Abstract
Background
There is currently no consensus as to a standardized tool for frailty measurement in any patient population. In the solid-organ transplantation population, routinely identifying and quantifying frailty in potential transplant candidates would support patients and the multidisciplinary team to make well-informed, individualized, management decisions. The aim of this scoping review was to synthesise the literature regarding frailty measurement in solid-organ transplant (SOT) candidates.
Methods
A search of four databases (Cochrane, Pubmed, EMBASE and CINAHL) yielded 3124 studies. 101 studies (including heart, kidney, liver, and lung transplant candidate populations) met the inclusion criteria.
Results
We found that studies used a wide range of frailty tools (N = 22), including four ‘established’ frailty tools. The most commonly used tools were the Fried Frailty Phenotype and the Liver Frailty Index. Frailty prevalence estimates for this middle-aged, predominantly male, population varied between 2.7% and 100%. In the SOT candidate population, frailty was found to be associated with a range of adverse outcomes, with most evidence for increased mortality (including post-transplant and wait-list mortality), post-operative complications and prolonged hospitalisation. There is currently insufficient data to compare the predictive validity of frailty tools in the SOT population.
Conclusion
Overall, there is great variability in the approach to frailty measurement in this population. Preferably, a validated frailty measurement tool would be incorporated into SOT eligibility assessments internationally with a view to facilitating comparisons between patient sub-groups and national and international transplant services with the ultimate goal of improved patient care.
Similar content being viewed by others
Introduction
Solid-organ transplantation (SOT) has evolved from an experimental to definitive treatment for patients with end-stage liver, kidney, pancreas, heart and lung dysfunction1. Advances in surgical techniques and immunosuppressive therapy have seen reduced perioperative and medical complications and improved graft survival [1]. In turn, patient survival rates are high; for example, in the United States, as of 2019, one-year patient survival rates were equal to or greater than 90% for all single SOT except intestine transplantation [2]. Improvements in other patient-important outcomes, such as quality of life and functional performance, have also been reported [3]. Consequently, demand for SOT now exceeds donor organ supply and the field must explore strategies to address this balance. One such strategy is to optimize organ allocation processes through greater understanding of potential transplant candidates’ risk profiles [4, 5].
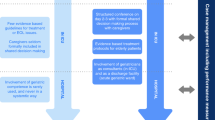
Determining transplant eligibility is a complex process undertaken by a multidisciplinary team, including transplant physicians and surgeons, specialist nurses and allied health professionals. Even though there are established scoring methods (such as the Model for End-Stage Liver Disease) and listing criteria for each organ, the decision to place a patient on the transplant waiting list often comes down to expert clinical judgement [6]. The multidisciplinary team must weigh the inherent risks of surgery and immunosuppression against the potential benefits for each individual, which in turn must be weighed against the need to ensure that there is maximum benefit derived from a finite resource. Moreover, transplant teams are increasingly being asked to evaluate older patients with more complex medical and functional needs [7]. Differences in health status, which corresponds with differences in risk of adverse outcomes, can be captured by measuring frailty [8]. Routinely identifying and quantifying frailty in potential transplant candidates would support patients and the multidisciplinary team to make well-informed, individualized, management decisions, a viewpoint shared by more than 250 kidney, liver, heart and lung transplantation specialists at the 2018 consensus conference on frailty [9].
Frailty is a state of increased vulnerability to stressors that is associated with adverse health outcomes, including death, disability and hospitalization [10, 11]. A frail individual has reduced physiological reserve and a reduced ability to compensate for disruptions to homeostasis [10]. Frailty increases with, but is not synonymous with, chronological age [12]. Frailty is prevalent in adults with organ failure and tends to develop at a younger age than the general population [13,14,15,16,17]. For example, in one study of dialysis-dependent patients, 73% of the entire cohort and 64% of those younger than 40 years of age were frail [18]. Even though kidney transplant candidates are likely to be a younger and healthier subset of patients with end-stage kidney disease, approximately 15% of wait-listed patients have been assessed as frail in recent studies [19, 20]. Similarly, frailty prevalence has been found to be high among younger heart failure patients, and the lack of relationship between age and frailty in this group indicates frailty in heart failure is not confined to older adults [16, 17].
Currently, there is no consensus as to a standardized tool for frailty measurement in any patient population. In a 2018 scoping review, 89 different measures were being utilized in the acute care setting alone [21]. To our knowledge, no systematic reviews have been conducted with a focus on the SOT population. The consensus conference concluded that a single frailty measure across all SOT candidates may not be appropriate due to different aspects of frailty being relevant to different patient (organ) groups [22]. However, we argue that frailty assesses intrinsic vulnerability rather than the impact of individual diseases and, as a result, there is merit in comparing frailty measurement across groups. The aim of our scoping review, therefore, was to synthesise the literature around frailty measurement in SOT candidate populations.
Materials and methods
Protocol and registration
This scoping review is reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) criteria [23]. The protocol was registered with Open science Framework (OSF; Digital Object Identifier https://doi.org/10.17605/OSF.IO/GZN38).
Search strategy
The search strategy was developed by JK, EG and NR, with the assistance of a librarian, and conducted by JK. We searched Cochrane (Cochranelibrary.com), Pubmed (PubMed.gov), EMBASE (Embase.com) and CINAHL (EBSCOhost) databases. Search terms used were broad and included ‘transplant*’ OR ‘allog*’ (title/abstract) and ‘frail*’ (title/abstract), which we deemed would capture all types of transplants and allograft studies, as well as any studies where frailty was an intended measure, regardless of which specific measure was used. The search included all studies up until 31st July 2022, and there were no limits placed. References lists of included full-text articles were searched for additional relevant studies.
Study selection
Studies were eligible for inclusion if they purported to measure ‘frailty’ in solid-organ (including kidney, liver, pancreas, heart or lung) transplant candidates. Additionally, studies of transplant recipients that measured frailty at, or just prior to, admission for transplant surgery were included as participants were ‘transplant candidates’ at the time of frailty assessment. Included studies could be of any design, but were to be conducted in an adult human population and published in English. Studies were excluded if they were not an original study (e.g., a protocol or review paper), were an abstract only or were not published in English. Abstract only studies were excluded as it was felt there would not be sufficient information for data analysis. Studies of non-solid organ transplant candidates (such as bone marrow transplant candidates) and studies that only measured frailty in transplant recipients were also excluded. As this review was interested in the methods by which these studies measured frailty, the measurement tool used was neither an inclusion nor exclusion criteria.
After removal of duplicates, reviewers JK and SK screened titles, abstracts and full-text of the studies. Any ambiguity regarding the study eligibility was resolved by an independent review of the study by a second and/or third reviewer (EG and/or NR).
Data extraction and analysis
A data extraction template was devised by three reviewers (JK, NR, EG) and imported into Covidence [24]. The following data was extracted from each included article: year and country of publication, transplant organ, study design, sample size, participant sex and age, frailty measurement tool used, timing of frailty measurement, reason for measuring frailty and, where relevant, adverse outcome measures examined in relation to frailty. Frailty tools were identified as ‘established’ if they were tools specifically developed and validated as a measure of frailty in a general population. ‘Other frailty tools’ referred to all other measures, including operational definitions developed for a SOT or disease-specific group (e.g., the Liver Frailty Index) and scales not specifically developed to measure frailty (e.g., the Short Physical Performance Battery).
These are definitions that have been employed in other studies of frailty measurement [21]. No specific adverse outcomes were of interest, we sought to identify all adverse outcomes that the included studies reported on. Data was extracted for all studies, and each reviewer (NR, EG, LS, EP, BL, SK, RH, SF) was given eight to ten unique studies to extract. Another reviewer (JK) independently extracted and cross-checked data for all studies. Disagreements were resolved by consensus.
Results
Search and study selection
The search strategy yielded 3124 articles, which were imported into Covidence (Fig. 1). After removal of duplicates, title and abstract screening and full-text review, a total of 101 studies were eligible for inclusion.
Study characteristics
The characteristics of included studies are presented in Table 1. The majority of studies were conducted in kidney (36 studies; [14, 19, 20, 25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57]) and liver (36 studies; [15, 58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92]) transplant candidates. Nine studies [93,94,95,96,97,98,99,100,101] and 16 studies [102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117] were conducted in heart and lung transplant candidates, respectively. Four studies [118,119,120,121] included double organ transplant candidates (i.e., kidney and liver transplant candidates or heart). No studies that met our inclusion criteria have been published in pancreas transplant candidates. The majority of studies (N = 93) were published within the last seven years, with 62.4% (N = 63) published between 2018 and 2022. The majority of the studies were published in North America (N = 74). Nearly all studies were observational in nature, with only three being experimental. Study sample sizes ranged from 15 participants to over 120,000 participants. The median age of participants was 56.4 years (IQR 53.1–59.0) and the majority of participants were male (median percentage male = 60.0% (IQR 57.9–66.7)).
Frailty measurement
Frailty measurement characteristics of the included studies are presented in Table 2. Most studies measured frailty at time of transplant eligibility assessment (N = 27) or at admission to hospital for transplant surgery (N = 16). Ten studies measured frailty retrospectively in transplant recipients and eight studies measured frailty when the participant was added to the transplant waitlist. Fifteen studies measured frailty at other times, such as four weeks after the participant was added to transplant waitlist. There were 18 studies that did not specify the point at which frailty was measured during the participant’s transplant journey. Seven studies measured frailty at more than one time point.
Overall, 22 different frailty measurement tools were used 123 times in the 101 included articles. The majority of studies used one frailty measurement tool (N = 83, 82.2%) and 18 studies utilized two or more frailty measurement tools. Of the 22 different measures used, four were ‘established frailty tools’ used in 51.2% of cases (N = 63) and the other 27 were ‘other frailty tools’ used in 48.8% of cases (N = 60). Descriptions of the frailty measurement tools are presented in Table 3. The most commonly used frailty tool across all included studies was the Fried Frailty Phenotype (standard and modified; N = 55/123, 44.7%). However, in liver transplant studies, the most commonly used frailty tool was the Liver Frailty Index (N = 19/43, 44.2%). Other established tools included the Clinical Frailty Scale, Rockwood’s Frailty Index and the Groningen Frailty Indicator. Other tools included validated scales such as the Short Physical Performance Battery and physical metrics such as grip-strength.
Eighty-two studies reported a frailty prevalence rate. Prevalence ranged from 2.7% in heart transplant candidates [96] to 100% in HIV-positive liver transplant candidates [118]. Risk stratification was the most common reason for frailty measurement (N = 74). Twenty studies had more than one purpose for frailty measurement. For example, some studies evaluated the feasibility of incorporating frailty assessment into transplant eligibility assessment as well as estimated frailty prevalence and its associated risk of adverse outcomes.
Adverse outcome measures examined in relation to frailty
The association between frailty and adverse outcomes was investigated in 74 studies. The majority of these studies measured more than one adverse outcome, which are listed in Table 4. The most commonly measured adverse outcomes were mortality (N = 29), hospital length of stay (N = 20), waitlist mortality (N = 21) and transplant status (e.g., de-listing; N = 17). Of these 74 studies, 70 (94.6%) found that pre-transplant frailty was predictive of at least one adverse outcome, where those who were frail (or had higher levels of frailty) were more likely to experience an adverse event (Fig. 2). For example, frailty was predictive of mortality in 23 of the 29 studies (79.3%) examining this outcome.
Discussion
This scoping review synthesizes the literature around frailty measurement in solid-organ transplant candidates. We found this to be an emerging field of research, with most studies conducted in kidney and liver transplant candidates in North America in the last seven years. Most studies were observational and examined the relationship between frailty and adverse outcomes, particularly mortality and hospital length of stay. Overall, there were 22 different frailty measures used across the 101 studies, including four ‘established frailty tools’. The two most commonly used tools were the Fried Frailty Phenotype and Liver Frailty Index. Frailty prevalence estimates for this middle-aged, predominantly male, population varied widely, from 2.7 to 100%.
In this review, we aimed to include any article claiming to measure frailty to ascertain the full range of frailty measurement tools utilized in the SOT literature. We demonstrated that whilst many articles purport to measure frailty, almost half did not use an established frailty measurement tool. Using non-established measures to identify frail patients is not unique to this population, and is problematic as it constrains the generalisability of studies [122].
Defined by Fried et al. in 2001, the Fried Frailty Phenotype conceptualizes frailty as a clinical syndrome (a set of signs and symptoms that tend to occur together, thus characterizing a specific medical condition) [123]. More specifically, it identifies frailty as the presence of ≥ 3 of 5 criteria: unintentional weight loss of ≥ 10lbs in the preceding year, self-reported exhaustion, weak grip strength, slow walking speed, low physical activity [123]. It is a well-known and widely-used tool in the non-transplant frailty literature and it has many strengths: it is clinically coherent, reproducible, and identifies frailty as a wasting disorder with sarcopenia as a key pathophysiological feature [123]. The most commonly used frailty measurement tool in liver transplant candidates, the Liver Frailty Index, also focuses on physical signs and symptoms of frailty. Developed only four years ago, it comprises three performance-based tests (of grip strength, sit-to-stand transfers and balance) and was originally designed to capture extrahepatic complications of cirrhosis and to enhance mortality prediction in patients with cirrhosis [64].
While the physical manifestations of frailty are important, the omission of disorders of cognition and mood from frailty assessment is controversial: frailty in the clinical setting consists of more than weakness, slowness and wasting [124]. In the non-transplant literature, an alternative conceptualization of frailty is a multidimensional risk state which can be quantified by the number rather than by the nature of health problems [125, 126]. The Frailty Index (FI) model, defined by Rockwood and colleagues, employs a well-defined methodology to create an index as a proportion of deficits [127]. Since FIs can be constructed from different numbers and types of deficits, a measure of frailty status can be derived from information routinely collected during patient assessment and, as a result, it is often touted as the ‘ideal’ frailty measure in the clinical setting. The FI methodology allows for organ-specific deficits to be included and as a result it may be a suitable standardized measure that addresses the concern that not all frailty measures are applicable to all patient (organ) groups [9], An added advantage of using the FI in studies of SOT populations would be the ability to compare prevalence levels and adverse outcomes across groups, which would unify the literature and generate greater impact and advocacy. There are many other tools that measure the ‘multidimensionality’ of frailty. For example, the Clinical Frailty Scale (CFS), also defined by Rockwood [128], is a judgement-based tool that estimates frailty severity according to increasing morbidity and functional dependence. Despite the prominence of these tools in the frailty literature, this scoping review found that they are infrequently utilized in the SOT population.
Different frailty tools do yield different estimates of prevalence. For example, in a meta-analysis of general population-based data from 62 countries and territories, frailty prevalence estimates ranged between 12% (Fried Frailty Phenotype) and 24% (Frailty Index) in adults aged 50 years and over [129]. While there is some overlap in identification of frailty, it is likely that the phenotypic and multidimensional models (and their associated measurement tools) capture different patient groups [130]. In the SOT literature, prevalence rates vary greatly, reflecting between-study methodological differences in sample size and participant inclusion criteria (such as HIV positive status) as well as frailty measurement tools. It is possible that the predominance of phenotypic frailty tools in this field of research may result in an underestimation of frailty burden in the SOT population. This in turn may lead to an underestimation of risk in this population. Consequently, we would further advocate that future SOT studies utilize an established multidimensional frailty tool, such as Rockwood’s Frailty Index, in their assessment of frailty in potential candidates.
In the SOT candidate population, this scoping review found frailty to be associated with a range of adverse outcomes, with most evidence for increased mortality (including post-transplant and wait-list mortality), post-operative complications and prolonged hospitalisation. Fewer studies explored the relationship between frailty and patient-centered outcomes, such as functional and cognitive decline, which are of critical importance to the informed decision-making process. In the general population literature, there has been an extensive evaluation of the ability of frailty tools to predict adverse outcomes, particularly survival. In comparison studies (wherein multiple frailty measures are applied to the same patient population), predictive power varies between tools, with a trend towards multidimensional frailty tools having higher predictive power for short-term survival [131]. At the present time there is limited data to compare the predictive validity of frailty tools in the SOT population.
SOT studies also varied greatly with respect to the timing of frailty measurement relative to transplantation. We would argue that measurement of frailty at transplant eligibility assessment would be most clinically informative, yet only approximately one quarter (N = 27; 26.7%) of studies measured frailty at this time-point. In the kidney transplant population, frail candidates experience a greater deterioration in health-related quality of life and face a higher risk of mortality while awaiting a transplant when compared with non-frail candidates [25, 132]. Similar associations between frailty, reduction of quality of life and wait-list mortality have been found in heart, lung, and liver transplant candidates [60, 69, 133,134,135]. Overall, frail candidates are less likely to receive a transplant than non-frail candidates [136]. While frailty at time of kidney transplantation has been associated with several post-transplantation complications, including delirium, prolonged hospitalisation, delayed graft function, immunosuppression intolerance and mortality [22], it remains unclear how frailty at time of transplant eligibility assessment relates to these outcomes.
This scoping review has a number of limitations. Firstly, the scope of this review was very broad, not only in terms of organ systems, but also frailty measures and time-points. As a result, quality assessments and meta-analysis were not conducted. Further, if a study used other measures of physical function, such as muscle strength or gait speed tests, but did not claim to measure frailty itself, it would not have been identified by our search. Additionally, our search terms were conducted in title/abstract only, however we consider this strategy would have captured almost all relevant studies. Secondly, non-English studies were not included and the grey literature was not reviewed. Thirdly, initial screening was performed by mainly one rather than two reviewers.
The broad scope of this review is also a strength. By synthesizing frailty data for all SOT groups, clinicians and researchers will be able to consider the evidence relevant to their specialty. It highlights gaps in frailty research in this field, such as the lack of experimental and qualitative studies addressing frailty and the small number of studies utilizing established multidimensional frailty tools such as the Frailty Index. Furthermore, it emphasizes the need for future research to address more focused research objectives such as determining the predictive power of different frailty tools in different organ groups for standard medical/surgical as well as patient-centered outcomes.
Ultimately, identifying frailty in potential SOT candidates may improve patient care. While the primary benefit may be in risk-stratification (i.e., defining an individual’s risk profile so to inform the decisions of patients and specialists), it is possible that, in the future, frailty measurement may also shed light on potentially reversible factors amenable to targeted interventions pre- and post-transplant. At a health service level, identifying frailty in SOT candidates has the potential to influence the balance of organ supply and demand by informing the clinician and the patient regarding the risks and benefits of transplantation. Since misclassification of frailty may have significant, negative implications for the patient and the health service, relying solely on clinical acumen is not ideal. Preferably, a validated frailty measurement tool would be incorporated into SOT eligibility assessments internationally. This would facilitate comparisons between patient sub-groups and national and international transplant services and would permit large-scale synthesis of relevant data, which in turn would foster improvements in SOT patient care.
Data availability
All data generated or analysed during this study are included in this published article.
Abbreviations
- CFS:
-
Clinical Frailty Scale.
- CINAHL:
-
Cumulative Index to Nursing and Allied Health Literature.
- EMBASE:
-
Excerpta Medica Database.
- FI:
-
Frailty Index.
- OHT:
-
Orthoptic Heart Transplant.
- OSF:
-
Open Science Framework.
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews.
- PRISMA-ScR:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews.
- SOT:
-
Solid Organ Transplant.
References
Black CK, Termanini KM, Aguirre O, Hawksworth JS, Sosin M. Solid organ transplantation in the 21(st) century. Ann Transl Med. 2018;6(20):409.
OPTN/SRTR. 2019 Annual Data Report: Introduction. Am J Transplant. 2021;21 Suppl 2:11–20.
Pinson CW, Feurer ID, Payne JL, Wise PE, Shockley S, Speroff T. Health-related quality of life after different types of solid organ transplantation. Ann Surg. 2000;232(4):597–607.
Jasseron C, Legeai C, Jacquelinet C, et al. Optimization of heart allocation: The transplant risk score. Am J Transplant. 2019;19(5):1507–17.
Lewis A, Koukoura A, Tsianos G-I, Gargavanis AA, Nielsen AA, Vassiliadis E. Organ donation in the US and Europe: The supply vs demand imbalance. Transplantation Reviews. 2021;35(2):100585.
Clinical Guidelines for Organ. Transplantation from Deceased Donors Version 1.5. The transplantation Society of Australia and New Zealand. 2021.
Hartmann EL, Wu C. The evolving challenge of evaluating older renal transplant candidates. Adv Chronic Kidney Dis. 2010;17(4):358–67.
Howlett SE, Rutenberg AD, Rockwood K. The degree of frailty as a translational measure of health in aging. Nat Aging. 2021;1(8):651–65.
Kobashigawa J, Dadhania D, Bhorade S, et al. Report from the American Society of Transplantation on frailty in solid organ transplantation. Am J transplantation: official J Am Soc Transplantation Am Soc Transpl Surg. 2019;19(4):984–94.
Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381(9868):752–62.
Xue QL. The frailty syndrome: definition and natural history. Clin Geriatr Med. 2011;27(1):1–15.
Mitnitski AB, Graham JE, Mogilner AJ, Rockwood K. Frailty, fitness and late-life mortality in relation to chronological and biological age. BMC Geriatr. 2002;2(1):1.
Kojima G. Prevalence of frailty in end-stage renal disease: a systematic review and meta-analysis. Int Urol Nephrol. 2017;49(11):1989–97.
Chu NM, Chen X, Norman SP, et al. Frailty Prevalence in Younger End-Stage Kidney Disease Patients Undergoing Dialysis and Transplantation. Am J Nephrol. 2020;51(7):501–10.
Haugen CE, McAdams-Demarco M, Holscher CM, et al. Multicenter study of age, frailty, and waitlist mortality among liver transplant candidates. Annals of Surgery. 2020;271(6):1132–6.
Denfeld QE, Winters-Stone K, Mudd JO, Gelow JM, Kurdi S, Lee CS. The prevalence of frailty in heart failure: A systematic review and meta-analysis. Int J Cardiol. 2017;236:283–9.
Jha SR, Ha HS, Hickman LD, et al. Frailty in advanced heart failure: a systematic review. Heart Fail Rev. 2015;20(5):553–60.
Bao Y, Dalrymple L, Chertow GM, Kaysen GA, Johansen KL. Frailty, dialysis initiation, and mortality in end-stage renal disease. Arch Intern Med. 2012;172(14):1071–7.
Haugen CE, Chu NM, Ying H, et al. Frailty and access to kidney transplantation. Clin J Am Soc Nephrol. 2019;14(4):576–82.
McAdams-DeMarco MA, Ying H, Thomas AG, et al. Frailty, Inflammatory Markers, and Waitlist Mortality among Patients with End-stage Renal Disease in a Prospective Cohort Study. Transplantation. 2018;102(10):1740–6.
Theou O, Squires E, Mallery K, et al. What do we know about frailty in the acute care setting? A scoping review. BMC Geriatr. 2018;18(1):139.
Kobashigawa J, Dadhania D, Bhorade S, et al. Report from the American Society of Transplantation on frailty in solid organ transplantation. Am J Transplant. 2019;19(4):984–94.
PRISMA Extension for Scoping Reviews (PRISMA-ScR). Checklist and Explanation. Annals of Internal Medicine. 2018;169(7):467–73.
Covidence systematic review software. Veritas Health Innovation, Melbourne, Australia..
Shrestha P, Haugen CE, Chu NM, et al. Racial differences in inflammation and outcomes of aging among kidney transplant candidates. BMC Nephrol. 2019;20(1):1–8.
Haugen CE, Gross A, Chu NM, et al. Development and Validation of an Inflammatory-Frailty Index for Kidney Transplantation. Journals of Gerontology Series A: Biological Sciences & Medical Sciences. 2021;76(3):470–7.
Pérez Fernández M, Martínez Miguel P, Ying H, et al. Comorbidity, Frailty, and Waitlist Mortality among Kidney Transplant Candidates of All Ages. Am J Nephrol. 2019;49(2):103–10.
McAdams-DeMarco MA, Isaacs K, Darko L, et al. Changes in Frailty After Kidney Transplantation. J Am Geriatr Soc. 2015;63(10):2152–7.
Schaenman J, Castellon L, Liang EC, et al. The Frailty Risk Score predicts length of stay and need for rehospitalization after kidney transplantation in a retrospective cohort: a pilot study. 2019;5(1).
Garonzik-Wang JM, Govindan P, Grinnan JW, et al. Frailty and delayed graft function in kidney transplant recipients. Archives of Surgery. 2012;147(2):190–3.
Lorenz E, Cosio F, Bernard S, et al. The relationship between frailty and decreased physical performance with death on the kidney transplant waiting list. Am J Transplantation. 2017;17:332.
Konel JM, Warsame F, Ying H, et al. Depressive symptoms, frailty, and adverse outcomes among kidney transplant recipients. Clinical Transplantation. 2018;32(10).
Schopmeyer L, El Moumni M, Nieuwenhuijs-Moeke GJ, Berger SP, Bakker SJL, Pol RA. Frailty has a significant influence on postoperative complications after kidney transplantation—a prospective study on short-term outcomes. Transpl Int. 2019;32(1):66–74.
Chu NM, Gross AL, Shaffer AA, et al. Frailty and changes in cognitive function after kidney transplantation. J Am Soc Nephrol. 2019;30(2):336–45.
McAdams-Demarco MA, Law A, King E, et al. Frailty and mortality in kidney transplant recipients. Am J Transplantation. 2015;15(1):149–54.
Chu NM, Deng A, Ying H, et al. Dynamic Frailty Before Kidney Transplantation: Time of Measurement Matters. Transplantation. 2019;103(8):1700–4.
dos Santos Mantovani M, de Carvalho NC, Archangelo TE, et al. Frailty predicts surgical complications after kidney transplantation. A propensity score matched study. PLoS ONE. 2020;15(2).
McAdams-Demarco MA, King EA, Luo X, et al. Frailty, Length of Stay, and Mortality in Kidney Transplant Recipients. Annals of Surgery. 2017;266(6):1084–90.
McAdams-DeMarco MA, Olorundare IO, Ying H, et al. Frailty and Postkidney Transplant Health-Related Quality of Life. Transplantation. 2018;102(2):291–9.
Haugen CE, Thomas AG, Chu NM, et al. Prevalence of frailty among kidney transplant candidates and recipients in the United States: Estimates from a National Registry and Multicenter Cohort Study. Am J Transplantation. 2020;20(4):1170–80.
Kosoku A, Uchida J, Iwai T, et al. Frailty is associated with dialysis duration before transplantation in kidney transplant recipients: A Japanese single-center cross-sectional study. Int J Urol. 2020;27(5):408–14.
Lorenz EC, Hickson LJ, Weatherly RM, et al. Protocolized exercise improves frailty parameters and lower extremity impairment: A promising prehabilitation strategy for kidney transplant candidates. Clinical Transplantation. 2020;34(9).
Quint EE, Schopmeyer L, Banning LBD, et al. Transitions in frailty state after kidney transplantation. Langenbeck’s Archives of Surgery. 2020;405(6):843–50.
McAdams-Demarco MA, Law A, Salter ML, et al. Frailty and early hospital readmission after kidney transplantation. Am J Transplantation. 2013;13(8):2091–5.
Manay P, Ten Eyck P, Kalil R, et al. Frailty measures can be used to predict the outcome of kidney transplant evaluation. Surgery. 2021;169(3):686–93.
Adlam T, Ulrich E, Kent M, Malinzak L. Frailty Testing Pilot Study: Pros and Pitfalls. J Clin Med Res. 2018;10(2):82–7.
Haugen CE, Mountford A, Warsame F, et al. Incidence, risk factors, and sequelae of post-kidney transplant delirium. J Am Soc Nephrol. 2018;29(6):1752–9.
McAdams-Demarco MA, Law A, Tan J, et al. Frailty, mycophenolate reduction, and graft loss in kidney transplant recipients. Transplantation. 2015;99(4):805–10.
McAdams-DeMarco MA, Ying H, Olorundare I, et al. Individual frailty components and mortality in kidney transplant recipients. Transplantation. 2017;101(9):2126–32.
Bozhilov K, Vo KB, Wong LL. Can the Timed Up & Go Test and Montreal Cognitive Assessment predict outcomes in patients waitlisted for renal transplant? Clinical Transplantation. 2021;35(1).
Chan GCK, Ng JKC, Chow KM, et al. Impact of frailty and its inter-relationship with lean tissue wasting and malnutrition on kidney transplant waitlist candidacy and delisting. Clin Nutr. 2021;40(11):5620–9.
Novais T, Pongan E, Gervais F, et al. Pretransplant Comprehensive Geriatric Assessment in Older Patients with Advanced Chronic Kidney Disease. Nephron. 2021;145(6):692–701.
Pérez-Sáez MJ, Dávalos-Yerovi V, Redondo-Pachón D, et al. Frailty in kidney transplant candidates: a comparison between physical frailty phenotype and FRAIL scales. Journal of Nephrology. 2022(Nephrology Department, Hospital del Mar, Passeig Maritim 25–29, Barcelona, Spain(Dávalos-Yerovi V).
Manay P, Ten Eyck P, Siniff E, et al. Psychosocial characteristics of patients evaluated for kidney transplant and associations with functional and frailty metrics at a veterans affairs hospital. Clinical Transplantation. 2022;36(2).
Worthen G, Vinson A, Cardinal H, et al. Prevalence of Frailty in Patients Referred to the Kidney Transplant Waitlist. Kidney360. 2021;2(8):1287–95.
Pérez-Sáez MJ, Arias-Cabrales CE, Dávalos-Yerovi V, et al. Frailty among chronic kidney disease patients on the kidney transplant waiting list: the sex–frailty paradox. Clin Kidney J. 2021;15(1):109–18.
Pérez-Sáez MJ, Redondo-Pachón D, Arias-Cabrales CE, et al. Outcomes of Frail Patients While Waiting for Kidney Transplantation: Differences between Physical Frailty Phenotype and FRAIL Scale. J Clin Med. 2022;11(3).
Ingraham NE, Tignanelli CJ, Menk J, Chipman JG. Pre- and Peri-Operative Factors Associated with Chronic Critical Illness in Liver Transplant Recipients. Surg Infections. 2020;21(3):246–54.
Raveh Y, Livingstone J, Mahan J, et al. Comprehensive Frailty Severity Index for End-Stage Liver Disease Predicts Early Outcomes After Liver Transplantation. JPEN J Parenter Enter Nutr. 2020;44(6):1079–88.
Haugen CE, McAdams-DeMarco M, Verna EC, et al. Association Between Liver Transplant Wait-list Mortality and Frailty Based on Body Mass Index. JAMA Surg. 2019;154(12):1103–9.
Dolgin NH, Martins PNA, Movahedi B, Lapane KL, Anderson FA, Bozorgzadeh A. Functional status predicts postoperative mortality after liver transplantation. Clin Transplantation. 2016;30(11):1403–10.
Dunn MA, Josbeno DA, Tevar AD, et al. Frailty as Tested by Gait Speed is an Independent Risk Factor for Cirrhosis Complications that Require Hospitalization. Am J Gastroenterol. 2016;111(12):1768–75.
Sinclair M, Poltavskiy E, Dodge JL, Lai JC. Frailty is independently associated with increased hospitalisation days in patients on the liver transplant waitlist. World J Gastroenterol. 2017;23(5):899–905.
Lai JC, Covinsky KE, Dodge JL, et al. Development of a novel frailty index to predict mortality in patients with end-stage liver disease. Hepatology. 2017;66(2):564–74.
Tapper EB, Konerman M, Murphy S, Sonnenday CJ. Hepatic encephalopathy impacts the predictive value of the Fried Frailty Index. Am J Transplantation. 2018;18(10):2566–70.
Bhanji RA, Narayanan P, Moynagh MR, et al. Differing Impact of Sarcopenia and Frailty in Nonalcoholic Steatohepatitis and Alcoholic Liver Disease. Liver Transplantation. 2019;25(1):14–24.
Dolgin NH, Smith AJ, Harrington SG, Movahedi B, Martins PNA, Bozorgzadeh A. Association between sarcopenia and functional status in liver transplant patients. Experimental and Clinical Transplantation. 2019;17(5):653–64.
Fozouni L, Lebsack A, Mohamad Y, Freise C, Stock P, Lai J. Frailty associated with increased rates of acute cellular rejection within 3 months after liver transplantation. American Journal of Transplantation. 2019;19((Fozouni L.; Lebsack A.; Mohamad Y.; Freise C.; Stock P.; Lai J.) UCSF, San Francisco, CA, United States):334.
Derck JE, Thelen AE, Cron DC, et al. Quality of life in liver transplant candidates: Frailty is a better indicator than severity of liver disease. Transplantation. 2015;99(2):340–4.
Cron DC, Friedman JF, Winder GS, et al. Depression and Frailty in Patients With End-Stage Liver Disease Referred for Transplant Evaluation. Am J Transplantation. 2016;16(6):1805–11.
Kulkarni SS, Chen H, Josbeno DA, et al. Gait Speed and Grip Strength Are Associated With Dropping Out of the Liver Transplant Waiting List. Transplantation Proceedings. 2019;51(3):794–797.
Lai JC, Rahimi RS, Verna EC, et al. Frailty Associated With Waitlist Mortality Independent of Ascites and Hepatic Encephalopathy in a Multicenter Study. Gastroenterology. 2019;156(6):1675–82.
Wang CW, Lebsack A, Chau S, Lai JC. The Range and Reproducibility of the Liver Frailty Index. Liver Transplantation. 2019;25(6):841–7.
DeMaria S, Khromava M, Schiano TD, Lin HM, Kim S. Standardized measures of frailty predict hospital length of stay following orthotopic liver transplantation for hepatocellular carcinoma. Clinical Transplantation. 2019;33(12).
Kardashian A, Ge J, McCulloch CE, et al. Identifying an Optimal Liver Frailty Index Cutoff to Predict Waitlist Mortality in Liver Transplant Candidates. Hepatology. 2020;73(3):1132–9.
Lai JC, Covinsky KE, McCulloch CE, Feng S. The liver frailty index improves mortality prediction of the subjective clinician assessment in patients with cirrhosis. Am J Gastroenterol. 2018;113(2):235–42.
Puchades L, Chau S, Dodson JA, et al. Association of Cardiac Abnormalities to the Frail Phenotype in Cirrhotic Patients on the Waitlist: From the Functional Assessment in Liver Transplantation Study. Transplantation. 2018;102(3):e101–7.
Lai JC, Segev DL, McCulloch CE, Covinsky KE, Dodge JL, Feng S. Physical frailty after liver transplantation. Am J Transplantation. 2018;18(8):1986–94.
Salim TI, Nestlerode LC, Lucatorto EL, et al. Frailty as Tested by Gait Speed Is a Risk Factor for Liver Transplant Respiratory Complications. Am J Gastroenterol. 2020;115(6):859–66.
Lai JC, Dodge JL, Kappus MR, et al. Changes in frailty are associated with waitlist mortality in patients with cirrhosis. J Hepatol. 2020;73(3):575–81.
Bittermann T, Dwinnells K, Chadha S, Wolf MS, Olthoff KM, Serper M. Low Health Literacy Is Associated With Frailty and Reduced Likelihood of Liver Transplant Listing: A Prospective Cohort Study. Liver Transplantation. 2020;26(11):1409–21.
Lai JC, Feng S, Terrault NA, Lizaola B, Hayssen H, Covinsky K. Frailty predicts waitlist mortality in liver transplant candidates. Am J Transplantation. 2014;14(8):1870–9.
Lai JC, Ganger DR, Volk ML, et al. Association of Frailty and Sex with Wait List Mortality in Liver Transplant Candidates in the Multicenter Functional Assessment in Liver Transplantation (FrAILT) Study. JAMA Surg. 2021;156(3):256–62.
Xu CQ, Yao F, Mohamad Y, et al. Evaluating the Associations Between the Liver Frailty Index and Karnofsky Performance Status With Waitlist Mortality. Transpl Direct. 2021;7(2):e651.
Johnston HE, de Crom T, Hargrave C, et al. The inter- and intrarater reliability and feasibility of dietetic assessment of sarcopenia and frailty in potential liver transplant recipients: A mixed-methods study. Clin Transpl. 2021;35(2):e14185.
Kuo SZ, Lizaola B, Hayssen H, Lai JC. Beta-blockers and physical frailty in patients with end-stage liver disease. World J Gastroenterol. 2018;24(33):3770–5.
Klein CG, Malamutmann E, Latuske J, et al. Frailty as a predictive factor for survival after liver transplantation, especially for patients with MELD ≤ 15—a prospective study. Langenbeck’s Archives of Surgery. 2021;406(6):1963–9.
Lin FP, Visina JM, Bloomer PM, et al. Prehabilitation-Driven Changes in Frailty Metrics Predict Mortality in Patients With Advanced Liver Disease. Am J Gastroenterol. 2021;116(10):2105–17.
Xu CQ, Mohamad Y, Kappus MR, et al. The relationship between frailty and cirrhosis etiology: From the Functional Assessment in Liver Transplantation (FrAILT) Study. Liver Int. 2021;41(10):2467–73.
Deng LX, Bischoff KE, Kent DS, O’Riordan DL, Pantilat SZ, Lai JC. Frailty is strongly associated with self-reported symptom burden among patients with cirrhosis. Eur J Gastroenterol Hepatol. 2021;33(1S Suppl 1):e395–400.
Berry K, Duarte-Rojo A, Grab JD, et al. Cognitive Impairment and Physical Frailty in Patients With Cirrhosis. Hepatol Commun. 2022;6(1):237–46.
Cullaro G, Verna EC, Duarte-Rojo A, et al. Frailty and the Risk of Acute Kidney Injury Among Patients With Cirrhosis. Hepatol Commun. 2022;6(4):910–9.
Jha SR, Hannu MK, Gore K, et al. Cognitive impairment improves the predictive validity of physical frailty for mortality in patients with advanced heart failure referred for heart transplantation. J Heart Lung Transplantation. 2016;35(9):1092–100.
Jha SR, Hannu MK, Chang S, et al. The Prevalence and Prognostic Significance of Frailty in Patients with Advanced Heart Failure Referred for Heart Transplantation. Transplantation. 2016;100(2):429–36.
Cacciatore F, Amarelli C, Maiello C, et al. Sacubitril/valsartan in patients listed for heart transplantation: effect on physical frailty. ESC Heart Failure. 2020;7(2):757–62.
Seese L, Hirji S, Sultan I, Gleason T, Kilic A. Frailty Screening Tool for Patients Undergoing Orthotopic Heart Transplant. Annals of Thoracic Surgery. 2021;111(2):586–93.
Macdonald PS, Gorrie N, Brennan X, et al. The impact of frailty on mortality after heart transplantation. J Heart Lung Transplantation. 2021;40(2):87–94.
Jha SR, Hannu MK, Newton PJ, et al. Reversibility of Frailty After Bridge-to-Transplant Ventricular Assist Device Implantation or Heart Transplantation. Transpl Direct. 2017;3(7):e167.
Ayesta A, Valero Masa MJ, Vidán MT, et al. Prevalence and characterization of frailty, depression, and cognitive impairment in patients listed for heart transplantation: Results of the FELICITAR prospective registry. Clinical Transplantation. 2021;35(9).
Aili SR, De Silva R, Wilhelm K, et al. Validation of Cognitive Impairment in Combination with Physical Frailty as a Predictor of Mortality in Patients with Advanced Heart Failure Referred for Heart Transplantation. Transplantation. 2022;106(1):200–9.
Lee YK, Shukman M, Biniwale R, et al. Benefits of both physical assessment and electronic health record review to assess frailty prior to heart transplant. Clinical Transplantation. 2022;36(3).
Montgomery E, Macdonald PS, Newton PJ, et al. Reversibility of Frailty after Lung Transplantation. Journal of Transplantation. 2020:1–10.
Baldwin MR, Singer JP, Huang D, et al. Refining Low Physical Activity Measurement Improves Frailty Assessment in Advanced Lung Disease and Survivors of Critical Illness. Annals of the American Thoracic Society. 2017;14(8):1270–9.
Singer JP, Diamond JM, Gries CJ, et al. Frailty Phenotypes, Disability, and Outcomes in Adult Candidates for Lung Transplantation. Am J Respiratory Crit Care Med. 2015;192(11):1325–34.
Layton AM, Armstrong HF, Baldwin MR, et al. Frailty and maximal exercise capacity in adult lung transplant candidates. Respiratory Med. 2017;131:70–6.
Wilson ME, Vakil AP, Kandel P, Undavalli C, Dunlay SM, Kennedy CC. Pretransplant frailty is associated with decreased survival after lung transplantation. J Heart Lung Transplantation. 2016;35(2):173–8.
Stanjek-Cichoracka A, Woźniak-Grygiel E, Łaszewska A, Zembala M, Ochman M. Assessment of Cytokines, Biochemical Markers of Malnutrition and Frailty Syndrome Patients Considered for Lung Transplantation. Transplantation Proceedings. 2019;51(6):2009–2013.
Venado A, McCulloch C, Greenland JR, et al. Frailty trajectories in adult lung transplantation: A cohort study. J Heart Lung Transplantation. 2019;38(7):699–707.
Courtwright AM, Zaleski D, Tevald M, et al. Discharge frailty following lung transplantation. Clinical Transplantation. 2019;33(10).
Anderson MR, Kolaitis NA, Gao Y, et al. A nonlinear relationship between visceral adipose tissue and frailty in adult lung transplant candidates. Am J Transplantation. 2019;19(11):3155–61.
Courtwright AM, Zaleski D, Gardo L, et al. Causes, Preventability, and Cost of Unplanned Rehospitalizations Within 30 Days of Discharge after Lung Transplantation. Transplantation. 2018;102(5):838–44.
Singer JP, Soong A, Bruun A, et al. A mobile health technology enabled home-based intervention to treat frailty in adult lung transplant candidates: A pilot study. Clinical Transplantation. 2018;32(6).
Singer JP, Diamond JM, Anderson MR, et al. Frailty phenotypes and mortality after lung transplantation: A prospective cohort study. Am J Transplantation. 2018;18(8):1995–2004.
Montgomery E, MacDonald PS, Newton PJ, et al. Frailty as a Predictor of Mortality in Patients with Interstitial Lung Disease Referred for Lung Transplantation. Transplantation. 2020;104(4):864–72.
Perez AA, Hays SR, Soong A, et al. Improvements in frailty contribute to substantial improvements in quality of life after lung transplantation in patients with cystic fibrosis. Pediatr Pulmonol. 2020;55(6):1406–13.
Rozenberg D, Mathur S, Wickerson L, Chowdhury NA, Singer LG. Frailty and clinical benefits with lung transplantation. J Heart Lung Transplantation. 2018;37(10):1245–53.
Montgomery E, Newton PJ, Chang S, et al. Frailty Measures in Patients Listed for Lung Transplantation. Transplantation. 2022;106(5):1084–92.
Guaraldi G, Dolci G, Zona S, et al. A frailty index predicts post-liver transplant morbidity and mortality in HIV-positive patients. AIDS Res Therapy. 2017;14:1–8.
Shamseddeen H, Pike F, Ghabril M, et al. Karnofsky performance status predicts outcomes in candidates for simultaneous liver-kidney transplant. Clin Transplantation. 2020;35(2):e14190.
Wickerson L, Rozenberg D, Gottesman C, Helm D, Mathur S, Singer LG. Pre-transplant short physical performance battery: Response to pre-habilitation and relationship to pre- and early post–lung-transplant outcomes. Clinical Transplantation. 2020;34(12).
Varughese RA, Theou O, Li Y, et al. Cumulative Deficits Frailty Index Predicts Outcomes for Solid Organ Transplant Candidates. Transpl Direct. 2021;7(3):e677.
King SJ, Raine KA, Peel NM, Hubbard RE. Interventions for frail older inpatients: A systematic review of frailty measures and reported outcomes in randomised controlled trials. Australasian J Ageing. 2021;40(2):129–44.
Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–56.
Fisher AL. Just what defines frailty? J Am Geriatr Soc. 2005;53(12):2229–30.
Rockwood K, Mitnitski A. Frailty defined by deficit accumulation and geriatric medicine defined by frailty. Clin Geriatr Med. 2011;27(1):17–26.
Wleklik M, Uchmanowicz I, Jankowska EA, et al. Multidimensional Approach to Frailty. Front Psychol. 2020;11:564–4.
Mitnitski AB, Song X, Rockwood K. The estimation of relative fitness and frailty in community-dwelling older adults using self-report data. J Gerontol A Biol Sci Med Sci. 2004;59(6):M627–32.
Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. Cmaj. 2005;173(5):489–95.
O’Caoimh R, Sezgin D, O’Donovan MR, et al. Prevalence of frailty in 62 countries across the world: a systematic review and meta-analysis of population-level studies. Age Ageing. 2021;50(1):96–104.
Theou O, Brothers TD, Mitnitski A, Rockwood K. Operationalization of frailty using eight commonly used scales and comparison of their ability to predict all-cause mortality. J Am Geriatr Soc. 2013;61(9):1537–51.
Pilotto A, Rengo F, Marchionni N, et al. Comparing the prognostic accuracy for all-cause mortality of frailty instruments: a multicentre 1-year follow-up in hospitalized older patients. PLoS One. 2012;7(1):e29090.
Quint E, Zogaj D, Banning W, et al. Frailty and kidney transplantation: A systematic review and meta-analysis. Transpl Int. 2021;34(SUPPL 1):114.
Yang X, Lupón J, Vidán MT, et al. Impact of Frailty on Mortality and Hospitalization in Chronic Heart Failure: A Systematic Review and Meta-Analysis. J Am Heart Association. 2018;7(23):e008251.
Singer JP, Katz PP, Dean MY, et al. Frailty is common in lung transplant candidates and associated with poorer health-related quality of life. J Heart Lung Transplantation. 2013;32(4):43.
Montgomery E, Macdonald PS, Newton PJ, Jha SR, Malouf M. Frailty in lung transplantation: a systematic review. Expert Rev Respiratory Med. 2020;14(2):219–27.
Kahn J, Wagner D, Homfeld N, Müller H, Kniepeiss D, Schemmer P. Both sarcopenia and frailty determine suitability of patients for liver transplantation—A systematic review and meta-analysis of the literature. Clin Transplantation. 2018;32(4):e13226.
Guaraldi G, Brothers TD, Zona S, et al. A frailty index predicts survival and incident multimorbidity independent of markers of HIV disease severity. Aids. 2015;29(13):1633–41.
Acknowledgements
There are no personal acknowledgments to report.
Funding
There are no funding sources to declare.
Author information
Authors and Affiliations
Contributions
JK, NR, EG designed study methodology including the data extraction template. Article screening and full text review completed by JK and SK, with assistance from NR and EG. Data extraction was completed by JK, NR, EG, RH, RH, LH, EP, BL, SK, SF. Manuscript write up including data analysis and preparation of tables and figures was completed by JK with major contribution from EG and NR. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors of this manuscript have no conflicts of interest to disclose as described by BMC Geriatrics.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Kao, J., Reid, N., Hubbard, R.E. et al. Frailty and solid-organ transplant candidates: a scoping review. BMC Geriatr 22, 864 (2022). https://doi.org/10.1186/s12877-022-03485-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12877-022-03485-7