Abstract
Background
Despite the availability of an effective vaccine, chronic hepatitis B virus (HBV) infections remain a major cause of liver cirrhosis and hepatocellular carcinoma. HBV burden in pregnancy, risk factors and the timing of mother to child transmission remain poorly described especially during this era of lifelong use of Tenofovir/Lamivudine/Efavirenz as firstline for HIV treatment. We aimed to determine the burden of HBV in pregnancy and infants receiving their first dose of HBV vaccine 6 weeks after birth in a high HIV-prevalence setting.
Methods
Pregnant women ≥ 20 weeks’ gestational age were enrolled and followed up as mother-infant dyads from delivery, 6, 24 and 96 weeks after birth. HBV surface antigen (HBsAg) was tested (fresh plasma, immunochromatography) in pregnancy. Women testing HBsAg-seropositive were further evaluated for other four HBV-biomarkers. Maternally HBV exposed babies were tested for HBsAg from birth and HBs-antibodies from 6 months of age. Maternal-infant factors were tested in univariable and multivariable analyses for predictors of HBsAg-seropositivity.
Results
Six hundred HIV-uninfected and 608 HIV-infected women on Tenofovir/Lamivudine/Efavirenz-regimen with median (interquartile range) 350: (87–1477) days of therapy use were enrolled. The overall HBsAg-seroprevalence was 32/1208: 2.65%, 95% confidence interval (CI) [1.74, 3.55]; being 7/600: 1.17%, 95% CI [0.37, 1.97] and 25/608: 4.11%, 95% CI [2.52, 5.68] in HBsAg-monoinfected and HBsAg/HIV-coinfected respectively, disproportionately detected in 31/32: 96.9%, 95% CI [90.8, 100] women presumably HBV-unvaccinated in infancy.
HBV exposed babies tended to be born prematurely (< 37 weeks); 15.2% versus 9.9% in the HBV-unexposed, p = 0.009.
In multivariate logistic regression-models with variable elimination, HIV-infection and reported tooth extractions predicted antenatal HBsAg-seropositivity; odds ratios (CI): 3.85 (1.61–10.7) and 2.46 (1.07–5.34), respectively.
None of the exposed infants were HBsAg-seropositive neither before nor after 6 weeks of age. No HBs-antibodies were detected in 23.3% of HBsAg-exposed infants at two years despite having successfully completed the HBV vaccination schedule.
Conclusion
Low and moderate HBV endemics were observed in HIV-uninfected and HIV-infected pregnant women, respectively. This underscores the need to routinely screen for HBV in pregnancy, especially the HIV-infected attending antenatal-care. Being HIV-infected and reported tooth extractions were independent risk factors for maternal HBsAg-seropositivity. Vertical and child horizontal transmissions were both absent, probably due to ~ the 50% frequency of antenatal anti-HBe-antibodies observed. Of concern was the absence of anti-HBs-antibodies in 23.3% of fully vaccinated/maternally HBV-exposed infants by two years. Absence of molecular diagnosis may have underestimated HBV burden.
Trial registration
www.clinicaltrials.gov, trial registration number: NCT04087239.
Similar content being viewed by others
Introduction
Hepatitis B virus (HBV) is a carcinogenic DNA virus that causes acute and chronic liver diseases, disproportionately affecting young African adults [1]. Chronic HBV infection is associated with a 15%-40% increased risk of the development of cirrhosis, hepatic failure and hepatocellular carcinoma (HCC) [2]. Such persistent viral infections are characterized by host weak immune responses with subsequent development of ineffective CD8 + T-lymphocyte responses [3, 4], a situation exacerbated by HIV infection endemic in sub-Saharan Africa (SSA). HIV accelerates progression to cirrhosis and liver cancer. On the other hand, HBV infection has been associated with poor CD4 + T-lymphocyte cell recovery despite successful HIV-RNA suppression [5, 6]. Understanding HBV/HIV dynamics in expecting mothers is essential since pregnancy as a condition is characterised by anti-inflammatory Th2-cytokines associated with increased risk of infections.
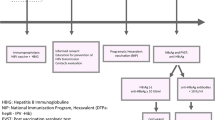
High sero-prevalence rates of hepatitis B surface antigen (HBsAg) in SSA of up to 20% in pregnant women [7, 8] remain a risk factor for onward vertical transmission. In these settings, HBV is commonly spread from an infected mother to her child at birth or by the horizontal route through mastication of foods, use of unsterile sharps or from an infected sibling to an uninfected child during the first 5 years of life [9]. However, exactly how and when African infants get infected remain poorly described. Elucidating this is critical since HBV infection acquired later in adulthood causes chronic hepatitis in only < 5% of cases, whereas infection in infancy and/or early childhood leads to chronic hepatitis in 95% of cases [10, 11]. This observation underscores the need to strengthen early-life HBV prevention strategies. Maternal screening programs are aimed at identifying the presence of HBsAg. Once the HBsAg-seropositive mothers are identified their exposed babies should receive passive immunoprophylaxis of specific hyperimmune immunoglobulin or monoclonal antibody preparations at birth to reduce the risk of vertical HBV transmission. Active immunoprophylaxis involves the administration of HBV vaccine birth dose followed by 2 doses given at least 4 weeks apart to complete the vaccination series for protection against HBV infections [11].
Universal HBV infant immunisation was instituted in Zimbabwe in 1988 followed by universal access since 1996. The vaccine is combined with the other four antigen preparations to form a pentavalent administered at 6, 10 and 14 weeks after birth. The Zimbabwe Demographic and Health Survey that assesses demography and health information of common infections through sampling a representative sample of ≥11,000 households nationwide every five years since 1988, reported the HBV vaccination coverage of 90% and 83% for the first and third dose, respectively [12]. However, the current administration of first dose of the HBV vaccine at 6 weeks of age rather than at birth remains a great concern as infants are thought to be vulnerable from birth until about 6 weeks of age, yet there is not much local evidence on the burden of perinatal transmission of HBV. Furthermore, there are research gaps in immunoprophylaxis failure rates of fully vaccinated maternally HBV-exposed babies.
The last comprehensive Zimbabwean report on the burden of HBV in pregnancy was described over 20 years ago before the advent of life-long antiretroviral therapy (ART) when HBsAg-seropositivity was 25% in the 1990s [13]. Around 2009, HBV sero-prevalence rates fell to ~ 3.3%, 3.0% and 3.7% in rural, peri-urban and urban pregnant women, respectively [14]. Thus, there is a paucity of contemporary and comprehensive data on HBV-infection in pregnant women and their infants including associated risk factors. Therefore, we aimed to determine the prevalence of HBsAg sero-status in pregnancy, the vertical and horizontal transmission rates in maternally exposed infants receiving their first dose of HBV vaccine at 6 weeks after birth.
We aimed to;
-
1.
Determine HBsAg sero-prevalence in pregnant women, and describe the associated risk factors.
-
2.
Characterise other HBV biomarkers; anti-HBs antibodies, HBeAg, anti-HBe antibodies, anti-HBc-antibodies in all HBsAg sero-positives. Women testing positive for just HBsAg marker only at baseline were re-tested every 6 months.
-
3.
Determine any associations between HBsAg seropositivity and adverse pregnancy outcomes (low birth weight; <2500g, prematurity; < 37 weeks).
-
4.
Determine HBsAg seropositivity rate in maternally HBV exposed babies from birth and 6 weeks of age (vertical transmission) and horizontal transmission from 6 months of age, i.e. at least 2 months following receipt of the last dose of HBV vaccine up until 2 years of age.
-
5.
Determine the immunoprophylaxis failure rate in maternally HBV-exposed but fully vaccinated babies from at least 6 months of age.
Methods
Design of University of Zimbabwe birth cohort study (UZBCS)
The University of Zimbabwe College of Health Sciences Birth Cohort study (UZBCS) is a prospective observational cohort of HIV-infected and HIV-uninfected pregnant women in a resource limited setting of high-density suburbs west of Harare, Zimbabwe. Kuwadzana, Dzivaresekwa (Rujeko), Glenview and Budiriro Harare Municipal polyclinics were selected based on higher volumes of maternal and child health services, frequency of HIV seropositivity, and lack of competing research activities targeting the same study population [15]. The UZBCS aims to investigate the role of maternal comorbidities, including co-infections with persistent viruses such as HIV, cytomegalovirus, HBV, and other infectious diseases like syphilis. Non-communicable diseases include maternal nutritional status, hypertensive disorders and their roles on pregnancy outcomes, infant mortality and general health [15]. By design at baseline approximately 50% of the expecting women in the UZBCS were HIV-infected as previously described [15]. In this cohort, lack of plasma HIV-RNA suppression is common within the HIV-infected subgroup mainly due to new HIV diagnosis, hence not being on ART and/or suboptimal duration of ART-exposure [16]. HIV-MTCT rate is low as previously reported [17].
Inclusion and exclusion criteria
Inclusion criteria included pregnant women ≥ 15 years of age, ≥ 20 weeks of gestation at enrolment, and planning to deliver at any of the selected 4 study sites.
Exclusion criteria was the presence of severe mental health disorders, rendering the women incapacitated to provide informed consent or comply with study procedures as assessed by the study Clinicians as previously described [15].
Study procedures
The study midwife administered a structured questionnaire to all pregnant women at enrolment aiming at a comprehensive clinical and socio-demographic characterisation. Socio-economic information assessed comprised of employment status, family monthly income, money set aside for food every month, general household characteristics and food security. Further questions addressing maternal life style such as alcohol use, concurrent medications, presence of comorbidities and sexually transmitted infection (STIs), awareness of spouse/intimate partner’s HIV status, including any missed ART doses were asked. At enrolment, the study midwife also performed a full maternal physical examination, including anthropometry of body mass index (BMI) and mid upper arm circumference (MUAC) to assess nutritional status as previously described [15].
Blood collection and analysis
Ten milliliters of maternal venous blood were collected in EDTA and plain tubes at enrolment. Maternal HIV diagnosis was done from an aliquot of whole blood EDTA using qualitative rapid immunochromatographic assays, SD Bioline HIV-1/2 3.0 (Standard Diagnostics Inc., Kyonggi-do, South Korea) and Abbott’s Determine® HIV-1/2. Western Blot was the tie breaker for any indeterminate test results as previously described [15].
Absolute CD4+ T-lymphocyte counts in whole blood EDTA samples were enumerated within a maximum of 6 h after sample acquisition using a Partec Cyflow counter (Cyflow, Partec, Munster, Germany) [15].
Plasma was processed for HIV RNA load testing and stored at -80 °C as previously described [15].
Assessment of serum liver function enzymes alkaline phosphatase (ALP), alanine transaminase (ALT), aspartate transaminase (AST), gamma-glutamyl transferase (GGT) including bilirubin was done using Beckman Coulter AU680 chemistry analyser (Krefeld, Germany) as previously described [15].
Testing of maternal HBsAg
Plasma from all the pregnant women were screened for HBsAg using immunochromatographic SD Bioline HBsAg WB test, Gyeonggi-do, Korea at concentration between 0.52–2.06 IU/ml in the World Health Organisation (WHO) International Biological Reference Preparation for HBsAg [03/262]. All samples were confirmed on a colloidal gold enhanced immunochromatographic assay Advanced Quality ™ one step HBsAg test with a lower detection limit of 1 ng/ml at 15 min as per the manufacturers’ instructions. The SD Bioline HBsAg test kit has a sensitivity and specificity of 100% and 99%, respectively as per the WHO evaluation. In addition, this kit was approved by the Ministry of Health and Child Care for diagnostic use in Zimbabwe. The One Step Advanced Quality HBsAg kit is ISO 13485 certified, and has a 100% sensitivity and a 99.4% specificity.
Testing of other HBV biomarkers in HBsAg seropositive women and infants
All women confirmed for the presence of HBsAg were further tested for the presence of (detection limit) HBsAg (1 ng/ml), HBsAb (30mIU/ml), HBeAg [2 National Clinical Units (NCU)/ml], HBeAb (2 NCU/ml) and HBcAb (2 NCU/ml) simultaneously in one cassette using a lateral flow OnSiteHBV-5 test, an antibody sandwich immunoassay (CTK Biotech, Inc, USA). The clinical performance characteristics of all the 5 HBV biomarkers are good with relative sensitivities of 100%, and relative specificities ≥ 99.5%.
Women testing positive for just the HBsAg biomarker only in pregnancy were re-tested for other HBV biomarkers seroconversions every 6 months until 24 months.
HBV-exposed babies were tested for HBsAg from birth to 6 weeks of age to determine the vertical HBV transmission rate, and further tested thereafter until 2 years of age to assess any presence of horizontal transmission. Anti-HBs antibody develops during convalescence following acute HBV infection or vaccination. Anti-HBs antibody seroconversion was assessed from 6 months of age until 2 years.
Data management and statistical analysis
Data were entered and managed using Research Electronic Data Capture (REDCap v 8.0, © 2020). Quality assurance on the accuracy of data entry included independent double entries and verification in cases of discrepancies.
Statistical analyses
For continuous variables, normalcy testing was performed using the Shapiro–Wilk test. Descriptive summary statistics were calculated for predictor and outcome variables of interest using frequencies and percentages for categorical variables, and medians and interquartile ranges, minimum–maximum for continuous variables. A p-value < 0.05 was considered significant.
In a univariate analysis, risk behavior, presence of an STI, common invasive medical procedures of HBsAg sero-positive and HBsAg sero-negative pregnant women were compared using the Kruskal Wallis test, Mann–Whitney U test or Fisher’s exact test where appropriate. Multivariable logistic regression analyses were performed to estimate the odds ratios (OR) and 95% confidence intervals (CI) for factors associated with HBsAg seropositivity. We used automated variable elimination to obtain simplified models with less parameters but high predictive power. Variable elimination was performed for maximizing the Akaike information criterion which considers both the model fit and the number of parameters (i.e. the Akaike information criterion increases with a better model fit but decreases according to the number of predictors) [18]. To test for predictors of HBsAg seropositivity, we carried out multivariate logistic regression analyses both without and with automated variable elimination. In the initial model (without variable elimination), independent variables such as HIV infection, liver enzymes, tooth extractions, blood transfusion and tattooing were included. Statistical analyses were performed in R Studio, version 4.1.1.
Ethical approval and consent to participate
UZBCS complied with the ethical principles of the Declaration of Helsinki (updated 2013) and was conducted in compliance with the international council for harmonisation of Good Clinical and Laboratory Practice guidelines and local regulatory requirements. Ethical approval was obtained from the Joint Research Ethics Committee (JREC) of the University of Zimbabwe, reference number; JREC/18/15 and the Medical Research Council of Zimbabwe; MRCZ/A/1968.
Results
HBsAg seropositivity rate in pregnancy
Overall antenatal HBsAg seropositivity was 32/1208: 2.65%, 95% CI [1.74, 3.55] being; 7/600: 1.17%, 95% CI [0.37, 1.97] versus 25/608: 4.11%, 95% CI [2.52, 5.68] in HIV-uninfected and -infected subgroups respectively. Among the 32 HBsAg seropositive pregnant women 25/32: 78.1%, 95% CI [63.8, 92.4] were HIV-infected compared to 7/32: 21.9%, 95% CI [7.6, 36.2] who were HIV-uninfected, p < 0.001. In addition, HBsAg presence divided the women on ethnic lines, with infections disproportionately present in Shona speaking women 27/32: 84.4%, 95% CI [71.8, 96.8] compared to 5/32: 15.6%, 95% CI [3.04, 28.2] in non–Shonas, p < 0.001, Table 1. As expected, 31/32: 96.9%, 95% CI [90.8, 100] HBsAg carriers were older, born before 1996, the year the universal hepatitis B vaccine was established in Zimbabwe.
As expected, none of the 32 HBsAg sero-positive women tested positive for anti-HBs-antibodies. In addition, none of these women were positive for the biomarker of viral replication, HBeAg. Interestingly, 47.9% tested positive for anti-HBe-antibodies, whilst 62.5% were positive for anti-HBc-total antibodies.
Of the 15 women who seroconverted from HBeAg to anti-HBe status, 11 (73.3%) were on ART, Table 1. One woman (6.7%) was HIV-infected but ART naïve (was yet to commence) and three (20%) were HIV-uninfected.
10/32: 31%, 95% CI [15.3, 47.2] were seropositive for HBsAg only with no other HBV biomarkers detected in pregnancy. However, HBs-antibodies seroconversions were later detected in 8/10 (80%) in 6–24 months’ later after delivery. The other 2 women continued testing HBsAg positive without seroconversion or presence of any other HBV biomarker(s) up until 2 years after delivery.
Comparison between HBsAg-seropositive and HBsAg-seronegatives
In a univariate analysis, HBsAg-seropositive women were older, median age 32 (27–35.3) versus 28 (23–33) years, p = 0.012. Furthermore, they were more likely to be married, p < 0.001 whilst the frequency of alcohol use, both in general and during pregnancy were significantly higher in the HBsAg negatives both p values < 0.001. The ages of sexual debut and at first pregnancy were comparable between the two groups, Table 1.
The frequency of reported presence of casual sexual partners, children/pregnancies not sharing the same father and use of sexual enhancing practices like vagina tightening herbs were significantly higher in HBsAg seropositive women relative to their seronegative peers, p values < 0.001 for all the 3 factors, but with comparable medians of total numbers of life time sexual partners, Table 1.
Frequency of reported tooth extraction(s) was higher in HBsAg-seropositive women compared to their HBsAg seronegative counterparts, p < 0.001. Interestingly, the latter group reported significantly higher frequency of small skin incision traditional procedures “kutemwa nyora” in vernacular p values < 0.001, Table 1. This involves use of razor blades to deliver powdery herbal medication under the skin.
HBsAg seropositive women were significantly more likely to have syphilis antibodies detected, and reported presence of previous STIs, p value < 0.001. Of concern were comparable rates of reported previous but untreated STIs in about 34% of women in both groups, Table 1.
Serum albumin levels < 35 g/L were observed in 71% versus 59.2% while lower total white blood counts, median (IQR) 5.8 (4.6–6.9) versus 6.8 (5.6–8.1) were observed in HBsAg seropositive compared to the HBsAg seronegative women, p = 0.017 and 0.019, respectively. However, liver enzymes, haemoglobin levels, MUAC and BMI were comparable.
Comparison between HBsAg/HIV coinfected and HBsAg mono-infected pregnant women
In a univariate analysis comparing 25 HBsAg/HIV coinfected, they were older 32 (29–36) versus 27 (26–31) years versus HBV mono-infected (without HIV infection), p = 0.049. Furthermore, they tended to report higher numbers of life time sexual partners to date median (IQR); mini-max of 2 (1–3); 1–30 versus 1 (1–1); 1–1, higher frequency of pregnancies/children not sharing the same father 56% versus 0%, and were more likely to be anemic, haemoglobin levels 10.45 g/dL (9.90–11.53) versus 11.70 g/dL (11.20–11.75), p = 0.010, 0.004 and 0.031, respectively. Otherwise, all other maternal factors including pregnancy outcome, infant mortality were comparable between the two groups, Table 2.
Of the 25 HBsAg/HIV coinfected 5/25: 20%, 95% CI [4.32, 35.68] were virally unsuppressed (HIV-RNA > 1000 copies/mL) with 13/25: 54.4%, 95% CI [25.7, 78.3] being immune compromised, with CD4 + T-lymphocyte counts < 350 cells/µL, not shown.
Due to the higher HIV seropositivity rates in HBsAg seropositive women the higher frequency of previous/current tuberculosis infection observed was not surprising compared to the HBsAg mono-infected, p < 0.001, Table 1.
Frequency of other HBV serological biomarkers; anti-HBsAb, HBeAg, anti-HBe, anti-HB-core-antibodies biomarkers, were comparable between the HBV-mono-infected and HBV/HBV co-infected pregnant women, not shown.
HBsAg seropositivity and adverse birth outcomes and mortality rates
Five of the 33 HBV-exposed babies (including 1 set of twins) were born prematurely (< 37 weeks) 15.2% versus 9.9% in HBsAg seropositive women compared to the HBsAg seronegatives, respectively p = 0.009, Table 1. All the 5 premature births were from the HBsAg positive/HIV-infected group, and of these women, 4 had HIV RNA load < 1000 copies/mL.
Median birth weight was significantly higher, median (IQR) 3200 g (2950–3425) versus 3100 g (2770–3400) in HBsAg seropositive women compared to seronegatives, p < 0.001. Interestingly, no mortality was observed in the former group compared to the 5% mortality rate in the latter. The positive impact of ART is not surprising as HBsAg seropositive women were on therapy for almost a year, median IQR 350 (87–1477) days of ART use, and 80% had HIV-RNA < 1000 copies per mL, Table 1.
Birth length, infant MUAC, including HIV-MTCT rates were comparable between the HBsAg-exposed and unexposed children over the 24 months’ follow-up period, Table 1.
HBV-MTCT rate and infant HBs-antibody seroconversion following vaccination
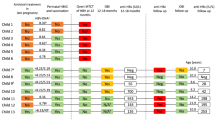
None of the HBV-exposed infants tested HBsAg positive from birth up until 2 years of age. Thus, both the vertical and horizontal HBV-MTCT rate were 0%. In HBV exposed infants with documented records for evidence of receipt of all the 3 doses of HBV vaccine in the Road to Heath Baby Cards, anti HBs-antibodies were detected in 23/30: 76.6%, 95% CI [55.17, 98.17] (3 babies were not tested). Thus, no HBs-antibodies were detected at 2 years of age in 23.3% of HBsAg-exposed infants, despite having successfully completed the HBV vaccination schedule.
There were no significant differences in maternal factors such as HIV status, MUAC including infant factors; sex, prematurity birth weight analysis between babies who had anti-HBs-antibodies present (3 babies were not tested) compared to those without anti HBs-antibodies being detected; Table 3.
Predictors of antenatal HBsAg seropositivity
In a multivariate logistic regression model, with variable elimination, we observed significant associations of a positive maternal HIV status and reported tooth extraction(s) with HBsAg-seropositive, odds ratio (CI): 3.85 (1.61–10.7) and 2.46 (1.07–5.34), respectively, Table 4.
Discussion
We report results on HBsAg seropositivity rates and associated risk factors in 608 HIV-infected pregnant on Tenofovir/Lamivudine/Efavirenz regimen with a median of 350 days since ART initiation alongside their 600 HIV-uninfected counterparts the UZBCS.
Maternally HBV-exposed babies were tested for HBsAg from birth, 6 weeks until 2 years. Presence of infants’ HBs-antibodies was evaluated from 6 months of age.
The following were key findings:
-
a.
In the study population of 1208 pregnant women, the overall HBsAg sero-prevalence was 2.65%, 95% CI [1.74, 3.55], being almost four times higher in the HIV-infected women 4.11%, 95% CI [2.52, 5.68] against 1.17%, 95% CI [0.37, 1.97] in their HIV-uninfected peers.
-
b.
The most important predictors for HBsAg seropositivity were HIV-infection status and reported tooth extraction(s), and these were associated with 3.85 and 2.46 times risks of HBsAg-seropositivity, respectively.
-
c.
The frequency of preterm births was significantly higher in HBsAg/HIV positive women.
-
d.
HBV-MTCT and horizontal transmission rates in maternally HBV-exposed babies receiving their first dose of HBV-vaccine at 6 weeks of age were both 0%.
-
e.
No anti-HBs-antibodies were detected at 2 years of age in 23.3% of fully vaccinated babies.
Overall HBsAg seropositivity in pregnancy
The overall prevalence of HBV was 2.65% in pregnancy; being 1.17% versus 4.1% in the HIV-uninfected and infected. The overall HBsAg sero-prevalence rate of 2.65% was within the intermediate prevalence category. Nevertheless, this figure was much lower compared to the high prevalence category of > 8% observed during the pre-ART era. This was a further drastic decline from very high prevalence rates reported in previous Zimbabwean studies done in the late 1990s where HBsAg seropositivity rate in pregnant women was 25% [13].
However, the HIV-uninfected subgroup’ HBsAg endemicity fell within the low prevalence category.
Our results are comparable to some regional findings, but not others. HBsAg seroprevalence rates in Cameroonian, Nigerian, Rwandan and Mozambican pregnant women ranged from 3.7%-18.4% [19,20,21,22]. Similarly, all these studies were also done during this option B + era of life long ART use. The overall prevalence rate of 2.65% observed in our study is the lowest of them all. This is evidence that Zimbabwe is working steadily towards the target 3.3 of the 2016 Sustainable Development Goals aiming to eliminate viral hepatitis as a public health problem by 2030 through optimising strategies for prevention, diagnosis and treatment of HBV infections [2].
In our study, HIV-positive status and reported tooth extraction(s) were independent risk factors for maternal HBsAg seropositivity. However, being born before the universal implementation of routine hepatitis B immunisation and CD4 count < 350 cells/mL predicted HBsAg positivity in a Sierra Leone study [23]. Bigger studies are warranted to further validate and understand modifiable risk factors around reported tooth extractions processes in our setting.
Previous studies have shown that considerable racial/ethnic disparities exist in HBV infection, exposure and immunity [24]. In our study, the Shona speaking women were more likely to test HBsAg positive compared to non-Shonas.
Encouragingly, our study findings were suggestive of safe traditional medical practices that use sharps. In addition, our blood and blood products from the national blood bank seem safe too.
The overall regional HBV-seroprevalence for SSA’s general population of 2.15% [25] is much higher compared to the 1.17% observed in our cohort among HIV-uninfected pregnant women, a figure well below the 2% threshold, meeting the low HBV prevalence classification. The WHO recommends HBV DNA testing and Tenofovir treatment from weeks 28 to 32 of pregnancy for HBV mono-infected women. However, securing access to the more sensitive HBV-DNA testing and the procurement of single dose Tenofovir for the mono-infected remain essential. There is need for HBsAg, testing, monitoring and treatment services in the public sector in the context of continuum of care and in accordance with universal health coverage. For affordability and more practical purposes, this could be done in synergy and coordination with the successful ongoing HIV prevention and control services to preclude new HBV infections.
HBsAg positive/HIV coinfection
In a Mozambican study, 36.3% of HBsAg-positive pregnant women were HIV coinfected [20]. This figure is much lower compared to the 78.1% observed in our cohort study. In our study, the HIV-infected pregnant women were 3.9 times more likely to be infected with HBV compared to their HIV-uninfected peers.
The HBsAg-positive/HIV coinfection rate of 4.11% observed in our study was very similar to the Rwandan seropositivity rate of 4.1% observed in a much bigger cohort of 13,121 pregnant women [19]. Interestingly, both studies were done during this era of life long ART. Both rates are much lower compared to the 12% rate observed in ART naïve Zimbabwean adults [26]. However, the rate of 4.11% observed in our study done in an urban setting was 5 times higher compared to the 0.8% observed in rural pregnant women in 2010 [14]. Probably the risks are lower for the rural peers. However, our observed rate of 4.11% was higher than the overall pooled prevalence of HBV/HIV co-infection among pregnant mothers in SSA which is 3.30% [25].
HBsAg seropositivity has been significantly associated with anemia and elevated ALT levels [27,28,29] at least in ART naïve adults. However, in our study no significant differences were observed in ALT levels between HBsAg seropositives and negatives. This is not surprising since previous studies demonstrated that serum ALT levels decreased following ART initiation in HIV-infected individuals [30].
ART exposures inhibit HBV replication, and hence could be the explanation underlying the gradual decline of HBV prevalence from as high as 6.36% [25] in reports published during the intrapartum single dose Nevirapine era done between years 2004–2010. The highest prevalence of up to 25% recorded during the pre-ART era in the 1990s [13]. Thus, our conclusion of the current lower HBV burden may not necessarily represent the natural results of transmission due to lifelong Tenofovir/Lamivudine/Efavirenz exposures for HIV therapy. In our study 78.1% of the HBV seropositive women were also coinfected with HIV and were on ART based regimen that also interrupt HBV replication. Tenofovir, which is included in the treatment combinations as first-line therapy for HIV infection, is also active against HBV [11].
HBsAg seropositivity and adverse birth/infant outcomes
There has been conflicting findings on the association of maternal HBV infection and adverse birth outcomes, especially on preterm deliveries [31]. Our study demonstrated that HIV/HBV-exposed babies tended to be born prematurely, but infant birth lengths were comparable. Interestingly, the maternally HIV exposed had heavier birth weights relative to their HBV-unexposed peers. This could be attributed to the positive impact of successful HIV therapy. About 80% HBsAg/HIV co-infected women were on ART for almost at least one year, hence generally were virally suppressed with a fair median CD4 counts of 305 cells/µL. This could also have been the reason behind comparable HBV-MTCT rates observed in HIV-infected/HBsAg seronegative and HIV infected/HBsAg seropositive women.
HBV-vertical and horizontal transmission rates
HBV infection in newborns is defined as HBsAg positivity within 6 months after birth. None of our babies were HBV-infected by 6 weeks of age even thereafter until 2 years in our cohort of babies receiving their first HBV vaccine at 6 weeks of age. The detection limits for the HBsAg screening, confirmatory and the third exploratory test were quite low in the 1 ng/mL ranges. Encouragingly all the three test kits had 100% concordance rates, and excellent test performances with respect to sensitivities and specificities.
This low transmission rate is comparable to only one case out of the 134 (0.7%) seropositive of maternally HBV-exposed Mozambican infants who received their first HBV vaccine at birth [20]. Previous studies have shown that delayed HBV vaccine birth dose is still effective in preventing perinatal infection, although at a reduced efficacy. This is even more important in our study setting where the third HBV vaccination dosage coverage is at least 83%, making our results less generalisable.
HBeAg (detected in serum of individuals with high HBV viral load), anti-HBe-antibodies and anti-HBc-antibodies (IgM/IgG total antibody) indicate recent or past infection. Anti-HBe antibody detection is generally associated with the disappearance of HBeAg, and indicates low infectivity. In our study, up to 47.9% of the pregnant women tested positive for anti-HBe-antibodies compared to only 3.3% observed in the late 1990s. This is critical, as lack of maternal anti-HBe antibodies predicts HBV infection in babies.
Ordinarily, the presence of HBsAg and/or HBV-DNA in neonates is the criteria for determining intrauterine, including perinatal transmissions that can occur before the administration of HBV vaccine at 6 weeks of age. Presence of HBsAg, HBeAg and anti-HBc IgM–antibodies from at least 6 months of age are potentially useful for simple and cost-effective algorithms for early HBV infant diagnosis in resource limited settings. HBV-MTCT rates vary with maternal HBeAg/anti-HBe status, being 70%-90% in HBeAg seropositives, 25% in HBeAg-seronegative/HBeAb negatives and 12% in HBeAg-negative/anti-HBe-positives [32,33,34]. Approximately 3%-13% of babies born of HBsAg-positive mothers, especially those carrying HBeAg become HBsAg carriers despite successful passive-active immunoprophylaxis [32, 33].
Perinatal HBV diagnosis remains a challenge as even more sensitive and reliable diagnostic platforms have shown that in some cases neonates born of HBV infected pregnant women test HBsAg positive at birth but with absence of infection simply because the small molecular HBsAg passes through the placenta. In addition, anti-HBe antibody and anti-(HBc antibody) also cross the placental barrier in nearly all babies before disappearing before 12 and 24 months, respectively [35, 36]. Again, this is just transplacental maternal antibodies transfers, but not indicators of infant HBV infection status.
Real-time polymerase chain reaction with two pairs of primers and probes has been shown to prevent the underestimation of HBV DNA levels [37], and can also detect occult HBV infection (i.e. HBsAg seronegative individuals but with detectable HBV DNA). Combining HBsAg and HBV DNA assays has been shown to increase prevalence of HBV from 4.8% (HBsAg alone) to 12.4% [38, 39]. Molecular diagnostic inadequacies may lead to significant underestimation of HBV infections. Furthermore, viral mutations, or genetic diversity that differ by geographic location may also contribute significantly to the occult HBV phenotype causing immune escape and diagnostic failures, vaccine escapes [40,41,42,43,44,45].
Infant immune response to HBV vaccination
Of concern were the 23.3% of HBV exposed infants that lacked HBs-antibodies at 2 years after receiving all the recommended 3 vaccine doses. Three exposed babies were not tested at 24 months because they had relocated to the rural areas due to economic hardships, a situation exacerbated by the covid-19 pandemic lockdowns. Previous findings suggested that age, obesity and gender differences may affect immune responses to HBV vaccine [46,47,48]. However, we did not observe these associations in our study, probably due to the small numbers of maternally HBV exposed babies. Host genetic variants may have played a role in this hypo-immune response to HBV vaccine as previously described [49,50,51]. Being non-cytopathic, liver injury following HBV infection is mostly due to host immune responses [52]. However, no risk factors associated with lack of HBs-antibodies seroconversion were observed, probably because again of the small numbers. This observation resonates well with previously studies’ findings that demonstrated that despite complete HBV vaccinations, a significant number of South African children were not immune to HBV [53]. Policy makers may have to consider revaccination for failed active immunoprophylaxis targeting HBV exposed babies. In addition, and more importantly it remains to be ascertained whether the infants with HBs-antibodies seroconversion had protective antibody levels of at least 10 IU/mL. Further investigations are necessary to ascertain whether lack of antibodies implies lack of immunity.
Birth dose of hepatitis B vaccine is currently not part of the immunization schedule in Zimbabwe, despite the fact that high proportion of deliveries occur within health care facilities [12], a situation that would facilitate its implementation. The current economic hardships now associated with increasing numbers of home births may be a challenge for HBV birth dose implementation if the situation continues. Paradoxically, are recent European studies that recorded perinatal HBV infections of 1% in infants receiving HBV birth dose [54, 55]. Given the foregoing, it may be worthwhile continuing with the current strategy of administering first HBV vaccine at 6 weeks of age.
Conclusion
In conclusion, in our cohort of 1208 pregnant women in a low-resource setting in SSA, the overall HBsAg sero-prevalence rate was 2.65% in women ≥ 20 weeks’ gestational age. The burden significantly different by HIV status; being 1.17% versus and 4.11% in the HIV-uninfected and infected subgroups, respectively. Thus, low and moderate HBV endemics were observed in HIV-uninfected and HIV-infected pregnant women, respectively, underscoring the need for routine HBV screening in pregnancy, especially the HIV-infected women attending antenatal care. Unlike for HIV, there is no population-based testing for HBV despite the fact that the two viruses share the same modes of transmission. There is no data on national HBV prevalence to help the readers to interpret the low and moderate HBV endemicity seen in our study in the background of the bigger national picture. HIV-infection and reported tooth extraction(s) were independent risk factors for HBsAg-seropositivity, warranting augmentation of respective preventive measures. Concerted efforts are necessary to optimise healthy pregnancy outcomes in the HIV/HBsAg coinfected for a greater impetus towards attaining the WHO strategy aiming to end the HBV epidemic by 2030. Vertical and child-horizontal transmissions were both absent, probably due to ~ 50% frequency of antenatal anti-HBe-antibodies. Of concern was the absence of anti-HBs-antibodies in about a quarter of the fully vaccinated/maternally HBsAg exposed infants. Finally, lack of adequate molecular diagnostic facilities in many African centres may lead to significant underestimation of HBV infections such that both vertical and horizontal transmissions are underappreciated, and hence may not be diagnosed until later in young adulthood.
Availability of data and materials
The datasets obtained during this study will be available upon reasonable request to the corresponding author.
Abbreviations
- ART:
-
Antiretroviral therapy
- BMI:
-
Body mass index
- CD4:
-
Cluster of differentiation-4
- CI:
-
Confidence interval
- DNA:
-
Deoxyribonucleic acid
- HBV:
-
Hepatitis B virus
- HBc-antibody:
-
Hepatitis B virus core antibody
- HIV:
-
Human immunodeficiency virus
- HBeAg:
-
Hepatitis B virus e antigen
- HBsAg:
-
Hepatitis B virus surface antigen
- HBs-antibody:
-
Hepatitis B virus surface antibody
- IQR:
-
Interquartile range
- MUAC:
-
Mid-upper arm circumference
- MTCT:
-
Mother to child transmission
- STIs:
-
Sexually transmitted infections
- UZBCS:
-
University of Zimbabwe Birth Cohort Study.
- WHO:
-
World Health Organisation
References
Rajbhandari R, Simon RE, Chung RT, Ananthakrishnan AN. Racial disparities in inhospital outcomes for hepatocellular carcinoma in the United States. Mayo Clin Proc. 2016;91(9):1173–82.
Seto WK, Lo YR, Pawlotsky JM, Yuen MF. Chronic hepatitis B virus infection. Lancet. 2018;392(10161):2313–24.
Floridia M, Masuelli G, Tamburrini E, Spinillo A, Simonazzi G, Guaraldi G, et al. HBV coinfection is associated with reduced CD4 response to antiretroviral treatment in pregnancy. HIV Clin Trials. 2017;18(2):54–9.
Matthews PC, Beloukas A, Malik A, Carlson JM, Jooste P, Ogwu A, et al. Prevalence and characteristics of hepatitis B Virus (HBV) Coinfection among HIV-positive women in South Africa and Botswana. PLoS ONE. 2015;10(7): e0134037.
Platt L, French CE, McGowan CR, Sabin K, Gower E, Trickey A, et al. Prevalence and burden of HBV co-infection among people living with HIV: a global systematic review and meta-analysis. J Viral Hepat. 2020;27(3):294–315.
Yang R, Gui X, Xiong Y, Gao SC, Yan Y. Impact of hepatitis B virus infection on HIV response to antiretroviral therapy in a Chinese antiretroviral therapy center. Int J Infect Dis. 2014;28:29–34.
Breakwell L, Tevi-Benissan C, Childs L, Mihigo R, Tohme R. The status of hepatitis B control in the African region. Pan Afr Med J. 2017;27(Suppl 3):17.
Chotun N, Preiser W, van Rensburg CJ, Fernandez P, Theron GB, Glebe D, et al. Point-of-care screening for hepatitis B virus infection in pregnant women at an antenatal clinic: A South African experience. PLoS ONE. 2017;12(7): e0181267.
Kiire CF. The epidemiology and prophylaxis of hepatitis B in sub-Saharan Africa: a view from tropical and subtropical Africa. Gut. 1996;38(Suppl 2):S5-12.
Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet. 2015;386(10003):1546–55.
World Health Organisation. Hepatitis b. 2022; https://www.who.int/news-room/fact-sheets/detail/hepatitis-b.
Zimbabwe National Statistics Agency and ICF International. Zimbabwe Demographic and Health Survey 2015: Final Report. Rockville: Zimbabwe National Statistics Agency (ZIMSTAT) and ICF International; 2016.
Madzime S, Adem M, Mahomed K, Woelk GB, Mudzamiri S, Williams MA. Hepatitis B virus infection among pregnant women delivering at Harare Maternity Hospital, Harare Zimbabwe, 1996 to 1997. Cent Afr J Med. 1999;45(8):195–8.
Mavenyengwa RT, Moyo SR, Nordbo SA. Streptococcus agalactiae colonization and correlation with HIV-1 and HBV seroprevalence in pregnant women from Zimbabwe. Eur J Obstet Gynecol Reprod Biol. 2010;150(1):34–8.
Duri K, Gumbo FZ, Munjoma PT, Chandiwana P, Mhandire K, Ziruma A, et al. The University of Zimbabwe College of Health Sciences (UZ-CHS) BIRTH COHORT study: rationale, design and methods. BMC Infect Dis. 2020;20(1):725.
Duri K, Munjoma PT, Mazhandu AJ, Marere T, Gomo E, Banhwa S, et al. Predictors and timing to viral suppression in HIV-infected pregnant women in the University of Zimbabwe Birth Cohort Study during the era of lifelong antiretroviral therapy (Option B+ treatment strategy). Front Virol. 2022;2:838234.
Duri K, Mataramvura H, Chandiwana P, Mazhandu AJ, Banhwa S, Munjoma PT, et al. Mother to child transmission of HIV within 24 months after delivery in women initiating lifelong antiretroviral therapy pre-/-post-conception or postnatally; effects of adolescent girl and young woman status and plasma viremia late in pregnancy. Front Virol. 2022;2:906271.
Akaike H. A new look at the statistical model identification. IEEE Trans Autom Control. 1974;19(6):716–23.
Mutagoma M, Balisanga H, Malamba SS, Sebuhoro D, Remera E, Riedel DJ, et al. Hepatitis B virus and HIV co-infection among pregnant women in Rwanda. BMC Infect Dis. 2017;17(1):618.
Loarec A, Nguyen A, Molfino L, Chissano M, Madeira N, Rusch B, et al. Prevention of mother-to-child transmission of hepatitis B virus in antenatal care and maternity services Mozambique. Bull World Health Organ. 2022;100(1):60–9.
Mawouma ARN, Djoulatou AH, Komnang EO, Kimessoukie EO. Factors associated with hepatitis B infection in pregnant women at health facilities in the health district of Mokolo/Far North of Cameroon. Pan Afr Med J. 2022;41:61.
Olakunde BO, Adeyinka DA, Olakunde OA, Uthman OA, Bada FO, Nartey YA, et al. A systematic review and meta-analysis of the prevalence of hepatitis B virus infection among pregnant women in Nigeria. PLoS ONE. 2021;16(10): e0259218.
Yendewa GA, Lakoh S, Yendewa SA, Bangura K, Lawrence H, Patino L, et al. Prevalence of hepatitis B surface antigen and serological markers of other endemic infections in HIV-infected children, adolescents and pregnant women in Sierra Leone: A cross-sectional study. Int J Infect Dis. 2021;102:45–52.
Kim HS, Rotundo L, Yang JD, Kim D, Kothari N, Feurdean M, et al. Racial/ethnic disparities in the prevalence and awareness of Hepatitis B virus infection and immunity in the United States. J Viral Hepat. 2017;24(11):1052–66.
Kafeero HM, Ndagire D, Ocama P, Walusansa A, Sendagire H. Sero-prevalence of human immunodeficiency virus-hepatitis B virus (HIV-HBV) co-infection among pregnant women attending antenatal care (ANC) in sub-Saharan Africa (SSA) and the associated risk factors: a systematic review and meta-analysis. Virol J. 2020;17(1):170.
Baudi I, Iijima S, Chin’ombe N, Mtapuri-Zinyowera S, Murakami S, Isogawa M, et al. Molecular epidemiology of co-infection with hepatitis B virus and human immunodeficiency virus (HIV) among adult patients in Harare. Zimbabwe J Med Virol. 2017;89(2):257–66.
Kurira P, Ndhlovu CE, Gomo ZA. Hepatitis B and C infection at a large public sector hospital clinic: is it a burden? Cent Afr J Med. 2014;60(9–12):56–62.
Price H, Dunn D, Zachary T, Vudriko T, Chirara M, Kityo C, et al. Hepatitis B serological markers and plasma DNA concentrations. AIDS. 2017;31(8):1109–17.
Goverwa-Sibanda TP, Mupanguri C, Timire C, Harries AD, Ngwenya S, Chikwati E, et al. Hepatitis B infection in people living with HIV who initiate antiretroviral therapy in Zimbabwe. Public Health Action. 2020;10(3):97–103.
Mata Marin JA, Martinez JG, Flores RA, Alvarez SM, de Jesus AM. I. Aminotransferase serum levels decrease after initiating antiretroviral treatment in HIV infected patients. Curr HIV Res. 2011;9(1):23–7.
Bierhoff M, Angkurawaranon C, Myat MA, Gilder ME, Win TN, Keereevijitt A, et al. Maternal hepatitis B infection burden, comorbidity and pregnancy outcome in a low-income population on the Myanmar-Thailand border: a retrospective cohort study. J Pregnancy. 2019;2019:8435019.
Wiseman E, Fraser MA, Holden S, Glass A, Kidson BL, Heron LG, et al. Perinatal transmission of hepatitis B virus: an Australian experience. Med J Aust. 2009;190(9):489–92.
Bai H, Zhang L, Ma L, Dou XG, Feng GH, Zhao GZ. Relationship of hepatitis B virus infection of placental barrier and hepatitis B virus intra-uterine transmission mechanism. World J Gastroenterol. 2007;13(26):3625–30.
Guo Z, Shi XH, Feng YL, Wang B, Feng LP, Wang SP, et al. Risk factors of HBV intrauterine transmission among HBsAg-positive pregnant women. J Viral Hepat. 2013;20(5):317–21.
Shepard CW, Simard EP, Finelli L, Fiore AE, Bell BP. Hepatitis B virus infection: epidemiology and vaccination. Epidemiol Rev. 2006;28:112–25.
Wang JS, Chen H, Zhu QR. Transformation of hepatitis B serologic markers in babies born to hepatitis B surface antigen positive mothers. World J Gastroenterol. 2005;11(23):3582–5.
Sun S, Meng S, Zhang R, Zhang K, Wang L, Li J. Development of a new duplex real-time polymerase chain reaction assay for hepatitis B viral DNA detection. Virol J. 2011;8:227.
Firnhaber C, Viana R, Reyneke A, Schultze D, Malope B, Maskew M, et al. Occult hepatitis B virus infection in patients with isolated core antibody and HIV co-infection in an urban clinic in Johannesburg. South Africa Int J Infect Dis. 2009;13(4):488–92.
Bivigou-Mboumba B, Amougou-Atsama M, Zoa-Assoumou S, M’boyis KH, Nzengui-Nzengui GF, Ndojyi-Mbiguino A, et al. Hepatitis B infection among HIV infected individuals in Gabon: occult hepatitis B enhances HBV DNA prevalence. PLoS One. 2018;13(1):e0190592.
Yucesoy B, Talzhanov Y, Johnson VJ, Wilson NW, Biagini RE, Wang W, et al. Genetic variants within the MHC region are associated with immune responsiveness to childhood vaccinations. Vaccine. 2013;31(46):5381–91.
Xie B, Zhang P, Liu M, Zeng W, Yang J, Liu H. Deltex1 polymorphisms are associated with hepatitis B vaccination non-response in Southwest China. PLoS ONE. 2016;11(2): e0149199.
Wang Y, Xu P, Zhu D, Zhang S, Bi Y, Hu Y, et al. Association of polymorphisms of cytokine and TLR-2 genes with long-term immunity to hepatitis B in children vaccinated early in life. Vaccine. 2012;30(39):5708–13.
Pan L, Zhang L, Zhang W, Wu X, Li Y, Yan B, et al. A genome-wide association study identifies polymorphisms in the HLA-DR region associated with non-response to hepatitis B vaccination in Chinese Han populations. Hum Mol Genet. 2014;23(8):2210–9.
Kim H, Lee SA, Do SY, Kim BJ. Precore/core region mutations of hepatitis B virus related to clinical severity. World J Gastroenterol. 2016;22(17):4287–96.
He F, Ma YJ, Zhou TY, Duan JC, Wang JF, Ji YL, et al. The serum anti-HBs level among children who received routine hepatitis B vaccination during infancy in Mianyang City, China: a cross-sectional study. Viral Immunol. 2016;29(1):40–8.
Fan W, Chen XF, Shen C, Guo ZR, Dong C. Hepatitis B vaccine response in obesity: a meta-analysis. Vaccine. 2016;34(40):4835–41.
Voysey M, Pollard AJ, Perera R, Fanshawe TR. Assessing sex-differences and the effect of timing of vaccination on immunogenicity, reactogenicity and efficacy of vaccines in young children: study protocol for an individual participant data meta-analysis of randomised controlled trials. BMJ Open. 2016;6(7): e011680.
Katoonizadeh A, Sharafkhah M, Ostovaneh MR, Norouzi A, Khoshbakht N, Mohamadkhani A, et al. Immune responses to hepatitis B immunization 10–18 years after primary vaccination: a population-based cohort study. J Viral Hepat. 2016;23(10):805–11.
Fan J, Huang X, Chen J, Cai Y, Xiong L, Mu L, et al. Host genetic variants in HLA Loci influence risk for hepatitis B virus infection in children. Hepat Mon. 2016;16(8): e37786.
Liu X, Yu L, Han C, Lu S, Zhu G, Su H, et al. Polymorphisms of HLA-DQB1 predict survival of hepatitis B virus-related hepatocellular carcinoma patients receiving hepatic resection. Clin Res Hepatol Gastroenterol. 2016;40:739.
Gao X, Liu W, Zhang X, Tang L, Wang L, Yan L, et al. Genetic polymorphism of HLA-DQ confers susceptibility to hepatitis B virus-related hepatocellular carcinoma: a case-control study in Han population in China. Tumour Biol. 2016;37(9):12103–11.
Chang KM. Hepatitis B immunology for clinicians. Clin Liver Dis. 2010;14(3):409–24.
Buchner A, Omar FE, Vermeulen J, Reynders DT. Investigating hepatitis B immunity in patients presenting to a paediatric haematology and oncology unit in South Africa. S Afr Med J. 2014;104(9):628–31.
Hoffmann CJ, Mashabela F, Cohn S, Hoffmann JD, Lala S, Martinson NA, et al. Maternal hepatitis B and infant infection among pregnant women living with HIV in South Africa. J Int AIDS Soc. 2014;17:18871.
Zhang L, Gui XE, Wang B, Fan JY, Cao Q, Mullane K, et al. Serological positive markers of hepatitis B virus in femoral venous blood or umbilical cord blood should not be evidence of in-utero infection among neonates. BMC Infect Dis. 2016;16(1):408.
Acknowledgements
We would like to thank the study participants and the staff of the UZBCS for their commitment, Dr. A Ziruma (Obstetrics and Gynecology Unit, FMHS, Harare), Dr. GQ Kandawasvika, Dr. P Kuona and Dr. S Chimhuya (Paediatrics and Child Health Unit, FMHS), Professor E Gomo, Ms. P Chandiwana, Mrs. P. Muzire and Mr. M Madhaka (Research Support Centre, FMHS), Sr. MM Chipiti, Sr M Ngoweni, Sr N Sibiya, Mr. N Taremeredzwa and Ms. E Mazengera (Immunology Unit-FMHS). The study has also been supported from the Midwives and Nurses working at Kuwadzana, Rujeko, Glen View and Budiriro City of Harare Poly Clinics.
Funding
The study was supported by the Wellcome Trust under the University Of Zimbabwe College Of Health Sciences Southern Africa Consortium for Research Excellence (SACORE) grant number 087537/F/08/A. Supplemental sponsorships were received from The Academy of Medical Sciences Global Challenges Research Fund Networking Grant Scheme (GCRFNGR2\10499), The Norwegian Programme for Capacity Development in Higher Education and Research for Development under the University of Zimbabwe NORHED grant (NORHED QZA-0484MWI-13/0032) and The Botnar Foundation and the Department of Visceral Surgery and Medicine, Bern University, Switzerland. None of the funding bodies were involved in the study design, data collection, data analysis, interpretation of findings and/or manuscript writing.
Author information
Authors and Affiliations
Contributions
The study was conceived by KD, designed by KD, FZG, LRM and TM. PTM, HM, PC, AJM, were responsible for data collection, entry and validation overseen by KD. PTM HM, PC and AJM performed the statistical analysis. KD, PTM, HM, PC and AJM were involved in the interpretation of findings. The manuscript was written by KD. All authors were involved in manuscript revisions and approved the final draft.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
UZBCS complied with the ethical principles of the Declaration of Helsinki and was conducted in compliance with the international council for harmonisation of Good Clinical and Laboratory Practice guidelines and local regulatory requirements. Ethical approval was obtained from the Joint Research Ethics Committee (JREC) of the University of Zimbabwe and Parirenyatwa Group of Hospitals reference number; JREC/18/15 and the Medical Research Council of Zimbabwe; MRCZ/A/1968. Literacy is nearly universal in Zimbabwe and all potential participants were able to read and comprehend the informed consent form. All study participants provided written informed consent.
Consent for publication
Not Applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Duri, K., Munjoma, P.T., Mataramvura, H. et al. Antenatal hepatitis B virus sero-prevalence, risk factors, pregnancy outcomes and vertical transmission rate within 24 months after birth in a high HIV prevalence setting. BMC Infect Dis 23, 736 (2023). https://doi.org/10.1186/s12879-023-08523-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-023-08523-2