 2.96–4.05, p < 0.001; stage III: HR 3.75; 95% CI 3.21–4.37, p < 0.001). Having Kaposi’s sarcoma was also associated with a higher risk of attrition (HR 1.99, 95% CI 1.65–2.39, p < 0.001).
Compared to a CD4 count of more than 500 cells/µL, a CD4 count of less than 50 cells/µL was associated with a higher risk of attrition (HR 1.91, 95% CI 1.63–2.24, p < 0.001), while a CD4 count of 350–499 cells/µL was associated with a lower risk (HR 0.79, 95% CI 0.65–0.97, p < 0.023) (Table 4).
Women, patients who were ART-naïve, those with CD4 count of more than 200 cells/µL at ART enrollment year, and certain drug combinations (ABC/AZT/D4T + 3TC + LPV/RTV,) were not associated with higher mortality (Table 4).
Survival estimates
Kaplan–Meier estimates for the probability of 18-year survival by CD4 cell count on ART initiation are presented in Fig. 3.
PLWHIV who had a CD4 count of more than 500 cells/µL had the highest probability of approximately 90% of survival at 18 years following ART initiation, while those with a CD4 count of less than 50 cells/µL had a survival probability of approximately 40% at 18 years in comparison.
Discussion
In our cohort, patients with CD4 counts of less than 50 cells/µL had almost double the attrition risk compared to those with CD4 counts of more than 500 cells/µL. These findings are a little higher than observed in other Mozambican literature21. An in-depth analysis of our cohort showed that the frequency of attrition in the patients with low CD4 cell counts was 26.3%; of these, 43.9% died, and 14.5% were lost to follow-up. Other studies have reported high mortality and LTFU in severely immunosuppressed patients22,23, highlighting LTFU as a fundamental problem for the health care in PLHIV. Risk factors for ART interruption are still poorly understood in many settings, including Mozambique.
The survival analysis shows that in our cohort, patients with CD4 counts under 50 cells/µL had an 18-year survival rate of less than 50%. These data are consistent with other reports24. The baseline CD4 cell count is the dominant prognostic factor for survival in PLHIV starting ART25. Our study showed that patients who started treatment at an advanced disease stage are at a higher risk of mortality than those who start treatment with higher CD4 cell counts, even if they survive the first 5 years of therapy. These findings are in keeping with studies from high-income settings25.
On analyzing the first 2 years of follow-up, we observed a considerable drop in the survival curve among people with CD4 counts of less than 50 cells/µL, from 100 to 60%; by 5 years, survival stood at just 50%. This finding is strongly related to the fact that severe immunosuppression in this population is not restricted to HIV infection alone; rather, multiple factors are at play, including hunger, adult malnutrition26, and poor nutritional supplementation27. These people are considered “immunological non-responders,” that is, HIV-infected individuals who fail to achieve normalization of CD4+ T-cell counts despite persistent virological suppression, which leads to AIDS and non-AIDS comorbidities28,29. This population continues to have a substantially increased mortality ratio even after 3 years of treatment. Thus, clinical factors such as immunological non-response, AIDS and non-AIDS-related multiple events predict a substantially increased risk of death after 3 years of ART treatment30,31.
Recovery of CD4 lymphocytes occurs in the first 2 years after starting ART and is associated with age and the pre-existing degree of HIV-1-related immunodeficiency, so long-term exposure to HIV-1 infection damages the immune system in ways that are difficult to correct due to exhaustion32,33,34.
Better understanding of patterns and risk factors that cause-specific mortality in the ART era can inform the development of appropriate care for HIV-infected individuals and guidelines for risk factor management35. This may include screening programs and intensive adherence counseling34.
We observed that patients who received ATT within the first 90 days of their ART initiation had a six-fold higher risk of attrition than those who were unexposed to ATT. These results are a little higher than those observed in other Mozambican studies36. In our cohort, the attrition rate on active TB patients was 24.13 per 100 person-years, and the frequency of attrition was 49.7%. The overwhelming majority of patients receiving ATT within 90 days (97.4%) died, while 25.3% of those LTFU had a fatal outcome. These results show high mortality but low LTFU, so having TB is a favorable predictor against LTFU.
In our sample, men were underrepresented (39%), as published elsewhere37. There were few men who went to health facilities to test for HIV and to initiate and adhere to ART38,39. We fully acknowledge that more women than men take an active role in accessing HIV services, and studies show that their adherence to ART is also higher40. This tendency may be rooted in different dimensions of gender norms in rural areas41.
Our cohort shows a higher ART initiation rate, of around 37.9%, and a higher death rate among 25–34-year olds (35.4%) compared to an Ethiopian University Teaching Hospital study, which reported an ART initiation rate of 27.9% in the same age group24. Just over 20% of men initiated ART as inpatients, compared to about 11% of women. Similarly, 22.1% of men started ART with CD4 counts of less than 50 cells/µL, compared to 12.8% of women, in keeping with reports elsewhere25.
With this advanced immune suppression, then, a high proportion of men started ART with advanced AIDS and AIDS Clinical Stage IV (45.0% in men vs. 25.8% in women). Immune reconstitution syndrome was closely associated with tuberculosis, confirming previous findings from our cohort and other resource-limited settings42,43. This syndrome was more frequent in men (40.8% vs 22.2%), and over the course of 18 years it was observed that the male population was always on a smaller scale.
Women comprised the majority of the population at 60% compared to men, but half (49%) were classified in the attrition group. Being a woman was not a statistically significant favorable predictor in our cohort.
Among the patient group with CD4 counts of less than 50 cells/µL, over half were men, 39.55% were aged 25–34 years, and 65% had started ATT within 90 days of initiating ART. These findings are consistent with data from other studies44,45, which reveal a greater predominance of men among patients with late HIV diagnosis as well as late-onset ART. The care, ART initiation and treatment monitoring are in accordance with the WHO and national ART guidelines, which were updated successively in 200246, 200947, 201348, 201649, 201750 and 201951. The number of participants changes over time according to these updates (Supplementary Table). From 2002 to 2008 (Era CD4 < 200 cell count46), there was a progressive increase in the number of patients starting ART and a stabilization in the number of new ART initiations in the latest years of the regimen. Both in Era CD4 < 250 cell count (years 2009–201247) and Era CD4 < 350 cell count (years 2013–2015)48), there was a considerable increase in recruitment at 1 year after the implementation of the new ART eligibility criteria, which subsequently decreased, in part due to the expansion of ART services in several other new peripheral health facilities. In the CD4 < 500 era (years 2017–201850) there was no increase but a continued decrease in recruitment, a reflection of the efforts of the Ministry of Health and cooperating partners (the Global Fund, and PEPFAR) to expand the health network that allows for the distribution of patients to other peripheral facilities.
In 2006/200746, the number of new patients who started ART doubled compared to the years 2004/2005 (Supplementary Table S1). This is partly due to community awareness campaigns on ART treatment, as well as the influx of patients into inpatient services. At that time, many patients were diagnosed at the advanced stage of the AIDS disease, consequently with severe immunosuppression.
The years 2014/201548 were the first following implementation of other eligibility criteria that defined co-infection with TB, Hepatitis B, cancer, and HTLV as criteria for starting ART, regardless of WHO stage or CD4 cell count (Supplementary Table S1). Thus, these data contributed to a substantial increase in patients with CD4 counts of 350–399 cells/µL.
The now outdated D4T-based regime was used as a first-line treatment from 2002 to 201846,47,48,49,50, and as an alternative line in patients with Hgb anemia of less than 8 g/dL at the time of screening. Another outdated initial regime, based on AZT, was used from 2009 to 201847,48,49,50. The updated TDF-based regime has been used since 201348, and the current DTG-based regime since 201951, a period characterized by the definitive discontinuation of INNRT (NVP/EFV) drugs.
The strengths of this study reside in the large sample size and the long study period. Limitations include those of many retrospective studies in which data collection is often inadequate and incomplete, without baseline viral load and data to monitor therapeutic failure. This may have an influence on the reported results, mainly for death and LTFU.
Conclusion
The results of this cohort study indicate that attrition after ART initiation is high. Nearly 40% of patients die or are LTFU. Low CD4 cell counts, starting ART as an inpatient, WHO clinical stage IV, and being a man were strongly associated with attrition. Although the mortality of patients diagnosed with tuberculosis after ART initiation was extremely high, patients who started anti-tuberculosis treatment before ART had similar attrition as patients without tuberculosis. The results of this study indicate that interventions aimed at initiating patients sooner after HIV seroconversion, improving the diagnosis and treatment of TB and other comorbidities (like Kaposi’s sarcoma), and giving extra support to patient groups at higher risk of attrition could potentially reduce the mortality and LTFU in HIV programs in Mozambique.
Data availability
The datasets analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
Abbreviations
- 3TC:
-
Lamivudine
- ABC:
-
Abacavir
- ART:
-
Antiretroviral therapy
- ATT:
-
Anti tuberculosis treatment
- AZT:
-
Zidovudine
- BMI:
-
Body mass index
- CHC:
-
Carmelo Hospital Chókwè
- D4T:
-
Stavudine
- DGT:
-
Dolutegravir
- EFV:
-
Efavirenz
- HR:
-
Hazard ratio
- LPV/RTV:
-
Lopinavir/ritonavir
- LTFU:
-
Loss to follow up
- NVP:
-
Nevirapine
- PEPFAR:
-
President’s emergency plan for AIDS relief
- PLWHIV:
-
People living with HIV
- Person-years:
-
Person-years of observed therapy
- UNAIDS:
-
United Nations Programme on HIV/AIDS
- WHO:
-
World Health Organization
References
Central Inteligence Agency, Library, publications, the world factbooks, Africa, Mozambique, Available: https://www.cia.gov/library/publications/the-world-factbook/geos/mz.html Accessed 29 July 2020
Ministerio de Saude (MISAU), Instituto Nacional de Estatıstica, ICF International. Inquerito de Indicadores de Imunização, Malaria e HIV/SIDA em Moçambique (IMASIDA) 2015. Maputo, Moçambique: Rockville, Maryland, EUA: INS, INE, and ICF; 2018 Feb. Available: http://dhsprogram.com/pubs/pdf/AIS12/ AIS12.pdf Accessed 29 July 2021
UNAID, Mozambique, report, Available: https://www.unaids.org/en/regionscountries/countries/mozambique Accessed : 29 January 2021
UNAIDS, AIDSinfo, Mozambique, People Living with HIV Available: https://aidsinfo.unaids.org/?did=5581277ae9beccab3bd5a44e&r=world&t=null&tb=c&bt=undefined&ts=0,0&cl=MOZ&cr=n&ct=2010&cav=Population: All&aid=5772878c9888f63937b6b1e5. Accessed 19 July 2021
Ministerio de Saude (MISAU). Relatorio Anual das Actividades Relacionadas ao HIV/SIDA 2013. Maputo, Moçambique: MISAU; 2014 Abril p. 50. Available: http://www.misau.gov.mz/index.php/relatorios-anuais? download=88:relatorio-anual-hiv-2013-final Accessed 29 July 2021
Ministerio de Saude (MISAU). Relatorio Anual das Actividades Relacionadas ao HIV/SIDA 2017. Maputo, Moçambique: MISAU; 2018 Abril p. 96. Available: http://www.misau.gov.mz/index.php/relatorios-anuais? download=145:relatorio-anual-hiv-2017-final Accessed 29 July 2021.
UNAIDS, Understanding_FastTrack Accelerating Action to End AIDS Epidemic by 2030, Available: https://www.unaids.org/sites/default/files/media_asset/201506_JC2743_Understanding_FastTrack_en.pdf Accessed 29 January 2021
Auld, A. F. et al. Four-year treatment outcomes of adult patients enrolled in Mozambique’s rapidly expanding antiretroviral therapy program. PLoS ONE 6(4), e18453. https://doi.org/10.1371/journal.pone.0018453 (2011).
Teasdale, C. A. et al. Expansion and scale-up of HIV care and treatment services in four countries over ten years. PLoS ONE 15(4), e0231667. https://doi.org/10.1371/journal.pone.0231667 (2020).
Agarwal, M. et al. Sex differences in mortality and loss among 21,461 older adults on antiretroviral therapy in Sub-Saharan Africa. J. Acquir. Immune Defic. Syndr. 73(2), e33–e35. https://doi.org/10.1097/QAI.0000000000001117 (2016) (PMID: 27632148).
Mecha, J. O. et al. Trends, treatment outcomes, and determinants for attrition among adult patients in care at a large tertiary HIV clinic in Nairobi, Kenya: a 2004–2015 retrospective cohort study. HIV AIDS (Auckl). 10, 103–114. https://doi.org/10.2147/HIV.S153185 (2018).
Wekesa, P. et al. Factors associated with 36-month loss to follow-up and mortality outcomes among HIV-infected adults on antiretroviral therapy in Central Kenya. BMC Public Health 20(1), 328. https://doi.org/10.1186/s12889-020-8426-1 (2020).
Onoya, D. et al. Attrition in HIV care following HIV diagnosis: a comparison of the pre-UTT and UTT eras in South. Africa. J Int AIDS Soc. 24(2), e25652. https://doi.org/10.1002/jia2.25652 (2021).
Ciccacci, F. et al. Hematologic alterations and early mortality in a cohort of HIV positive African patients. PLoS ONE 15(11), e0242068. https://doi.org/10.1371/journal.pone.0242068 (2020).
World Health Organization. Antiretroviral therapy for HIV infection in adults and adolescents in resource-limited settings: towards universal access. Recommendations for a public health approach 2006 revision. Available: https://www.who.int/hiv/pub/guidelines/artadultguidelines.pdf?ua=1 Accessed 29 January 2021
Minn, A. C. et al. Attrition among HIV positive children enrolled under integrated HIV care programme in Myanmar: 12 years cohort analysis. Glob Health Action. 11(1), 1510593. https://doi.org/10.1080/16549716.2018.1510593 (2018).
Silverman, R. A. et al. Predictors of mortality within the first year of initiating antiretroviral therapy in urban and rural Kenya: a prospective cohort study. PLoS ONE 14(10), e0223411. https://doi.org/10.1371/journal.pone.0223411 (2019).
Gesesew, H. A., Ward, P., Woldemichael, K. & Mwanri, L. Early mortality among children and adults in antiretroviral therapy programs in Southwest Ethiopia, 2003–15. PLoS ONE 13(6), e0198815. https://doi.org/10.1371/journal.pone.0198815 (2018).
Gaifer, Z. & Boulassel, M. R. Comparative analysis of two methods of measuring antiretroviral therapy adherence in HIV-infected omani patients. J. Int. Assoc. Provid. AIDS Care. https://doi.org/10.1177/2325958219867316 (2019).
Basu, S., Garg, S., Sharma, N. & Singh, M. M. Improving the assessment of medication adherence: challenges and considerations with a focus on low-resource settings. Ci Ji Yi Xue Za Zhi. 31(2), 73–80. https://doi.org/10.4103/tcmj.tcmj_177_ (2019).
Molfino, L. et al. High attrition among HIV-infected patients with advanced disease treated in an intermediary referral center in Maputo, Mozambique. Glob Health Action. 8(7), 23758. https://doi.org/10.3402/gha.v7.23758 (2014).
Silverman, R. A. et al. Predictors of mortality within the first year of initiating antiretroviral therapy in urban and rural Kenya: a prospective cohort study. PLoS ONE https://doi.org/10.1371/journal.pone.0223411 (2019).
Telele, N. F. et al. Baseline predictors of antiretroviral treatment failure and lost to follow up in a multicenter countrywide HIV-1 cohort study in Ethiopia. PLoS ONE 13(7), e0200505. https://doi.org/10.1371/journal.pone.0200505 (2018).
Fekade, D. et al. Predictors of survival among adult Ethiopian patients in the national ART program at seven university teaching hospitals: a prospective cohort study. Ethiop. J. Health Sci. 27(Suppl 1), 63–71. https://doi.org/10.4314/ejhs.v27i1.7s (2017).
Ren, L. et al. Prognosis of HIV patients receiving antiretroviral therapy according to CD4 counts: a long-term follow-up study in Yunnan, China. Sci. Rep. 7(1), 9595. https://doi.org/10.1038/s41598-017-10105-7 (2017).
Alebel, A., Demant, D., Petrucka, P. & Sibbritt, D. Effects of undernutrition on mortality and morbidity among adults living with HIV in sub-Saharan Africa: a systematic review and meta-analysis. BMC Infect. Dis. 21(1), 1. https://doi.org/10.1186/s12879-020-05706-z (2021).
Fuseini, H., Gyan, B. A., Kyei, G. B., Heimburger, D. C. & Koethe, J. R. Undernutrition and HIV infection in sub-Saharan Africa: health outcomes and therapeutic interventions. Curr HIV/AIDS Rep. 18(2), 87–97. https://doi.org/10.1007/s11904-021-00541-6 (2021).
Shete, A. et al. Incomplete functional T-cell reconstitution in immunological non-responders at one year after initiation of antiretroviral therapy possibly predisposes them to infectious diseases. Int. J. Infect. Dis. 81, 114–122. https://doi.org/10.1016/j.ijid.2019.01.017 (2019).
Yang, X. et al. Incomplete immune reconstitution in HIV/AIDS patients on antiretroviral therapy: challenges of immunological non-responders. J. Leukoc. Biol. 107(4), 597–612. https://doi.org/10.1002/JLB.4MR1019-189R (2020).
Lilian, R. R. et al. CD4 testing after initiation of antiretroviral therapy: analysis of routine data from the South African HIV programme. South Afr. J. HIV Med. 21(1), 1165. https://doi.org/10.4102/sajhivmed.v21i1.1165 (2020).
Han, W. M. et al. Characteristics of suboptimal immune response after initiating antiretroviral therapy among people living with HIV with a pre-treatment CD4 T cell count <200 cells/mm3 in Thailand. J. Virus Erad. 6(3), 100005. https://doi.org/10.1016/j.jve.2020.100005 (2020).
Song, A. et al. Effects of early and delayed antiretroviral therapy on plasma anti-CD4 autoreactive IgG and its association with CD4+ T-cell recovery in acute HIV-infected individuals. Front. Pharmacol. 11, 449. https://doi.org/10.3389/fphar.2020.00449 (2020).
Stirrup, O. T. et al. CASCADE Collaboration in EuroCoord. Predictors of CD4 cell recovery following initiation of antiretroviral therapy among HIV-1 positive patients with well-estimated dates of seroconversion. HIV Med 19(3), 184–194. https://doi.org/10.1111/hiv.12567 (2018).
Gunda, D. W., Kilonzo, S. B., Kamugisha, E., Rauya, E. Z. & Mpondo, B. C. Prevalence and risk factors of poor immune recovery among adult HIV patients attending care and treatment centre in northwestern Tanzania following the use of highly active antiretroviral therapy: a retrospective study. BMC Res. Notes. 10(1), 197. https://doi.org/10.1186/s13104-017-2521-0.PMID:28595630;PMCID:PMC5465538 (2017).
Portilla-Tamarit, J., Reus, S., Portilla, I., Fuster Ruiz-de-Apodaca, M. J. & Portilla, J. Impact of advanced HIV disease on quality of life and mortality in the era of combined antiretroviral treatment. J. Clin. Med. 10(4), 716. https://doi.org/10.3390/jcm10040716 (2021).
Nacarapa, E. et al. Effect of Xpert MTB/RIF testing introduction and favorable outcome predictors for tuberculosis treatment among HIV infected adults in rural southern Mozambique, a retrospective cohort study. PLoS ONE 15(3), e0229995. https://doi.org/10.1371/journal.pone.0229995 (2020).
Baisley, K. et al. High HIV incidence and low uptake of HIV prevention services: the context of risk for young male adults prior to DREAMS in rural KwaZulu-Natal, South Africa. PLoS ONE 13(12), e0208689. https://doi.org/10.1371/journal.pone.0208689 (2018).
Medina-Marino, A. et al. Outcomes from a multimodal, at-scale community-based HIV counselling and testing programme in twelve high HIV burden districts in South Africa. J. Int. AIDS Soc. 24(3), e25678. https://doi.org/10.1002/jia2.25678 (2021).
Shamu, S. et al. Comparison of community-based HIV counselling and testing (CBCT) through index client tracing and other modalities: outcomes in 13 South African high HIV prevalence districts by gender and age. PLoS ONE 14(9), e0221215. https://doi.org/10.1371/journal.pone.0221215 (2019).
Kehoe, K. et al. Long-term virologic responses to antiretroviral therapy among HIV-positive patients entering adherence clubs in Khayelitsha, Cape Town, South Africa: a longitudinal analysis. J Int AIDS Soc. 23(5), e25476. https://doi.org/10.1002/jia2.25476 (2020).
Pulerwitz, J. et al. Gender norms and HIV testing/treatment uptake: evidence from a large population-based sample in South Africa. AIDS Behav. 23(Suppl 2), 162–171. https://doi.org/10.1007/s10461-019-02603-8 (2019).
Stek, C. et al. Diagnostic accuracy of the INSHI consensus case definition for the diagnosis of paradoxical tuberculosis-IRIS. J. Acquir. Immune Defic. Syndr. 86(5), 587–592. https://doi.org/10.1097/QAI.0000000000002606 (2021).
Namale, P. E. et al. Paradoxical TB-IRIS in HIV-infected adults: a systematic review and meta-analysis. Future Microbiol. 10(6), 1077–1099. https://doi.org/10.2217/fmb.15.9 (2015).
Wandeler, G. et al. Outcomes of antiretroviral treatment programs in rural Southern Africa. J. Acquir. Immune Defic. Syndr. 59(2), e9-16 (2012).
Gupta, A. et al. Early mortality in adults initiating antiretroviral therapy (ART) in low- and middle-income countries (LMIC): a systematic review and meta-analysis. PLoS ONE 6(12), e28691 (2011).
World Health Organization. (2002). Scaling up antiretroviral therapy in resource-limited settings: guidelines for a public health approach: executive summary (No. WHO/HIV/2002.01). World Health Organization. Accessed 22 June 2021
Guia de tratamento antiretroviral e infecções oportunistas no adulto, adolescente e grávida. 2009. https://www.who.int/hiv/pub/guidelines/mozambique_art.pdf Accessed 22 June 2021
Orientação novas normas TARV adultos 23–04–2013. https://comitetarvmisau.co.mz/docs/orientacoes_nacionais/Orientacao%20novas%20normas%20TARV%20pediatrico%2029-05-2013.pdf Accessed 22 june 2021
Norma TARV adultos Março 2016 https://comitetarvmisau.co.mz/docs/orientacoes_nacionais/NORMA_TARV_ADULTOS_MARCO_2016.pdf Accessed 22 June 2021
Circular Testar e Tratar fase 2 Fevereiro 2017 https://comitetarvmisau.co.mz/docs/orientacoes_nacionais/circular_testar_e_Iniciar_fase%202.pdf Accessed 22 June 2021
Circular Normas Clínicas 08.03.19 https://comitetarvmisau.co.mz/docs/orientacoes_nacionais/Circular_Normas_Cl%C3%ADnicas_Actualizadas_29_11_19.pdf Accessed 22 June 2021
Acknowledgements
The authors thank the patients and staff of Carmelo Hospital of Chókwè, Gaza Province, Mozambique, for their co-operation. The authors also want to express their gratitude to the Daughters of Charity of Saint Vincent of Paul for granting the researchers access to hospital facilities and patient records. Many thanks also to Isabelle Casavant, Marcelo de Almeida, and Meggan Harris for additional support.
Author information
Authors and Affiliations
Contributions
E.N., contribute on study design, data acquisition, study implementation, analysis and implementation of data, major contribution to writing, read an approved final version. E.V., J.N., A.M., & B.C., contributed equally on data acquisition, study implementation, read and approved final version. D.O., D.M., R.P., D.T. & I.M., contribute equally on study design, read an approved final version. A.C., B.R., S.A. & M.S. contribute equally on study design, analysis and implementation of data, major contribution to writing, read an approved final version. D.T. & J.M.R.R. contribute on study design, analysis and implementation of data, major contribution to writing, read an approved final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nacarapa, E., Verdu, M.E., Nacarapa, J. et al. Predictors of attrition among adults in a rural HIV clinic in southern Mozambique: 18-year retrospective study. Sci Rep 11, 17897 (2021). https://doi.org/10.1038/s41598-021-97466-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-97466-2
- Springer Nature Limited