Abstract
Background
In Switzerland, there is a lack of adequate rehabilitation services, and effective coordination, that take into account the multifactorial health risks of older people. The literature shows that the hospitalisation rate in rehabilitation facilities has increased in recent years and that a gender bias exists. Additionally, there is little or no evidence available on the effect that a post-acute care programme might have over an extended period on functioning, quality of life and the informal network of older people. Therefore, the aim of this trial is to evaluate the sustainability of post-acute care within three nursing homes in Zurich, Canton of Zurich, Switzerland.
Methods
The Prevention Admission into Nursing homes (PAN) study is a explorative, prospective, longitudinal pilot trial based on a convenience sample of three long-term care facilities in the Swiss Canton of Zurich. The proposed pilot study will examine the effects of a post-acute care programme on people aged ≥65 years with a post-acute care potential ≥ three admitted to any of the three post-acute care units (n = 260). Older people of all sexes admitted to one of the post-acute care units and likely to be discharged to home within 8 weeks will be eligible for participation in the study. The primary endpoint is functionality based on the Barthel Index. The secondary endpoints are independency based on delirium, cognition, mobility, falling concerns, frailty, weight/height/body mass index, post-acute care capability, quality of life, and lastly, the informal network. As part of process evaluation, a qualitative evaluation will be conducted based on constructive grounded theory to specifically analyse how the experience of informal caregivers (n = 30) can contribute to a successful daily life 6 months after discharge.
Discussion
We expect to observe improved functional status and independence after the post-acute care programme. The qualitative evaluation conducted with caregivers will complement our description of the transition of older people towards living at home.
Trial registration
This study is registered in the German Clinical Trials Register under DRKS00016647 (registered on 23.05.2019).
Similar content being viewed by others
Background
The World Health Organization recommends strengthening the availability of rehabilitation services in health systems [1]. It is generally accepted that rehabilitation should improve the functions and adaptability of those affected through the coordinated use of medical, social, occupational, technical and pedagogical interventions [2, 3].
Various terms for rehabilitation can be found in the literature: post-acute care (PAC), rehabilitative transitional care, geriatric rehabilitation, slow stream rehabilitation and acute transitional care. This study focuses on PAC. PAC takes into account that low-intensity therapies can be used to individually prepare older people for their return home, specifically those who are unable to return home immediately after hospital discharge [4]. Therefore, PAC can occur in many settings, such as inpatient rehabilitation facilities, long-term care facilities or home care [5]. Since the introduction of Diagnosis Related Groups, it has been observed that acute hospitals are increasingly referring older people who need follow-up treatment to rehabilitation facilities, in addition to homes and outpatient facilities [6]. Conducting a comprehensive geriatric assessment is standard practice in PAC facilities for determining a treatment plan, which increases the probability that people will not be institutionalised [7,8,9]. Those directly affected want their care to focus on a well-functioning social network and improve their mobility [10]. However, the extent to which PAC positively affects patient outcomes over an extended period has not been extensively investigated [8].
Although improved functional status does not guarantee a return home, it is a central outcome of rehabilitation and subsequent treatments [11]. A decline in functional capacity can lead to undesirable events, such as hospitalisation. Monitoring functional status can help detect changes in the health status of chronically ill people at an early stage [12], and the functional status of older people is an essential indicator for rehospitalisation [13,14,15,16]. It has also been shown that older people with reduced functional status and cognitive abilities often need long-term care [15, 17,18,19]. Hence, maintaining functional status is a central influencing factor of the ability to live independently at home or with the assistance of a home-care organisation [12, 16, 20, 21]. Functionality in rehabilitation care is predominantly measured using the Barthel Index (BI). However, although the BI is widely used in rehabilitation care, it is not described within the PAC context [22].
PAC is becoming increasingly important in international health care settings [23,24,25]. PAC and rehabilitation services for older people after hospital stays in Switzerland were therefore implemented in 2011 and are governed by cantonal policies [26]. Some cantons authorise nursing homes to deliver PAC, while other cantons opt for PAC delivery by hospitals or home-care providers [27]. The Canton of Zurich has opted to provide PAC in nursing homes and does so successfully [22]. However, equitable access and rehospitalisation issues remain [28]. Moreover, unlike in rehabilitation clinics and hospitals, quality improvement and control for PAC in nursing homes is not centralised [29].
The hospitalisation rate in rehabilitation facilities in Switzerland has increased in recent years and currently stands at 10.1 per 1000 inhabitants [30]. In 2017, the most frequent reasons for admission of older people to rehabilitation facilities were injuries, musculoskeletal diseases and cardiovascular diseases [31]. Older people referred to inpatient rehabilitation have more illnesses that require more intensive rehabilitation [32, 33], while the average length of stay has decreased in recent years [30]. Older people undergoing rehabilitation have a primary diagnosis linked with an average of six secondary diagnoses [31]. This indicates a high co-morbidity rate among older Swiss people in rehabilitation.
Furthermore, in Switzerland, there is a lack of adequate rehabilitation services, and effective coordination, that take into account the multifactorial health risks of older people [34, 35]. Problems associated with the discharge of older people from Swiss hospitals may be related to the study results of Koné and colleagues [34]. They found that discharge planning in Swiss hospitals is not standardised. Also, hospitals refer women less frequently to rehabilitation institutions than men [34]. It is unclear why women are less often rehabilitated than men of the same age with similar conditions.
In summary, based on this multifaceted background, there is a lack of evidence as to a) to what extent a PAC programme positively affects patient outcomes, specifically the functional status, and b) how sustainable these effects are over an extended period. This knowledge is pivotal in informing future care pathways for this co-morbid population and enhancing adequate rehabilitation services with an effective PAC programme.
Aims and hypothesis
The aim of this study is to evaluate the sustainability of PAC using functionality as the primary endpoint, measured using the BI. We hypothesise that the PAC programme improves a patient’s functional status (measured using the BI) to a level that allows them to return home after completing PAC in the nursing home and maintain a moderate increase in BI for at least 6 months after discharge (odds ratio = 1.5). Based on the study’s exploratory nature, we assume that moderate improvements are associated with a substantial gain in independence.
Methods
The SPICE framework is used to describe the study components [36]. The version adapted for this study contains the following elements: S = study design, setting, sample size, P = participants, I = intervention/issue of interest, E = Endpoints, including their specific analyses. Comparison (C) has been omitted, as this is not a clinical trial.
Study design and setting
The Prevention Admission into Nursing homes (PAN) study is a three-site, multicentre, exploratory, prospective, longitudinal pilot study in Swiss nursing homes without a control group. All centres (n = 3) are located in Zurich and the study will examine older people (n = 260) enrolled in PAC treatment. Additionally, a qualitative process evaluation will assess relatives’ perspectives (n = 30).
This study will be led by Zurich University of Applied Science’s health department and the department of Nursing Homes of the City of Zurich (PZZ) and will last approximately 24 months.
Sample size
The sample size calculation was based on the primary endpoint, the BI, for which we model the cumulative probability that the i-th BI Yit will fall into the j-th category or below:
i corresponds to the index for observations at time t, j represents the index for categories, {θ} is the cut-points, δ1 corresponds to the change in BI between PAC entry (baseline) and PAC exit, and δ2 is the change in BI between PAC entry and follow-up measurement at home after 6 months. Patient effects (random effects) are designed as independent and identically normally distributed:
Of vital importance for the evaluation of the sustainability of PAC is parameter δ2, for which the following hypothesis will be tested:
The expected improvement in BI under the alternative hypothesis is >0.4 logits, which corresponds to an odds ratio (OR) of about 1.5. The required sample size to detect an effect under the alternative hypothesis was determined by simulation. We assumed a significance level of α = 0.05 (two-sided test) and a target power of 0.8 (β = 0.2). The obtained power is based on 500 iterative analogue model estimates and was performed for different levels of patient-specific autocorrelation (intra-class correlation [ICC] = 0.1, 0.2, 0.4). As is usual with mixed-effects models, the sample size decreases with increasing ICC (see Fig. 1).
Since no empirical values are available for the ICC from similar studies with comparable endpoints, we assumed a relatively conservative ICC of 0.2 to determine the sample size. Our assumption resulted in a calculation of 200 older people being needed to achieve the targeted power. Taking into account the dropout rate, which we estimate to be 30% due to the advanced age of the study population, the study requires a sample size of n = 260.
The coloured curves show three different estimates of obtained power based on our simulations. Each curve assumed another level of patient-specific autocorrelation. Our assumed target power of 0.8 is highlighted with a horizontal line.
Participants
The study population will consist of older people enrolled in a PAC treatment programme and their informal caregiver network.
-
1.
Older people
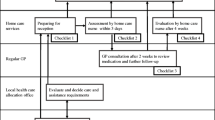
The following inclusion criteria will be applied to the older people: must be ≥65 years old; admitted to one of the nursing homes and participating in the running PAC programme; assessed with a PAC potential ≥3 (see Fig. 2) and conditions impairing physical functioning; able to communicate in German; and must have provided informed consent.
-
2.
Informal caregiver network
The following inclusion criteria will be applied to the informal caregiver network (i.e., primary relatives in charge or trusted persons): must have the capacity and the capability to provide informed consent; be able to communicate in German; and be of legal age.
For a description of the recruitment, refer to Fig. 2. The department of Nursing Homes of the City of Zurich (PZZ), namely the third author, will be in charge and administrate the study participants’ enrolment.
Intervention
Typically, patients are selected for geriatric rehabilitation during their acute hospital stay (i.e., usual rehabilitation care). PAC provides a differentiated geriatric assessment and estimates the PAC potential for those affected by musculoskeletal diseases, injuries and circulatory diseases. PAC provides rehabilitation for older people who leave an acute hospital and are not sufficiently independent to resume living at home or can no longer cope at home. The aim is to help them reach the point at which they can reintegrate into their homes. Interprofessional teams conduct PAC programmes in nursing homes (see Fig. 2). Unfortunately, no uniform international definition of PAC exists [5]. Wang and colleagues (2019) proposed a clinical perspective with five goals: 1) the potential to live independently, 2) a comprehensive assessment, 3) enhancing patients independent living, 4) program duration 6 weeks, and 5) individual assessment mechanisms [5]. PAC treatment and plans with individual goals are developed according to the comprehensive assessment of cognition, nutritional status, physical function and mobility and basic activities of daily living [22]. Nursing, medical and therapeutic interventions and treatments support those affected in regaining the greatest possible independence. PAC gives older people more time than in a traditional rehabilitation setting to take on as much as possible by themselves and thus strengthen their self-care abilities.
Treatments with a rehabilitative approach are characterised by close interprofessional cooperation focusing on functional activities, education and psycho-social support [37]. Physicians are primarily responsible for PAC assessment, drug treatment, follow-up and evaluation. Therapeutic specialists, such as physiotherapists and occupational and speech therapists, are tasked with improving functioning, and nurses transfer and integrate (re)gained functions into the daily lives of older people. For example, the handgrip strength regained through targeted physiotherapy is transferred to everyday life through targeted nursing interventions. Patients are given sufficient time and individual support to select, prepare and eat their bread rolls for breakfast independently instead of simply feeding them. Overall, providing advice, particularly on mobility and self-care, to patients and their caregivers is an essential nursing intervention in PAC [37]. Social service providers carefully plan the return home and subsequent outpatient care. Furthermore, they initiate necessary measures in collaboration with the primary relatives.
A PAC programme lasts an average of 8 weeks and a maximum of 10 weeks based on the PAC potential assessment. If, after this time span, a return home is not possible, even with appropriate support, suitable alternatives are explored together with the affected person. In cases where a return home is likely after an extended stay, the older person is transferred to a specialised nursing ward to attain adequate independence and functioning.
Endpoints
The following outcomes will guide the evaluation of the PAC programme.
-
1.
Functional status as the primary endpoint
Functional status will be measured with the BI [38] at PAC entry (T0), PAC exit (T1) and 6 months after PAC discharge to the home environment (T3). The BI is a ten-item scale with either 0, 5, 10 or 15 points allocated for each item (maximum total score of 100 points = maximum functional status). The BI is a validated and common instrument used to assess functionality in rehabilitation care [38, 39]. For use with older and multi-diagnosed people, there might be imprecision when testing takes place by different observers [40]. To the best of our knowledge, little is known about the sensitivity to changes in the BI [41]. A clinically meaningful change is to be determined by the patients themselves. Recommended BI cut-off points to determine overall independence are problematic. Overall, a five-point difference can have completely different meanings for individual daily life independence. Collin and colleagues [39, 41] recommended that a 20-point change be considered as clinically significant. For the individual use of BI, other endpoints must also be considered.
All items will be collected through direct observation by study personnel or interviews with those affected, their relatives or the nursing staff.
-
2.
Independence as the secondary endpoint
Independence will be measured via the informal network and the following nine criteria: delirium, cognition, mobility, falling concerns, frailty, body mass index (BMI), PAC capability and quality of life. The details of each criterion are as follows:
-
Delirium: The assessment of delirium will be performed with the Delirium Observational Scale (DOS) at PAC entry (T0) [42]. The instrument has shown a high internal consistency with a reported Cronbach’s alpha = 0.96 and is considered valid and reliable for the early detection of delirium in older people [37, 38]. Delirium per shift will be evaluated using 13 items and four levels (never, sometimes, always or do not know). The occurrence of more than three criteria indicates a positive delirium screening and will trigger additional assessment.
-
Cognition: The degree of cognitive performance will be measured using the Mini-Mental State Examination (MMSE) at admission (T0) and at home (T3) [43]. The reliability of the MMSE has been reported with a reported Cronbach’s alpha = 0.91 [44]. The sum of the MMSE spans from zero to 30 points, with 30 being the optimal result. Cognitive impairment is diagnosed at an MMSE sum of less than 24 points [43, 45, 46].
-
Mobility: Mobility will be measured using the Short Physical Performance Battery (SPPB) at PAC entry and 6 months after release from the PAC to the home environment (T0, T3) [47]. SPPB has proven reliable with a Cronbach’s alpha = 0.87 [48]. The SPPB includes balance, a four-metre walking test and the stand-up test. A score of 10–12 points represents normal mobility, a score of 7–9 points indicates an increased risk of gait disorder and a score of less than 6 points indicates a very high risk of increasing gait disorder and loss of independence [47].
-
Falling concerns: Concerns regarding falls will be measured with the German version of the Falls Efficacy Scale-International (FES-I) at PAC entry and 6 months after release from the PAC to the home environment (T0, T3). The German version of the FES-I has a high internal consistency (Cronbach’s α = 0.96) and retest-reliability (r = 0.96) [49]. The FES-I questionnaire consists of 16 items in which concerns about different activities and cases are raised. The answers are graded as follows: one = no concerns; two = some concerns; three = moderate concerns; and four = very great concerns. A minimum score of 16 indicates no concerns about falling, and a maximum score of 64 indicates severe concern about falling [49, 50].
-
Frailty: Frailty will be assessed using the frailty screening questionnaire [51, 52] at PAC entry and 6 months after release from PAC to the home environment (T0, T3). The questionnaire has been shown to be a valid and reliable instrument with a test-retest reliability total score of r = 0.937 [53]. The frailty screening questionnaire examines five factors (weight loss, fatigue, walking speed, physical weakness and activity level). The assessment is “met” or “not met”, with zero factors met indicating robustness, one to two factors met indicating a pre-frail status and less than three factors met indicating frailty [51, 52].
-
Weight/height/BMI: Weight, height and BMI measures will be used as co-variates of frailty [8, 54]. All three elements will be measured at entry and 6 months after release from PAC to the home environment (T0, T3) [8].
-
PAC capability: PAC capability assessment will be conducted using the Resident Assessment Instrument for Nursing Homes (RAI_NH) to determine the degree of care dependency at PAC entry (T0) and at PAC discharge (T1). The “Minimum Data Set” category of the Swiss RAI_NH is a needs assessment tool [55]. The RAI_NH care dependency score ranges from tier 1 (lowest) to 12 (highest) [55]. All items will be collected through direct observation or interviews with those affected, their relatives or the nursing staff.
-
Quality of life: Quality of life will be measured as a subjective screening of health status on a numeric rating scale ranging from “excellent (10)” to “poor (0)” as an influence on functionality. It will be measured at entry, discharge and 6 months after release from the PAC to the home environment (T0, T1 and T3).
-
Informal network: The frequency of use of formal and informal caregiver networks to secure the home environment will be measured by interviewing the primary relative in charge. The frequency of informal network use will be measured 6 months after discharge from PAC to the home environment (T3).
Data from the study participants will be routinely collected four times (see Table 1). Socio-demographic data of the participants will also be collected, particularly to analyse sex-specific aspects.
Ninety days after PAC discharge, telephone contact will be established with the older person to ask whether they want to continue participating in the study and to verify their address.
As part of the process evaluation, the primary relatives (n = 30) will be individually interviewed 6 months after PAC discharge using constructive grounded theory to identify impacts of the PAC programme on their quality of life during their daily routines [56]. These interviews will be conducted by trained PZZ staff members not involved in the PAC programme.
The timepoints are labelled T0 to T3. The plus symbol (“+”) indicates the timepoints at which each instrument will be applied. At T2, no outcomes will be evaluated, but the participants’ residence and continued participation will be verified.
Statistical analysis
Data analysis will be performed using R-Project software version 3.5.2. The socio-demographic data will be analysed descriptively (frequencies, central tendencies, standard deviations). For the primary endpoint, the BI, we will estimate unadjusted cumulative odds using the basic model presented in the sample size section to detect BI changes between baseline and follow-up measures. The model will further be adjusted for age, sex, and secondary endpoints mobility and frailty, which have been identified as driving factors leading to institutionalisation in a nursing home. Both unadjusted and adjusted OR with corresponding 95% confidence intervals will be reported. Similarly, unadjusted and adjusted generalised linear mixed-effects models of the appropriate family and link function will be used to estimate the parameters of the secondary endpoints. Statistical significance will be established at p < 0.05.
Analysis of interviews
Process evaluation analysis of the informal caregiver networks will apply constructive grounded theory [56], run through the Atlas.ti® software Version 8.4.4. Within constructive grounded theory, data analysis and data collection take place simultaneously, which is why data analysis will begin with the first interviews. Sample size depends on theoretical saturation. This leading criterion for the grounded theory method can vary [56]. In this case, we assume that we will interview up to 30 relatives. Using comparative strategies (so-called “constant comparisons”), text passages, events, strategies and persons will be compared to identify content similarities and differences. Further analysis will be conducted by employing two analytical steps: initial and focused coding. A data segment will be coded row by row during initial coding to develop categories. In the next step (focused coding), the properties and connections of the codes will be developed so that an explanatory model of daily routines after the PAC programme is created in an inductive process. The process will be documented using memos to ensure the credibility and quality of the analysis [56].
Discussion
The complexity of older people’s treatment requirements and the lack of information about the sustainability of PAC in Switzerland highlight the urgent need for an evaluation of PAC. Current data do not permit any further differentiation of geriatric rehabilitation programmes and, more specifically, of PAC treatment programmes for people with injuries, musculoskeletal diseases and circulatory diseases [31,32,33]. Older people are often multimorbid. In Swiss university hospitals, 79% of patients (median age: 68 years old) are multimorbid and have seven types of illnesses (median), four of which are chronic (median): chronic heart disease, chronic kidney disease, solid malignancy and substance-related disorders. Each of these four types of illness comprises two to eight comorbidities. The most common are neurological diseases, heart/kidney diseases, malignancy, miscellaneous diseases and psychiatric diseases [57]. Therefore, this study aims to evaluate functional status using the BI as the primary endpoint. In addition, quality of life and the informal network (relatives) will be assessed as secondary endpoints.
Nevertheless, there are some points to discuss. First, a programme of 6 weeks for multimorbid patients is often too short to gain independence and depends on the severity of the impairments. Second, improved functions are not always clinically significant. There may be a difference between performing in a laboratory setting and coping with daily life. An effective program always takes place in interaction with the environment [58]. Third, for people with neurological diseases, for example, dealing emotionally with role changes due to physical limitations or reduced social participation is essential for living as independently as possible at home [59, 60].
PAC programmes include repeated assessments, individual treatment and therapy plans (e.g. physiotherapy, occupational therapy and nursing). The therapies take place individually and in groups. In assessment interviews, older people and their family members are informed about their status, treatment progress and plan. Discharge planning is also important. Older people in PAC receive information and documents on continuing care, medications, therapies and aids as needed. Their general practitioners are also informed so that further medical care is ensured.
If the results of this study show that PAC programmes delivered in nursing homes improve the participants’ functional status to the point that they can return home, then the tested programmes could be considered an effective way to delay the elderly from permanently residing in a nursing home. Furthermore, having evidence of a successful PAC programme will offer specialised nursing homes the opportunity to differentiate their services and transform their image from the “last station in life”. Having access to a comprehensive geriatric assessment tool will also enable PAC staff to develop treatment plans, which decreases the probability that older people will be institutionalised [7, 9].
According to the British Medical Research Framework for complex interventions [61], this study is categorised as a pilot study to determine whether the PAC programme can improve older people’s functional status, as measured using the BI. Moreover, the repeated measures study design allows the assessment of functionality over several months and ensures that the intervention’s short-term and long-term effects can be adequately captured and differentiated [62].
In sum, we will determine the sustainability of the PAC programme from the perspectives of the elderly and their relatives over 6 months. We expect that the PAC programme will improve the functional status of older people and reduce the burden on their relatives.
Availability of data and materials
The datasets produced as part of the study are available on request from the corresponding author.
Abbreviations
- BI:
-
Barthel Index
- BMI:
-
Body Mass Index
- DOS:
-
Delirium Observation Scale
- FES-I:
-
Falls Efficacy Scale-International
- MMSE:
-
Mini-Mental State Examination
- PAN:
-
Prevention Admission into Nursing homes
- PAC:
-
Post-acute Care
- PI:
-
Principal Investigator
- SPPB:
-
Short Physical Performance Battery
- RAI_NH:
-
Resident Assessment Instrument for Nursing Homes
References
World Health Organization, Violence and Injury Prevention. Rehabilitation in health systems. 2017 [cited 2020 Oct 24]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK552492/
World Health Organisation W. Concept paper: WHO Guidelines on Health-Related Rehabilitation (Rehabilitation Guidelines) [Internet]. 2005 [cited 2020 Oct 24]. Available from: https://www.who.int/disabilities/care/rehabilitation_guidelines_concept.pdf
Kieser U. Rehabilitation - eine Bilanz der bundesgerichtlichen Rechtsprechung zum Krankenversicherungsrecht. In: Oggier W, editor. 20 anni LAMal: retrospettive e prospettive per la riabilitazione : 20 jahre KVG: Rück- und ausblick für die rehabilitation. Bern: Schweizerische Gesellschaft für Gesundheitspolitik SGGP; 2017. p. 23–40. (Schriftenreihe der SGGP; vol. 132).
Leung G, Katz PR, Karuza J, Arling GW, Chan A, Berall A, et al. Slow stream rehabilitation: a new model of post-acute care. J Am Med Dir Assoc 2015/12/15 ed. 2016;17(3):238–43.
Wang Y-C, Chou M-Y, Liang C-K, Peng L-N, Chen L-K, Loh C-H. Post-acute care as a key component in a healthcare system for older adults. Ann Geriatr Med Res 2019/06/30 ed. 2019;23(2):54–62.
Bieri-Brüning G. Pflegeheimplazierung statt ‘bloody exit’? Schweiz Ärzteztg. 2013;94(23):893–6.
Ellis G, Gardner M, Tsiachristas A, Langhorne P, Burke O, Harwood RH, et al. Comprehensive geriatric assessment for older adults admitted to hospital. Cochrane Database Syst Rev. 2017/09/13 ed. 2017;9:Cd006211.
Tröster T, Thalmann M, Fischer K, Bieri-Brüning G, Beeler PE, Bischoff-Ferrari HA, et al. Frailty, underweight and impaired mobility are associated with institutionalisation after post-acute care. Swiss Med Wkly. 2020;150(2526) [cited 2020 Oct 24]. Available from: https://smw.ch/article/doi/smw.2020.20276.
Zeeh J. Das geriatrische Assessment. MMW Fortschr Med. 2016;158(2):52–9.
Frost R, Kharicha K, Jovicic A, Liljas AEM, Iliffe S, Manthorpe J, et al. Identifying acceptable components for home-based health promotion services for older people with mild frailty: a qualitative study. Health Soc Care Community 2017/12/07 ed. 2018;26(3):393–403.
Lancaster CW, DiMaggio C, Marshall G, Wall S, Ayoung-Chee P. Functional outcomes after inpatient rehabilitation for trauma-improved but unable to return home. J Surg Res. 2017/11/07 ed. 2018;222:187–194.e3.
St John PD, Tyas SL, Menec V, Tate R, Griffith L. Multimorbidity predicts functional decline in community-dwelling older adults: prospective cohort study. Can Fam Physician 2019/02/16 ed. 2019;65(2):e56–63.
Depalma G, Xu H, Covinsky KE, Craig BA, Stallard E, Thomas J, et al. Hospital readmission among older adults who return home with unmet need for ADL disability. The Gerontologist. 2013;53(3):454–61.
Greysen SR, Stijacic Cenzer I, Auerbach AD, Covinsky KE. Functional impairment and hospital readmission in Medicare seniors. JAMA Intern Med 2015/02/03 ed. 2015;175(4):559–65.
Luppa M, Luck T, Matschinger H, Konig HH, Riedel-Heller SG. Predictors of nursing home admission of individuals without a dementia diagnosis before admission - results from the Leipzig longitudinal study of the aged (LEILA 75+). BMC Health Serv Res 2010/06/30 ed. 2010;10:186.
Shih AF, Buurman BM, Tynan-McKiernan K, Tinetti ME, Jenq G. Views of primary care physicians and home care nurses on the causes of readmission of older adults. J Am Geriatr Soc. 2015;63(10):2193–6.
Nuutinen M, Leskela RL, Suojalehto E, Tirronen A, Komssi V. Development and validation of classifiers and variable subsets for predicting nursing home admission. BMC Med Inf Decis Mak 2017/04/15 ed. 2017;17(1):39.
Chen C, Naidoo N, Er B, Cheong A, Fong NP, Tay CY, et al. Factors associated with nursing home placement of all patients admitted for inpatient rehabilitation in Singapore community hospitals from 1996 to 2005: a disease stratified analysis. PLoS One 2014/01/01 ed. 2013;8(12):e82697.
von Bonsdorff M, Rantanen T, Laukkanen P, Suutama T, Heikkinen E. Mobility limitations and cognitive deficits as predictors of institutionalization among community-dwelling older people. Gerontology. 2006/08/15 ed. 2006;52(6):359–65.
Fried TR, McGraw S, Agostini JV, Tinetti ME. Views of older persons with multiple morbidities on competing outcomes and clinical decision-making. J Am Geriatr Soc 2008/09/06 ed. 2008;56(10):1839–44.
Fried TR, Tinetti M, Agostini J, Iannone L, Towle V. Health outcome prioritization to elicit preferences of older persons with multiple health conditions. Patient Educ Couns 2010/06/24 ed. 2011;83(2):278–82.
Thalmann M, Tröster T, Fischer K, Bieri-Brüning G, Beeler PE, Bischoff-Ferrari HA, et al. Do older adults benefit from post-acute care following hospitalisation? A prospective cohort study at three Swiss nursing homes. Swiss Med Wkly. 2020;150(0910) [cited 2021 May 16]. Available from: https://smw.ch/article/doi/smw.2020.20198.
Peel NM, Hubbard RE, Gray LC. Impact of post-acute transition Care for Frail Older People: a prospective study. J Frailty Aging. 2013;2(3):165–71.
McGilton KS, Vellani S, Krassikova A, Robertson S, Irwin C, Cumal A, et al. Understanding transitional care programs for older adults who experience delayed discharge: a scoping review. BMC Geriatr. 2021;21(1):210.
Hang J-A, Francis-Coad J, Naseri C, Waldron N, Hill A-M. Effects of facility-based transition care programs on health-related outcomes in older adults: a systematic review protocol. JBI Evid Synth. 2020;18(11):2425–34.
Bundesversammlung der Schweizerischen Eidgenossenschaft. Pflegeleistungen bei Krankheit [Internet]. SR83210 Art. 25a 2011. Available from: https://www.fedlex.admin.ch/eli/cc/1995/1328_1328_1328/de
Monego R, Golay Y, Streit C, Berger S. Akut- und Übergangspflege (AÜP): Mängel, Handlungsbedafr und Forderung einer Neuregelung; 2018.
Zimmermann BM, Koné I, Rost M, Leu A, Wangmo T, Elger BS. Factors associated with post-acute discharge location after hospital stay: a cross-sectional study from a Swiss hospital. BMC Health Serv Res. 2019;19(1):289.
Charité - Universitätsmedizin Berlin. Nationaler Vergleichsbericht 2019 Geriatrische Rehabilitation [Internet]. Bern: Nationaler Verein für Qualitätsentwicklung in Spitälern und Kliniken; 2020. [cited 2021 May 16]. Available from: https://www.anq.ch/de/downloads/?category=3027
OBSAN. Hospitalisierungsrate in Rehabilitationseinrichtungen. 2017;
Bundesamt für Statistik (BFS). Rehabilitation in Schweizer Spitälern 2017. Neuchâtel: Schweizerische Eidgenomssenschaft; 2019.
Büsching G, Hollander, Schmidt, Frey M, Steurer J. Finanzierung nach DRG: Einfluss auf die kardiovaskuläre und pulmonale Rehabilitation. Schweiz Ärzteztg Für Standesfragen Bull Prof Médecins Suisses Boll Dei Medici Svizzeri Interes Prof. 1;2014:3.
W. von E, Schüring S, Greitemann B, Karoff M. REDIA - Auswirkungen der DRG-Einführung auf die Rehabiliation. Rehabil Stuttg. 2011;50:214–21.
Koné I, Zimmermann B, Wangmo T, Richner S, Weber M, Elger B. Hospital discharge of patients with ongoing care needs: a cross-sectional study using data from a city hospital under SwissDRG. Swiss Med Wkly 2018/01/30 ed. 2018;148:w14575.
Manville M, Klein MC, Bainbridge L. Improved outcomes for elderly patients who received care on a transitional care unit. Can Fam Physician Med Fam Can. 2014;60(5):e263–71.
Booth A. Clear and present questions: formulating questions for evidence based practice. Cleyle S, editor. Libr Hi Tech. 2006;24(3):355–68.
Kannisto K, Hirvonen E, Koivuniemi M, Teeri S, Asikainen P, Koivunen M. Daily functioning support – a qualitative exploration of rehabilitative approach in acute hospitalised care. Scand J Caring Sci. 2021;35(4):1342–51.
Mahoney F, Barthel D. Functional evaluation: the Barthel index. Md State Med J. 1995;14:56–61.
Collin C, Wade DT, Davies S, Horne V. The Barthel ADL index: a reliability study. Int Disabil Stud. 1988;10(2):61–3.
Sainsbury A, Seebass G, Bansal A, Young JB. Reliability of the Barthel index when used with older people. Age Ageing. 2005;34(3):228–32.
Dromerick AW, Edwards DF, Diringer MN. Sensitivity to changes in disability after stroke: a comparison of four scales useful in clinical trials. J Rehabil Res Dev. 2003;40(1):1–8.
Schuurmans MJ, Shortridge-Baggett LM, Duursma SA. The delirium observation screening scale: a screening instrument for delirium. Res Theory Nurs Pr. 2003/05/20 ed. Spring. 2003;17(1):31–50.
Brühwiler J. Die Zürcher Variante des Mini-Mental-Status nach Folstein : eine Validationsstudie an 1200 Zürcher Krankenheimpatienten; 2019.
Marioni RE, Chatfield M, Brayne C, Matthews FE. Medical Research Council cognitive function and ageing study. The reliability of assigning individuals to cognitive states using the Mini mental-state examination: a population-based prospective cohort study. BMC Med Res Methodol. 2011;11(1):127.
Hajek A, Brettschneider C, Lange C, Posselt T, Wiese B, Steinmann S, et al. Longitudinal predictors of institutionalization in old age. PLoS One. 2015;10(12):e0144203.
Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–98.
Mijnarends DM, Meijers JMM, Halfens RJG, ter Borg S, Luiking YC, Verlaan S, et al. Validity and reliability of tools to measure muscle mass, strength, and physical performance in community-dwelling older people: a systematic review. J Am Med Dir Assoc. 2013;14(3):170–8.
Gómez JF, Curcio C-L, Alvarado B, Zunzunegui MV, Guralnik J. Validity and reliability of the Short Physical Performance Battery (SPPB). Colomb Méd. 2013;44(3):165–71.
Dias N, Kempen GIJM, Todd CJ, Beyer N, Freiberger E, Piot-Ziegler C, et al. Die Deutsche Version der Falls Efficacy Scale-International Version (FES-I). Z Gerontol Geriatr. 2006;39(4):297–300.
Kempen GI, Yardley L, van Haastregt JC, Zijlstra GA, Beyer N, Hauer K, et al. The short FES-I: a shortened version of the falls efficacy scale-international to assess fear of falling. Age Ageing 2007/11/23 ed. 2008;37(1):45–50.
de Vries NM, Staal JB, van Ravensberg CD, Hobbelen JSM, Olde Rikkert MGM, Nijhuis-van der Sanden MWG. Outcome instruments to measure frailty: a systematic review. Ageing Res Rev. 2011;10(1):104–14.
Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol Biol Sci Med Sci 2001/03/17 ed. 2001;56(3):M146–56.
Zhang Y, Zhang Y, Li Y, Chan P, Ma L. Reliability and validity of the self-reported frailty screening questionnaire in older adults. Ther Adv Chronic Dis. 2020;11:2040622320904278.
Verlaan S, Ligthart-Melis GC, Wijers SLJ, Cederholm T, Maier AB, de van der Schueren MAE. High prevalence of physical frailty among community-dwelling malnourished older adults-a systematic review and Meta-analysis. J Am Med Dir Assoc. 2017;18(5):374–82.
Anliker M, Bartlete G, Du Pasquier J, Gilgen R, Müller P, Mylaeus-Renggli M, et al. Handbuch RAI-Home-Care Schweiz. St. Gallen; 2009. (AG. Q-S, editor. Handbuch RAI-Home Care)
Charmaz K. Constructing grounded theory. Vol. 2nd. Los Angeles: SAGE; 2014.
Aubert CE, Fankhauser N, Marques-Vidal P, Stirnemann J, Aujesky D, Limacher A, et al. Patterns of multimorbidity in internal medicine patients in Swiss university hospitals: a multicentre cohort study. Swiss Med Wkly 2019/07/01 ed. 2019;149:w20094.
Khan F, Amatya B, Elmalik A, Lowe M, Ng L, Reid I, et al. An enriched environmental programme during inpatient neuro-rehabilitation: a randomized controlled trial. J Rehabil Med. 2016;48(5):417–25.
Ysrraelit MC, Fiol MP, Gaitán MI, Correale J. Quality of life assessment in multiple sclerosis: different perception between patients and neurologists. Front Neurol. 2018;8:729.
Luo Y, Yang J, Zhang Y. Development and validation of a patient-reported outcome measure for stroke patients. Health Qual Life Outcomes. 2015;13:53.
Moore G, Audrey S, Barker M, Bonell C, Hardeman W, Moore L, et al. UK Medical Research Council (MRC) guidance. 2015;134.
Maximos M, Seng-iad S, Tang A, Straford P, Bello-Haas VD. Slow stream rehabilitation for older adults: a scoping review. Can J Aging. 2019;0(0):1–21.
World Medical Association. World medical association declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–4.
Acknowledgements
We would like to thank the participating nursing homes of the City of Zurich (PZZ).
Funding
The project is funded by the Foundation of the School of Nursing Zurich, Switzerland. The department of Nursing Homes of the City of Zurich (PZZ) provided internal resources for proposal development. None of the authors have agreements with the funding organisation that would limit their ability to complete the research as planned and publish the results.
Author information
Authors and Affiliations
Contributions
A. K.: ultimate authority over any study design activity; data collection, management, analysis and interpretation; writing of the study protocol; preparation of publication draft; shared decision to submit the study protocol for publication. S. S.: study design; preparation of publication draft; shared decision to submit the study protocol for publication. G. B.: data collection; literature search; preparation of publication draft. H. G.: draft of method paragraph; preparation of publication draft. A. K.: ethical submission; preparation of publication draft. F. S.: preparation of publication draft. T. V.: literature search; preparation of publication draft; shared decision to submit the study protocol for publication. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study will follow the Swiss law on research with human subjects. Internationally recognised good clinical practice guidelines will be followed [63]. The trial has been approved by the Ethics Committee of the Canton of Zurich (03/28/2019), with clearance certification number “ZH BASEC 2019–00143”. All participants are requested to provide consent and sign an institutional consent form for publication before data collection. The consent form has been approved by the Ethics Committee of the Canton of Zurich.
Consent for publication
All participants will provide consent for publication via an institutional consent form for publication before data collection.
Competing interests
The authors declare no financial and non-financial competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Koppitz, A.L., Suter-Riederer, S., Bieri-Brünig, G. et al. Prevention Admission into Nursing homes (PAN): study protocol for an explorative, prospective longitudinal pilot study. BMC Geriatr 22, 227 (2022). https://doi.org/10.1186/s12877-022-02885-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12877-022-02885-z