Abstract
Background
Use of anticholinergic (ACH) medications is associated with increased risk of cognitive decline in the elderly. However, little is known about this association from a health plan perspective.
Methods
This retrospective cohort study used the Humana Research Database to identify individuals with at least one ACH medication dispensed in 2015. Patients were followed until incidence of dementia/Alzheimer’s disease, death, disenrollment or end of December 2019. Multivariate Cox regression models were used to assess the association between ACH exposure and study outcomes, adjusting for demographics and clinical characteristics.
Results
A total of 12,209 individuals with no prior ACH use or dementia/Alzheimer’s disease diagnosis were included. As ACH polypharmacy increased (i.e., from no ACH exposure, to one, two, three, and four or more ACH medications), there was a stair-step increase in the incidence rate of dementia/Alzheimer’s disease (15, 30, 46, 56 and 77 per 1,000 person-years of follow-up) and in the incidence of mortality (19, 37, 80, 115 and 159 per 1,000 person-years of follow-up). After adjusting for confounders, ACH exposure to one, two, three and four or more ACH medications was associated with a 1.6 (95% CI 1.4–1.9), 2.1 (95% CI 1.7–2.8), 2.6 (95% CI 1.5–4.4), and 2.6 (95% CI 1.1–6.3) times, respectively, increased risk of a dementia/Alzheimer’s disease diagnosis compared to periods of no ACH exposure. ACH exposure to one, two, three and four or more medications was associated with a 1.4 (95% CI 1.2–1.6), 2.6 (95% CI 2.1–3.3), 3.8 (95% CI 2.6–5.4), and 3.4 (95% CI 1.8–6.4) times, respectively, increased risk of mortality compared to periods of no ACH exposure.
Conclusions
Reducing ACH exposure may potentially minimize long-term adverse effects in older adults. Results suggest populations which may benefit from targeted interventions to reduce ACH polypharmacy.
Similar content being viewed by others

Background
The 2015 American Geriatrics Society Beers Criteria recommend against concurrent use of three or more central nervous system and two or more anticholinergic (ACH) medications in the elderly due to increased risk of cognitive decline [1]. The updated 2019 Beers Criteria for potentially inappropriate medication use in older adults added drugs with strong ACH properties to their list based on more recent evidence [2], which continues to accumulate to support these recommendations. For example, in a diverse population of 6,249 Latino adults residing in four major U.S. cities, ACH drug use at two points in time during an average 7-year follow-up was associated with lower global cognition, learning, memory, and executive functioning [3]. Additionally, among 8,216 older adults in Great Britain, recurrent use of ACH medications that were scored 3 on the Anticholinergic Cognitive Burden (ACB3) scale was linked with dementia by year 10 of cohort follow-up [4]. However, the impact on dementia was not observed in patients on ACH medications characterized with a lower anticholinergic burden of ACB 1 or 2 or in those who had no prior use.
Due to the possible negative effects of ACH exposure, medications with higher ACH activity such as oxybutynin now include a caution for patients with preexisting dementia treated with cholinesterase inhibitors because adding ACH drugs may exacerbate cognitive symptoms [5]. Prospective data also support the connection between increasing ACH exposure and increasing rates of cognitive decline and dementia in older populations [6], and two recent systematic reviews and meta-analyses found the risk of incident dementia and cognitive decline increased with increasing ACH exposure [5, 7]. Despite these potential risks, older individuals may be initiated to or continued on these agents because the ACH medication class is broad and includes therapies for several conditions affecting older individuals such as depression [8], overactive bladder [9, 10], and Parkinson’s disease [11].
The issue of polypharmacy may further complicate this clinical picture when assessing the possible risks and benefits of ACH medications for older adults. Clinical consequences of polypharmacy in older adults include cognitive impairment, disability, falls, frailty, increased healthcare use, and mortality [12]. For example, an Italian multicenter cohort study found approximately half of the cohort of 342 older adults with mild cognitive impairment took three or more drugs per day, and at the end of one year, the odds of dementia were six times higher for those taking more than three medications compared with similar adults taking fewer than three drugs. Further evidence demonstrated dementia risk was five times higher for those assessed with Anticholinergic Risk Scale (ARS) ≥ 1 [13]. In another example, a polypharmacy study of a population of patients aged ≥ 65 years from an outpatient multi-morbidity clinic in Australia found exposure to polypharmacy in the sedative and ACH medication classes in particular was linked to future mortality [14].
While the impact of ACH burden on cognition is well documented globally [15,16,17,18,19], less is known regarding the prevalence and intensity of ACH use in large, U.S. health plan populations, and of how ACH polypharmacy in particular may affect the risk of dementia, Alzheimer’s disease, and mortality in this population. Health plans may play a unique role in helping to mitigate medication-related problems such as polypharmacy in the elderly, adding further value to considering the issue through a health plan lens. To address this issue, we used real-world claims to describe the demographics and clinical characteristics of a large population of Medicare Advantage prescription drug (MAPD) plan beneficiaries newly dispensed ACH medications and analyzed the relationship between polypharmacy and cumulative ACH therapy exposure on incident dementia/Alzheimer’s disease and mortality.
Methods
Study design
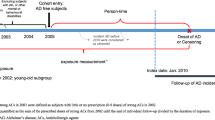
This retrospective cohort study used the Humana Research Database to identify MAPD patients, 65–89 years of age, newly initiated on an ACH medication with an Anticholinergic Cognitive Burden scale (ACB) score 2 or 3 [20, 21] between January 1, 2015 and December 31, 2015 (index date = first ACH exposure). The cut point of 89 years was chosen to reflect organizational data and privacy policies requiring exclusion of patients 90 years and older from research studies. The ACB score is a high-quality anticholinergic burden scale [22] and is based on a systematic review by Boustani et al. which ranks drugs from a scale of one to three [20]. An ACB score of 2 or 3 indicates drugs with established and clinically relevant cognitive ACH effects, as compared to a score of one, which indicates drugs with potential ACH effects. Eligible patients had 12-months pre-index continuous enrollment, and were followed up until the end of enrollment, death, or end of study period (December 31, 2019) whichever occurred first (Fig. 1).
Patients with any exposure to a prescription ACH medication or any evidence of a diagnosis or medication use for dementia/Alzheimer’s disease any time prior to 2015 (until 2007) were excluded from the study.
Study exposure
Using pharmacy claims data, we measured time-varying ACH exposure to account for changes in medication regimens over extended periods of follow-up. ACH exposure was measured in two ways: i. Time-varying person-time level ACH polypharmacy measure for medication exposure on each follow-up day (i.e., no exposure, 1, 2, 3, ≥ 4 ACH medications), and ii. Cumulative ACH exposure measure derived by summing each person’s standardized daily ACH exposure intensity during the follow-up, and categorized as low, moderate, high and very high.
Exposure intensity was measured taking into account both drug-specific properties (i.e., anticholinergic activity) and patient-specific dosing. It was measured with medication coverage arrays using Defined Daily Dose (DDD) [23] to standardize dosing across different medications and the drug-specific ACB score to provide a scale by strength of anticholinergic activity. The DDD is the assumed average maintenance dose per day for a drug used for its main indication in adults. For each anticholinergic dispensing, the Standardized Daily Dose (SDD) of the medication was calculated by taking the estimated number of dosage units consumed in a day multiplied by the unit strength then divided by the DDD, as described by the following equation:
For example, if 90 tablets of oxybutynin-immediate release 5 mg were dispensed with a days’ supply of 30, where the DDD for oxybutynin is 15 mg, then the calculation would be: [(90 tablets/30 days’ supply)*5 mg]/15 mg = 1.0 SDD. A value of 1.0 SDD indicates that for the period of time covered by the prescription dispensing, the patient received 1.0 standardized dose per day covered with medication. Once the SDD for an individual dispensing is determined, the SDD was multiplied by the ACB scale score of the medication dispensed to yield a drug and patient–specific measure of the Standardized Daily Anticholinergic Exposure (SDACE). This value was input into a medication days’ coverage array for the period of time including the index date through the last day covered based on the days’ supply for the dispensing. A grace period of 15 days’ supply was added to each anticholinergic dispensing to account for partial adherence to treatment and residual anticholinergic effect, unless the days’ supply is ≤ 7 then 3 days was added. Using array-based methods, the drug-specific SDACE was aggregated at the patient level to account for coverage with multiple medications on a given day, yielding a Summated Standardized Daily Anticholinergic Exposure (SumSDACE) measure that reflected the total anticholinergic intensity of exposure for each patient on each day of follow-up, taking into account all anticholinergic medication that the individual was receiving.
Cumulative anticholinergic exposure was then measured as the cumulative SumSDACE for all preceding days during the follow-up period. Breakpoints for categorization of cumulative anticholinergic exposure (i.e., into low, moderate, high, and very-high cumulative exposure categories) were determined using all post-index follow-up times and were based on initial assessment of the distribution of the data and then informed by clinical judgment and existing literature.
Study outcomes
The dementia/Alzheimer’s disease study outcome was defined by the presence of ≥ 2 relevant diagnosis codes on medical claims or by the presence of ≥ 1 relevant diagnosis code on a medical claim and ≥ one prescription claim for a cognition-enhancing medication (rivastigmine, galantamine, donepezil or memantine). Mortality was measured using information from the enrollment data provided by the Centers for Medicare and Medicaid Services.
Statistical analysis
Crude incidence rates were reported as rate per 1,000 person-years. Since patients dynamically start and stop treatment with ACH medications over the course of an extended period of follow-up, a series of extended Cox regression models were computed accounting for time-varying ACH exposure when examining the relationship to incident dementia/Alzheimer’s disease and mortality outcomes. Regression models were adjusted for patient demographics, Elixhauser Comorbidity Index [24,25,26], RxRisk-V score [27], baseline medication use, baseline healthcare resource use, and time-varying use of other high-risk non-ACH medications associated with cognitive impairment (i.e., non-ACB drugs included in the drug classes anxiolytic benzodiazepines, anticonvulsant benzodiazepines, anti-depressants, anti-convulsants, anti-psychotics, barbiturate hypnotics, sedative hypnotics, anti-Parkinson drugs and anti-nausea/vertigo agents). [28,29,30,31] The Elixhauser comorbidity index uses 31 categories of ICD-9 and ICD-10 diagnosis codes [24, 26] to calculate a score that is associated with hospital charges, length of stay, and mortality [26]. The RxRisk score is a pharmacy-based measure of comorbidity burden that was originally developed to assess medication burden in the Veterans Health Administration (VHA) population [27], ranges from 0–45, and has been shown to be predictive of healthcare costs and mortality in a range of populations [27, 31,32,33,34,35,36,37,38]. Two of the original RxRisk-V categories (neurogenic bladder and ostomy) are not included in the current implementation because products associated with these conditions (urinary catheters and colostomy supplies, respectively) are not captured in outpatient pharmacy claims. Hence the composite score in the present study ranges from 0 to 43.
Results
We identified 12,209 individuals with no prior ACH therapy use and no prior diagnoses of dementia or Alzheimer’s disease (Fig. 2). The cohort was 57.6% female, 80.6% White, 13.2% with low-income subsidy and dual Medicare-Medicaid eligibility status, with a mean age of 72.2 years. Prevalence of non-ACH high-risk medication use was 51.2% (Table 1). We observed maximum concurrent ACH use of one, two, three, or four or more therapies during the follow-up period in 59%, 30%, 8%, and 3% of the cohort, respectively. Individuals exposed to four or more ACH medications had the highest baseline utilization of inpatient stays (16.7%) and emergency department visits (29.7%, Table 1).
As ACH polypharmacy increased, there was a stair-step increase in the incidence of dementia/Alzheimer’s disease (Table 2) and in the incidence of mortality (Table 3). For example, moving from periods of no ACH exposure to 1, 2, 3 and ≥ 4 ACH medications, the incidence rate of dementia/Alzheimer’s disease increased from 15 per 1,000 person-years to 31, 46, 56 and 77 (Table 2) and the incidence rate for mortality increased from 19 per 1,000 person-years to 37, 79, 115 and 159 (Table 3), respectively. A similar stair-step increase in the incidence rate of both outcomes was observed as cumulative ACH exposure increased from moderate to high to very high compared to the period of low ACH exposure.
After adjusting for confounders, ACH exposure to one, two, three and four or more ACH medications was associated with a 1.6 (95% CI 1.4–1.9), 2.1 (95% CI 1.7–2.8), 2.6 (95% CI 1.5–4.4), and 2.6 (95% CI 1.1–6.3) times, respectively, increased risk of a dementia/Alzheimer’s disease diagnosis compared to periods of no ACH exposure (Table 2). Similarly, ACH exposure to one, two, three and four or more medications was associated with a 1.4 (95% CI 1.2–1.6), 2.6 (95% CI 2.1–3.3), 3.8 (95% CI 2.6–5.4), and 3.4 (95% CI 1.8–6.4) times, respectively, increased risk of mortality compared to periods of no ACH exposure (Table 3).
Discussion
Greater ACH burden was associated with an increased rate of dementia/Alzheimer’s disease and mortality in this study of seniors enrolled in a Medicare Advantage health plan. While the magnitude of effect differed across our results, the directionality is in line with a recent meta-analysis that documented a 46% increased odds of incident dementia in patients with ≥ 3 months of ACH medication use [5]. Additionally, reflecting on our results, a Cochrane Database review and meta-analysis with 968,428 participants in total suggested a possible doubling of the risk of dementia with ACH drug exposure and that a greater ACB score increases the risk of dementia [39]. Furthermore, two other systematic reviews and meta-analyses reported a positive association between ACH burden and risk of mortality across the majority of the included studies spanning from 1990 to 2018 [40, 41]. The authors of those studies acknowledged short follow-up time as an important limitation, with only a few of the studies meeting the criteria for superior quality. In our study, we were able to follow patients for four years enabling a more robust data collection in comparison to many existing studies. With this longer-term evaluation, we found the risk of cognitive decline and mortality with ACH exposure may apply across a wide range of populations and settings.
Additionally, we considered the intersection of polypharmacy with ACH drug exposure and found that significant portions of this population were prescribed more than one ACH medication. Of note, 30% of this population was prescribed two or more ACH therapies, 8% used three or more, and 3% used four or more drugs with ACH activity on at least one day during their follow-up. These results should be concerning, as a recently published meta-analysis of 2.9 million elderly individuals reported a 28% increased risk of mortality associated with use of five or more drugs [42]. These results represent a significant number of older individuals exposed to the well-documented risks of cognitive decline and increased mortality that are associated with this therapeutic class of medications [43,44,45]. Health plans have an opportunity and important role in minimizing ACH exposure for enrolled patients. For example, coordinated educational outreach efforts can be made to pharmacy, provider, and patients on the safety concerns with the use of multiple anticholinergics.
While we did not specifically assess the impact of ACH therapy on other treatment outcomes, more than 50% of our cohort was exposed to non-ACH, high-risk therapy. These results point toward the importance of assessing both ACH and total polypharmacy in future research and when designing de-prescribing programs with the intention of lowering the risk of cognitive decline. Future research should continue to further define these relationships and provide insights into optimal policies and programs to address ACH exposure/polypharmacy in the elderly, particularly with the understanding that cognitive decline and its attendant sequelae can lead to a loss of independence [46].
Limitations
Dementia may not be well represented in administrative claims data, and diagnosis codes do not provide information on severity of dementia. This study used a broad range of diagnosis codes and an extended follow-up period (3–5 years) to identify new onset cases. Additionally, this study focuses only on prescription medication utilization which is well captured using claims data; however, over-the-counter medications, such as some antihistamines, may have strong anticholinergic effects and are not captured in prescription claims, which would lead to underestimating ACH polypharmacy. It is not possible to know from claims data if the drug was indicated for as needed versus daily use. Hence, DDD was an assumed average maintenance dose per day for a drug used for its main indication in adults. We assumed that a patient took the medications based on the days’ supply variable. This may lead to an overestimate of ACH exposure. Similar to other retrospective database studies, this study was subject to limitations including coding errors of omission and commission, incomplete claims, unreliable clinical coding, and unobservable factors that also may have influenced the outcomes. For example, while pharmacy claims and days’ supply on prescription fills indicate the possession of pharmacotherapy, we are unable to verify if the patient took the medication. This study used data from a single data source, and as such the results may not be generalized to the general population. However, Humana is a large national health plan with beneficiaries residing in a broad array of geographic regions.
Conclusions
ACH medication use is common among health plan members residing in broad geographic regions of the United States. ACH drugs were associated with significantly increased risk of dementia and mortality in this real-world population of older adults. Reducing ACH exposure may potentially minimize long-term adverse effects in older adults. Results suggest populations which may benefit from targeted interventions to reduce ACH polypharmacy.
Availability of data and materials
The datasets generated and analyzed during the current study are not publicly available due the proprietary nature of the work but may be available in summary form from the corresponding author on reasonable request.
Abbreviations
- ACB:
-
Anticholinergic Cognitive Burden
- ACH:
-
Anticholinergic
- ARS:
-
Anticholinergic Risk Scale
- COPD:
-
Chronic obstructive pulmonary disease
- DDD:
-
Defined Daily Dose
- SDACE:
-
Standardized Daily Anticholinergic Exposure
- SDD:
-
Standardized Daily Dose
- SumSDACE:
-
Summated Standardized Daily Anticholinergic Exposure
References
American Geriatrics Society 2015 Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2015;63(11):2227–46.
American Geriatrics Society 2019 Updated AGS Beers Criteria® for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2019;67(4):674–94.
Posis AIB, Tarraf W, Gonzalez KA, Soria-Lopez JA, Léger GC, Stickel AM, et al. Anticholinergic drug burden and neurocognitive performance in the study of latinos-investigation of neurocognitive aging. J Alzheimers Dis. 2022;86(1):53–65.
Grossi CM, Richardson K, Fox C, Maidment I, Steel N, Loke YK, et al. Anticholinergic and benzodiazepine medication use and risk of incident dementia: a UK cohort study. BMC Geriatr. 2019;19(1):276.
Dmochowski RR, Thai S, Iglay K, Enemchukwu E, Tee S, Varano S, et al. Increased risk of incident dementia following use of anticholinergic agents: a systematic literature review and meta-analysis. Neurourol Urodyn. 2021;40(1):28–37.
Gray SL, Anderson ML, Dublin S, Hanlon JT, Hubbard R, Walker R, et al. Cumulative use of strong anticholinergics and incident dementia: a prospective cohort study. JAMA Intern Med. 2015;175(3):401.
Taylor-Rowan M, Edwards S, Noel-Storr AH, McCleery J, Myint PK, Soiza R, et al. Anticholinergic burden (prognostic factor) for prediction of dementia or cognitive decline in older adults with no known cognitive syndrome. Cochrane Database Syst Rev. 2021;5(5):Cd013540.
Jaffe RJ, Novakovic V, Peselow ED. Scopolamine as an antidepressant: a systematic review. Clin Neuropharmacol. 2013;36(1):24–6.
Thiagamoorthy G, Cardozo L, Robinson D. Current and future pharmacotherapy for treating overactive bladder. Expert Opin Pharmacother. 2016;17(10):1317–25.
Matta R, Gomes T, Juurlink D, Jarvi K, Herschorn S, Nam RK. Receipt of Overactive Bladder Drugs and Incident Dementia: A Population-based Case-control Study. Eur Urol Focus. 2021.
Ghossein N, Kang M, Lakhkar AD. Anticholinergic Medications. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022. https://www.ncbi.nlm.nih.gov/books/NBK555893/. Accessed 2 Jan 2023.
Chippa V, Roy K. Geriatric Cognitive Decline and Polypharmacy. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022. https://www.ncbi.nlm.nih.gov/books/NBK574575/. Accessed 2 Jan 2023.
Trevisan C, Limongi F, Siviero P, Noale M, Cignarella A, Manzato E, et al. Mild polypharmacy and MCI progression in older adults: the mediation effect of drug-drug interactions. Aging Clin Exp Res. 2021;33(1):49–56.
Masnoon N, Kalisch Ellett L, Shakib S, Caughey GE. Predictors of mortality in the older population: the role of polypharmacy and other medication and chronic disease-related factors. Drugs Aging. 2020;37(10):767–76.
Kalisch Ellett LM, Pratt NL, Ramsay EN, Barratt JD, Roughead EE. Multiple anticholinergic medication use and risk of hospital admission for confusion or dementia. J Am Geriatr Soc. 2014;62(10):1916–22.
Joung KI, Kim S, Cho YH, Cho SI. Association of anticholinergic use with incidence of Alzheimer’s disease: population-based cohort study. Sci Rep. 2019;9(1):6802.
Fox C, Richardson K, Maidment ID, Savva GM, Matthews FE, Smithard D, et al. Anticholinergic medication use and cognitive impairment in the older population: the medical research council cognitive function and ageing study. J Am Geriatr Soc. 2011;59(8):1477–83.
Campbell NL, Perkins AJ, Bradt P, Perk S, Wielage RC, Boustani MA, et al. Association of anticholinergic burden with cognitive impairment and health care utilization among a diverse ambulatory older adult population. Pharmacotherapy. 2016;36(11):1123–31.
Egberts A, Moreno-Gonzalez R, Alan H, Ziere G, Mattace-Raso FUS. Anticholinergic drug burden and delirium: a systematic review. J Am Med Dir Assoc. 2021;22(1):65-73.e4.
Boustani M, Campbell N, Munger S, Maidment I, Fox C. Impact of anticholinergics on the aging brain: a review and practical application. Aging Health. 2008;4(3):310–20.
Salahudeen MS, Duffull SB, Nishtala PS. Anticholinergic burden quantified by anticholinergic risk scales and adverse outcomes in older people: a systematic review. BMC Geriatr. 2015;15:31.
Lisibach A, Benelli V, Ceppi MG, Waldner-Knogler K, Csajka C, Lutters M. Quality of anticholinergic burden scales and their impact on clinical outcomes: a systematic review. Eur J Clin Pharm. 2021;77(2):147–62.
WHO Collaborating Centre for Drug Statistics Methodology. Norwegian Institute of Public Health. Defined Daily Dose Definition and General Considerations. 2018. https://www.whocc.no/ddd/definition_and_general_considera/. Accessed 2 Jan 2023.
Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–9.
Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53(12):1258–67.
Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27.
Sloan KL, Sales AE, Liu CF, Fishman P, Nichol P, Suzuki NT, et al. Construction and characteristics of the RxRisk-V: a VA-adapted pharmacy-based case-mix instrument. Med Care. 2003;41(6):761–74.
Suehs BT, Caplan EO, Hayden J, Ng DB, Gaddy RR. The relationship between anticholinergic exposure and falls, fractures, and mortality in patients with overactive bladder. Drugs Aging. 2019;36(10):957–67.
Moore AR, O’Keeffe ST. Drug-induced cognitive impairment in the elderly. Drugs Aging. 1999;15(1):15–28.
Tannenbaum C, Paquette A, Hilmer S, Holroyd-Leduc J. Carnahan R A systematic review of amnestic and non-amnestic mild cognitive impairment induced by anticholinergic, antihistamine. GABAergic and opioid drugs Drugs Aging. 2012;29(8):639–58.
Zhang YR, Xu W, Zhang W, Wang HF, Ou YN, Qu Y, et al. Modifiable risk factors for incident dementia and cognitive impairment: an umbrella review of evidence. J Affect Disord. 2022;314:160–7.
Sales AE, Liu C-F, Sloan KL, Malkin J, Fishman PA, Rosen AK, et al. Predicting costs of care using a pharmacy-based measure risk adjustment in a veteran population. Med Care. 2003;41(6):753.
Fishman PA, Goodman MJ, Hornbrook MC, Meenan RT, Bachman DJ, Rosetti MCO. Risk adjustment using automated ambulatory pharmacy data: the RxRisk model. Med Care. 2003;41(1):84.
Farley JF, Harley CR, Devine JW. A comparison of comorbidity measurements to predict healthcare expenditures. Am J Manag Care. 2006;12(2):110.
Johnson ML, El-Serag HB, Tran TT, Hartman C, Richardson P, Abraham NS. Adapting the Rx-Risk-V for mortality prediction in outpatient populations. Med Care. 2006;44(8):793–7.
Fan VS, Maciejewski ML, Liu C-F, McDonell MB, Fihn SD. Comparison of risk adjustment measures based on self-report, administrative data, and pharmacy records to predict clinical outcomes. Health Serv Outcomes Res Methodol. 2006;6(1):21–36.
Vitry A, Wong SA, Roughead EE, Ramsay E, Barratt J. Validity of medication-based co-morbidity indices in the Australian elderly population. Aust NZ J Public Health. 2009;33(2):126–30.
Lu CY, Barratt J, Vitry A, Roughead E. Charlson and Rx-Risk comorbidity indices were predictive of mortality in the Australian health care setting. J Clin Epidemiol. 2011;64(2):223–8.
Woodford HJ, Stevenson JM. Anticholinergic drugs and dementia: time for transparency in the face of uncertainty. Cochrane Database Syst Rev. 2021;9:Ed000154.
Graves-Morris K, Stewart C, Soiza RL, Taylor-Rowan M, Quinn TJ, Loke YK, et al. The prognostic value of anticholinergic burden measures in relation to mortality in older individuals: a systematic review and meta-analysis. Front Pharmacol. 2020;11:570.
Ali S, Peterson GM, Bereznicki LR, Salahudeen MS. Association between anticholinergic drug burden and mortality in older people: a systematic review. Eur J Clin Pharmacol. 2020;76(3):319–35.
Li Y, Zhang X, Yang L, Yang Y, Qiao G, Lu C, et al. Association between polypharmacy and mortality in the older adults: A systematic review and meta-analysis. Arch Gerontol Geriatr. 2022;100: 104630.
Coupland CAC, Hill T, Dening T, Morriss R, Moore M, Hippisley-Cox J. Anticholinergic Drug Exposure and the Risk of Dementia: A Nested Case-Control Study. JAMA Intern Med. 2019;179(8):1084–93.
Fox C, Smith T, Maidment I, Chan WY, Bua N, Myint PK, et al. Effect of medications with anti-cholinergic properties on cognitive function, delirium, physical function and mortality: a systematic review. Age Ageing. 2014;43(5):604–15.
Sørensen SR, Frederiksen JD, Anru PL, Masud T, Petrovic M, Rosholm JU, et al. Use of drugs with anticholinergic properties at hospital admission associated with mortality in older patients: a danish nationwide register-based cohort study. Drugs Real World Outcomes. 2022;9(1):129–40.
Marshall GA, Amariglio RE, Sperling RA, Rentz DM. Activities of daily living: where do they fit in the diagnosis of Alzheimer’s disease? Neurodegener Dis Manag. 2012;2(5):483–91.
Acknowledgements
The authors would like to thank Amy Hu, PharmD, and Joanna Peyron, BS for their consultation work and Mary Costantino, PhD for her contributions editing.
Funding
Humana Healthcare Research funded the research and manuscript development. No external funds were used in the creation of this work.
Author information
Authors and Affiliations
Contributions
IBP, RG, MR, SB, YX and BTS conceptualized and designed the study. YX created the data sets and analyzed the data. SWD created the first manuscript draft. SWD, IBP, YX, RG, AJ, MR, SB, and BTS reviewed and edited the manuscript. All authors have read and agreed to the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. The Humana Healthcare Research Human Subject Protection Office (HHR HSPO) used the US Department of Health and Human Services regulations 45 CFR 46 and the Office of Human Research Protections Guidance on Coded Private Information or Specimens Use in Research, Guidance (2008) to grant this study a waiver from informed consent and ethics approval because it did not constitute human subjects research and did not require institutional review board oversight. The decision was based on the determination that the research involved only analysis of anonymized and coded information, and the researchers could not readily identify the individuals from which the information was derived. Analysis was performed on a limited data set, and additional administrative permissions were not required to access the data used in the study.
Consent for publication
Not applicable.
Competing interests
I declare that the authors have no competing interests as defined by BMC, or other interests that might be perceived to influence the results and/or discussion reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Poonawalla, I.B., Xu, Y., Gaddy, R. et al. Anticholinergic exposure and its association with dementia/Alzheimer's disease and mortality in older adults. BMC Geriatr 23, 401 (2023). https://doi.org/10.1186/s12877-023-04095-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12877-023-04095-7