Abstract
Background
Phenylephrine (PE) and norepinephrine (NE) may be used to maintain adequate blood pressure and tissue perfusion in patients with septic shock, but the effect of NE combined with PE (NE-PE) on mortality remains unclear. We hypothesized that NE-PE would not inferior to NE alone for all-cause hospital mortality in patients with septic shock.
Methods
This single-center, retrospective cohort study included adult patients with septic shock. According to the infusion type, patients were divided into the NE-PE or NE group. Multivariate logistic regression, propensity score matching and doubly robust estimation were used to analyze the differences between groups. The primary outcome was the all-cause hospital mortality rate after NE-PE or NE infusion.
Results
Among 1, 747 included patients, 1, 055 received NE and 692 received NE-PE. For the primary outcome, the hospital mortality rate was higher in patients who received NE-PE than in those who received NE (49.7% vs. 34.5%, p < 0.001), and NE-PE was independently associated with higher hospital mortality (odds ratio = 1.76, 95% confidence interval = 1.36–2.28, p < 0.001). Regarding secondary outcomes, patients in the NE-PE group had longer lengths of stay in ICU and hospitals. Patients in the NE-PE group also received mechanical ventilation for longer durations.
Conclusions
NE combined with PE was inferior to NE alone in patients with septic shock, and it was associated with a higher hospital mortality rate.
Similar content being viewed by others
Introduction
Septic shock is a severe consequence of infection that typically has an extremely high mortality rate of 35–40% [1, 2]. Due to its clinical urgency, immediate treatment and resuscitation are required. Fluid resuscitation and vasoactive medication therapy are two important components of septic shock resuscitation [3]. Norepinephrine (NE) was recommended as the first selective vasopressor for septic shock [4], but there is increasing evidence that excessive dosing or duration of NE infusion can adversely affect patient outcomes due to its multiple effects on immunity, metabolism, and coagulation [5,6,7]. The Surviving Sepsis Campaign(SSC) guidelines therefore recommended adding other vasoactive drugs to decrease the adverse effects from NE [4, 8].
As a pure α-adrenergic agonist, phenylephrine (PE) has been suggested as potentially beneficial in achieving heart rate (HR) control [9, 10], and for reversing hemodynamic and metabolic abnormalities [11, 12]. However, it has also been found that PE has the potential to induce splanchnic vasoconstriction [13], decreased cerebral perfusion [14], and even an increased mortality risk [15, 16].
Currently, the effects of PE in treating septic shock are uncertain. We therefore hypothesized that the combined use of NE and PE has a similar effect to NE on patients with septic shock. We therefore conducted retrospective cohort research to compare the effectiveness of NE-PE with that of NE alone on hospital mortality and on other secondary outcomes in septic shock.
Methods
Data source and study populations
This study used the Medical Information Mart for Intensive Care-III (MIMIC-III) database, which is a freely available large database that contains deidentified data information of 46,476 patients who were admitted to the intensive care unit (ICU) of the Beth Israel Deaconess Medical Center between 2001 and 2012 [17, 18]. The true identity information of all patients is hidden in this database, and so informed consent was therefore not required from the patients. The author passed the relevant course training and obtained a database access certificate (number: 47907567).
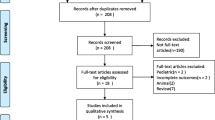
The study cohort was constructed using the STROBE checklist [19]. The study was designed to compare the prognosis and outcomes of continuous infusions of NE-PE versus NE in ICU patients with septic shock based on the definition by Angus et al. [3] using the diagnostic codes of the ninth revision of ICD (78,552). The inclusion and exclusion criteria were as follows: only first ICU admissions; only patients treated with NE or NE-PE; only lengths of stay (LOSs) in an ICU exceeding 24 h; and only adult patients. Finally, 1, 747 patients were selected for the study cohort: 1, 055 patients in the NE group and 692 in the NE-PE group.
Data extraction
Data were extracted using Structured Query Language [20], NE exposure was defined as the continuous intravenous infusion of NE during the ICU admission, and NE-PE exposure was defined as the continuous intravenous infusion of NE combined with PE. The variables included demographic information, vital signs, comorbidities, the score for disease severity, laboratory tests, medications, and other information about the patients. Basic information included age, sex, weight, ethnicity, admission type, and first care unit. Vital signs were the mean values on the first day of the ICU which included HR, mean blood pressure (MBP), respiratory rate (RR), temperature, and SpO2. The score for disease severity included the SOFA score [21], APSIII [22], and Elixhauser Comorbidity Index [23]. Comorbidities included congestive heart failure (CHF), cardiac arrhythmias, pulmonary circulation disorder, peripheral vascular disorder, neurological diseases, chronic pulmonary disease, hypertension, diabetes, renal failure, liver disease, solid tumor without metastasis, metastatic cancer, fluid and electrolyte disorders, drug abuse, and alcohol abuse. Laboratory tests on the first day of ICU admission included white blood cells (WBCs), hemoglobin, platelets, bilirubin, serum creatinine, urea nitrogen, glucose, lactate, bicarbonate, international normalized ratio (INR), potassium, and sodium. Medications included the use of vasopressin, dobutamine, and epinephrine. Other information included firstday urine output, firstday mechanical ventilation (MV), firstday renal replacement therapy (RRT), microorganism, and vasopressor use during ICU stays.
Primary and secondary outcomes
The primary outcomes were the all-cause hospital and ICU mortality rate. Hospital mortality and ICU mortality were defined as death during hospitalization and ICU, respectively. Secondary outcomes included ICU LOS and hospital LOS, and MV duration. Acute kidney injury (AKI) at 48 h and 7 days were defined as whether AKI had occurred at 48 h or 7 days after ICU admission.
Statistical analysis
The proportion of missing values for each variable did not exceed 20%. Variables with missing data were estimated and filled using the multiple imputation method [24]. Continuous parameters were presented as mean ± standard-deviation or median and interquartile range (25%–75%) values. Continuous variables were analyzed using variance or nonparametric tests. Categorical variables were analyzed using chi-square tests. Odds ratios (ORs) and 95% confidence intervals (CIs) were used to analyze outcomes between groups. SPSS software (version 27.0) and R software (version 4.1.3) were used for the statistical analyses.
Univariate and multivariate logistic regression model were established to assess the independent association of exposure with the primary endpoint. Furthermore, to ensure the stability of the results for the primary and secondary outcomes, propensity score matching (PSM) was used to balance the confounding factors between groups. The following variables were included in the PSM analysis: age, gender, weight, ethnicity, admission type, first care unit, HR, MBP, RR, temperature, SpO2, SOFA score, APSIII, Elixhauser Comorbidity Index, CHF, cardiac arrhythmias, peripheral vascular disorder, chronic pulmonary disease, pulmonary circulation disorders, hypertension, diabetes, renal failure, liver disease, solid tumor without metastasis, metastatic cancer, fluid and electrolyte disorders, drug abuse, alcohol abuse, WBCs, hemoglobin, hematocrit, platelets, bilirubin, serum creatinine, urea nitrogen, glucose, lactate, bicarbonate, INR, potassium, sodium, vasopressin, dobutamine, dopamine and epinephrine, microorganism, first-day urine output, firstday MV, firstday RRT, and initial NE dose. A 1:1 ratio was applied to matching using a 0.1 caliper [25]. The standardized mean difference (SMD) was calculated before and after matching to assess the differences between the two groups. When the SMD of a variable is less than 0.1, it can be considered that balance was obtained between the groups [26].
Sensitivity analysis
Several sensitivity analyses were used to estimate the robustness of the results in two different models: PSM cohort with multivariate logistic regression model and inverse probability weighting with multivariate logistic regression model (doubly robust model). We also performed a sensitivity analysis to compare the mortality risk for ICU mortality between NE and NE-PE groups.
Subgroup analysis
Subgroup analyses were implemented to further investigate the mortality rate between PSM groups. We included six subgroups: age (≥ 65 or < 65 years), gender, SOFA score (≥ 8 or < 8), HR (≥ 100 or < 100 bpm), CHF, arrhythmias, hypertension, diabetes, renal failure, and liver disease.
Results
Baseline characteristics
As shown in Fig. 1, 1, 747 patients diagnosed with septic shock [3] in the MIMIC-III database were included, comprising 1055 NE users (60.4%) and 692 NE-PE users (39.6%). The basic characteristics of the cohort are listed in Table 1. Patients who were exposed to NE-PE generally differed from those exposed to NE in most aspects during hospitalization. The NE patients had a higher proportion of Emergency admissions (97.7% vs 94.4%), MICU admissions (78.3% vs 67.9%), hypertension (53.6% vs 51.6%). The NE-PE group also had a higher percentage of CHF (43.6% vs 37.7%), cardiac arrhythmias (53.3% vs 36.4%), pulmonary circulation disorder (10.3% vs 7.3%), liver disease (28.5% vs 20.5%), fluid and electrolyte disorders (62.3% vs 56.7%). NE-PE group presented with higher severity scores after ICU admission: APSIII (75.05 ± 24.75 vs 63.45 ± 22.95), SOFA score (9.49 ± 3.94 vs 8.35 ± 3.39), and Elixhauser Comorbidity Index (14.95 ± 8.85 vs 12.63 ± 8.60). Patients in the NE-PE group also had higher HRs (98.16 ± 18.27 vs 89.40 ± 16.81 bpm) and RRs (22.26 ± 4.76 vs 21.10 ± 4.50 breaths/min). More NE-PE patients received MV (73.6% vs 56.0%) during the first 24 h of their ICU stay, and a higher proportion of NE-PE patients received other vasoactive drugs treatment during their ICU stay: vasopressin (60.3% vs 20.9%), dobutamine (9.4% vs 4.6%), dopamine (20.2% vs 16.2%), and epinephrine (7.2% vs 0.7%).
In the PSM cohort, 486 patients exposed to NE-PE were matched with 486 patients in the NE group at a 1:1 ratio. As indicated in Table 2, covariates for the matching cohorts were balanced between NE-PE and NE( all covariates SMD < 0.1).
Primary outcome
The hospital mortality rate was higher in patients who received NE-PE than in those who received NE (51.0% vs. 38.7%, P < 0.001) in the propensity score matching cohort, the univariate logistic regression analysis showed the NE-PE group has a higher hospital mortality rate compared to NE group (OR = 3.16, 95% CI = 2.59–3.88, p < 0.001), and the multivariate stepwise logistic regression analysis also demonstrated a higher hospital mortality rate in NE-PE versus NE (OR = 1.76, 95% CI = 1.36–2.28, p < 0.001). For the sensitivity analysis, as listed in Table 3, all two models yielded a similar result: patients in the NE-PE group had higher hospital and ICU mortalities compared to NE alone.
Secondary study outcomes in the PSM cohort
We assessed several secondary outcomes to explore potential reasons accounting for the higher mortality rate in the NE-PE group. A few differences were observed in secondary outcomes (Table 4). First, ICU LOS (12.18 vs 9.25 days, p < 0.001) and hospital LOS (17.35 vs 15.19 days, p = 0.019) were significantly longer in the NE-PE group. Second, the NE-PE group had longer durations of MV (160.26 vs 132.74 h, p = 0.042). The percentages of AKI at 48 h and 7 days after ICU admission did not differ between the groups.
Subgroup analyses for hospital mortality rate
As shown in Fig. 2, the hospital mortality rate did not differ significantly among the subgroups.
Discussion
This retrospective, single-center cohort study found that NE-PE infusion did not lead to the same outcomes compared with NE in patients with septic shock. On the contrary, NE-PE administration increased the mortality risk, which was confirmed by the univariate and multivariate stepwise logistic regression model, PSM model, and doubly robust model. The total mortality rate in our study was 38.52%, which was consistent with an epidemiological survey by Bauer and Vincent et al. [1, 2]. In addition, NE-PE use was associated with longer ICU LOS, hospital LOS, and duration of mechanical ventilation, but there were no differences in the incidence rates of AKI at 48 h and 7 days after ICU admission.
The increased mortality of NE-PE may be attributable to several factors. As a selective α1-receptor agonist, PE increases systemic vascular resistance and arterial blood pressure while decreasing splanchnic perfusion and increasing arterial lactate in septic shock [27]. Splanchnic hypoperfusion may cause cytokine release into the systemic circulation in septic shock, which potentially leads to a vicious circle of inflammatory responses, culminating in multiple organ dysfunction syndromes [27, 28]. PE may also reduce cardiac output and cerebral oxygen saturation [14, 29]. Patients with septic shock often also experience cardiac dysfunction; PE leads to increased afterload, which offsets the benefits of cardiac output through arterial vasodilation caused by septic shock [30, 31]. NE has a lesser effect on cardiac output than PE due to its β1-adrenergic agonism. Moreover, a retrospective study found that patients who received PE had a greater decrease in HR over 24 h, which may have resulted in a decrease in cardiac output [15]. The above potential reasons are consistent with the SSC guidelines that do not recommend the use of PE [16]. The same results regarding the increased mortality in PE were also observed in studies by Hawn et al. [15] and Patel et al. [32].
Our study had the following specific strengths: we included a large population of 1, 747 patients with septic shock in a public database, and we performed multivariate stepwise logistic regression, PSM, doubly robust and subgroup analyses to improve the robustness of the results. However, the study also had several limitations. First, it had a retrospective design and was therefore subject to selection bias; although we used PSM and a multivariate model to control bias, other unknown confounding factors may remain. Second, we could not identify the specific clinical decision that would lead to the selection of PE for an individual patient, such as whether it was for a specific disease, during NE shortages, or based on the personal experience of the physician. Third, different therapeutic targets of NE, NE combined with PE, and other vasopressors in septic shock may leads to different endings, and this was not clear in our study. Fourth, although we have balanced the initial dose of NE by PSM, the subsequent infusion dose of NE and other vasopressors were not clear, and these differences may have an impact on outcomes. Fifth, the study was limited to a septic shock population from a single center, which makes it difficult to extrapolate our findings to different shock syndromes in critical care units.
Conclusion
This study found that the infusion of NE combined with PE was associated with a higher hospital mortality rate in comparison with NE alone for patients with septic shock. This result reminds us that PE should be used with caution in patients with septic shock. The results need to be confirmed in multicenter prospective randomized clinical trials.
Availability of data and materials
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: The data were available on the MIMIC-III website at https://mimic.physionet.org/, http://dx.doi.org/10.13026/C2XW26.
References
Bauer M, Gerlach H, Vogelmann T, Preissing F, Stiefel J, Adam D. Mortality in sepsis and septic shock in Europe, North America and Australia between 2009 and 2019— results from a systematic review and meta-analysis. Crit Care. 2020;24(1):239. https://doi.org/10.1186/s13054-020-02950-2.
Vincent JL, Jones G, David S, Olariu E, Cadwell KK. Frequency and mortality of septic shock in Europe and North America: a systematic review and meta-analysis. Crit Care. 2019;23(1):196. https://doi.org/10.1186/s13054-019-2478-6.
Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):801. https://doi.org/10.1001/jama.2016.0287.
Rhodes A, Evans LE, Alhazzani W, et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med. 2017;43(3):304–77. https://doi.org/10.1007/s00134-017-4683-6.
Stolk RF, van der Poll T, Angus DC, van der Hoeven JG, Pickkers P, Kox M. Potentially Inadvertent Immunomodulation: Norepinephrine Use in Sepsis. Am J Respir Crit Care Med. 2016;194(5):550–8. https://doi.org/10.1164/rccm.201604-0862CP.
Uhel F, van der Poll T. Norepinephrine in Septic Shock: A Mixed Blessing. Am J Respir Crit Care Med. 2020;202(6):788–9. https://doi.org/10.1164/rccm.202006-2301ED.
Andreis DT, Singer M. Catecholamines for inflammatory shock: a Jekyll-and-Hyde conundrum. Intensive Care Med. 2016;42(9):1387–97. https://doi.org/10.1007/s00134-016-4249-z.
Levy MM, Evans LE, Rhodes A. The Surviving Sepsis Campaign Bundle: 2018 update. Intensive Care Med. 2018;44(6):925–8. https://doi.org/10.1007/s00134-018-5085-0.
Haiduc M, Radparvar S, Aitken SL, Altshuler J. Does Switching Norepinephrine to Phenylephrine in Septic Shock Complicated by Atrial Fibrillation With Rapid Ventricular Response Improve Time to Rate Control? J Intensive Care Med. 2021;36(2):191–6. https://doi.org/10.1177/0885066619896292.
Law AC, Bosch NA, Peterson D, Walkey AJ. Comparison of Heart Rate After Phenylephrine vs Norepinephrine initiation in patients with septic shock and atrial fibrillation. Chest. Published online May 2022:S0012369222008911. https://doi.org/10.1016/j.chest.2022.04.147
Singh DK, Jain G. Comparison of phenylephrine and norepinephrine in the management of dopamine-resistant septic shock. Indian J Crit Care Med. 2010;14(1):29–34. https://doi.org/10.4103/0972-5229.63033.
Kalmar AF, Allaert S, Pletinckx P, et al. Phenylephrine increases cardiac output by raising cardiac preload in patients with anesthesia induced hypotension. J Clin Monit Comput. 2018;32(6):969–76. https://doi.org/10.1007/s10877-018-0126-3.
Morelli A, Lange M, Ertmer C, et al. Short-term effects of phenylephrine on systemic and regional hemodynamics in patients with septic shock: a crossover pilot study. Shock. 2008;29(4):446–51. https://doi.org/10.1097/SHK.0b013e31815810ff.
Larson S, Anderson L, Thomson S. Effect of phenylephrine on cerebral oxygen saturation and cardiac output in adults when used to treat intraoperative hypotension: a systematic review. JBI Evid Synth. 2021;19(1):34–58. https://doi.org/10.11124/JBISRIR-D-19-00352.
Hawn JM, Bauer SR, Yerke J, et al. Effect of Phenylephrine Push Before Continuous Infusion Norepinephrine in Patients With Septic Shock. Chest. 2021;159(5):1875–83. https://doi.org/10.1016/j.chest.2020.11.051.
Vail E, Gershengorn HB, Hua M, Walkey AJ, Rubenfeld G, Wunsch H. Association between US norepinephrine shortage and mortality among patients with septic shock. JAMA. 2017;317(14):1433. https://doi.org/10.1001/jama.2017.2841.
Wu WT, Li YJ, Feng AZ, et al. Data mining in clinical big data: the frequently used databases, steps, and methodological models. Mil Med Res. 2021;8(1):44. https://doi.org/10.1186/s40779-021-00338-z.
Johnson AEW, Pollard TJ, Shen L, et al. MIMIC-III, a freely accessible critical care database. Sci Data. 2016;3(1):160035. https://doi.org/10.1038/sdata.2016.35.
von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–7. https://doi.org/10.1016/S0140-6736(07)61602-X.
Yang J, Li Y, Liu Q, et al. Brief introduction of medical database and data mining technology in big data era. J Evid-Based Med. 2020;13(1):57–69. https://doi.org/10.1111/jebm.12373.
Vincent JL, de Mendonça A, Cantraine F, et al. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on “sepsis-related problems” of the European Society of Intensive Care Medicine. Crit Care Med. 1998;26(11):1793–800. https://doi.org/10.1097/00003246-199811000-00016.
Vazquez G, Benito S, Rivera R, Spanish Project for the Epidemiological Analysis of Critical Care Patients. Simplified Acute Physiology Score III: a project for a new multidimensional tool for evaluating intensive care unit performance. Crit Care Lond Engl. 2003;7(5):345–6. https://doi.org/10.1186/cc2163.
Menendez ME, Neuhaus V, van Dijk CN, Ring D. The Elixhauser comorbidity method outperforms the Charlson index in predicting inpatient death after orthopaedic surgery. Clin Orthop. 2014;472(9):2878–86. https://doi.org/10.1007/s11999-014-3686-7.
Sterne JAC, White IR, Carlin JB, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338:b2393–b2393. https://doi.org/10.1136/bmj.b2393.
Rassen JA, Shelat AA, Myers J, Glynn RJ, Rothman KJ, Schneeweiss S. One-to-many propensity score matching in cohort studies: ONE-TO-MANY MATCHING IN COHORT STUDIES. Pharmacoepidemiol Drug Saf. 2012;21:69–80. https://doi.org/10.1002/pds.3263.
Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivar Behav Res. 2011;46(3):399–424. https://doi.org/10.1080/00273171.2011.568786.
Morelli A, Ertmer C, Rehberg S, et al. Phenylephrine versus norepinephrine for initial hemodynamic support of patients with septic shock: a randomized, controlled trial. Crit Care. 2008;12(6):R143. https://doi.org/10.1186/cc7121.
Tamion F, Richard V, Sauger F, et al. Gastric mucosal acidosis and cytokine release in patients with septic shock. Crit Care Med. 2003;31(8):2137–43. https://doi.org/10.1097/01.CCM.0000079600.49048.28.
Asfar P, De Backer D, Meier-Hellmann A, Radermacher P, Sakka SG. Clinical review: influence of vasoactive and other therapies on intestinal and hepatic circulations in patients with septic shock. Crit Care. 2003;8(3):170. https://doi.org/10.1186/cc2418.
Walley KR. Sepsis-induced myocardial dysfunction. Curr Opin Crit Care. 2018;24(4):292–9. https://doi.org/10.1097/MCC.0000000000000507.
Yamazaki T, Shimada Y, Taenaka N, Ohsumi H, Takezawa J, Yoshiya I. Circulatory responses to afterloading with phenylephrine in hyperdynamic sepsis. Crit Care Med. 1982;10(7):432–5. https://doi.org/10.1097/00003246-198207000-00003.
Patel VV, Sullivan JB, Cavanaugh J. Analysis of mortality in patients treated with phenylephrine in septic shock. J Pharm Pract. Published online March 23, 2021:089719002110002. https://doi.org/10.1177/08971900211000218
Acknowledgements
We thank the staff members of the collaborative research team at the Laboratory for Computational Physiology, Massachusetts Institute of Technology.
Funding
This study was supported by the National Natural Science Foundation of China (82072232 and 81871585), the Clinical Frontier Technology Program of the First Affiliated Hospital of Jinan University, China (JNU1AF-CFTP-2022-a01235), the Science and Technology Projects in Guangzhou, China (202201020054 and 2023A03J1032), the Appropriate Technology of Hunan Health Commission, China (202218015798).
Author information
Authors and Affiliations
Contributions
Dan He and Hai Hu created the protocol, performed the statistical analysis, and wrote the first draft of the manuscript. Hong Liang conceived the study and critically revised the manuscript. Xuehao Lu assisted with data extraction. Luming Zhang assisted in revising the manuscript and confirming the data. Wan-jie Gu assisted with manuscript editing, and Jun Lyu and Haiyan Yin were responsible for the study protocol, data interpretation, and manuscript revision. All authors reviewed the manuscript. The authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study was an analysis of a third-party anonymized publicly available database with pre-existing institutional review board (IRB) approval. Data extracted from the MIMIC III database do not require individual informed consent because MIMIC III database research data is publicly available and all patient data are de-identified.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing financial interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
He, D., Hu, H., Hong, L. et al. Norepinephrine combined with phenylephrine versus norepinephrine in patients with septic shock: a retrospective cohort study. BMC Infect Dis 23, 221 (2023). https://doi.org/10.1186/s12879-023-08142-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-023-08142-x