Abstract
Background
The significance of human epidermal growth factor receptor 2 (Her2) and nucleus-associated antigen Ki-67 expression remains controversial in gastric adenocarcinoma (GaC). The aim of this study was to investigate the expression and clinicopathologic and prognostic significance of Her2 and Ki-67 in resected GaC without distant metastasis.
Methods
Malignant tissues and clinicopathologic data were obtained from 195 patients with resected non-metastatic GaC. Immunohistochemistry staining was performed to examine the expression of Her2 and Ki-67; their association with clinicopathologic factors were investigated using logistic regression, and their association with survival was explored using Kaplan–Meier analysis and Cox proportional hazards regression.
Results
Her2 was majorly expressed in cell membrane and Ki-67 in cell nucleus in non-metastatic GaC. Stronger Her2 expression was significantly associated with better tumor differentiation, neurovascular invasion, less advanced pathological tumor (pT) stage, and more advanced pathological node (pN) stage; while Ki-67 expression was not significantly associated with any investigated clinicopathologic factors. Patients with both negative Her2 and negative Ki-67 expression had poorer tumor differentiation, and more advanced pT and pathological tumor-node-metastasis (pTNM) stages; the association with pT and pTNM stages were further confirmed by multivariable analyses, especially in node-negative disease. Her2 or Ki-67 alone was not significantly associated with pTNM stage. A strongly positive (+++) Her2 expression was associated with poorer survival in multivariable analysis only (P = 0.047); while Ki-67 or combined expression was not significantly associated with prognosis.
Conclusions
In non-metastatic GaC, Her2 expression and combined expression of Her2 and Ki-67 were associated with several clinicopathologic factors including tumor differentiation and stage, and only a +++ Her2 expression was associated with poorer prognosis in multivariable analysis with marginal significance in this study; while Ki-67 alone had both limited clinicopathologic and prognostic values.
Similar content being viewed by others
Background
Gastric cancer, the majority of which is gastric adenocarcinoma (GaC), is one of the most prevalent and lethal digestive malignancies worldwide [1, 2]. Although the diagnosis and treatment modalities have improved, the overall 5-year survival rates of GaC remain poor [3,4,5]. GaC is highly malignant, invasive, and chemo-resistant, and rapidly progressive. Recurrence and metastasis occur frequently after surgery and chemo(radio)therapy, which impacts patients’ survival time and quality of life greatly. Tumor-node-metastasis (TNM) stage well differentiates prognoses and guides therapy in GaC [3,4,5].
The occurrence and development of GaC is a multi-stage process which involves multiple gene dysregulations (oncogene activation and tumor-suppressor gene inactivation) and the resulting changes in cell biology behaviors [6, 7]. Human epidermal growth factor receptor 2 (Her2), which belongs to the ErbB/Her family of receptor tyrosine kinases, is an oncogene and predictive biomarker in various cancers [8, 9]. Nucleus-associated antigen Ki-67 is an important cell proliferation-associated protein marker, and its expression is correlated with invasion depth, lymphatic metastasis, and differentiation grade in various solid tumors, especially in colorectal cancer, breast cancer, and liver carcinoma [10,11,12].
Her2 is commonly tested for metastatic GaC. These two markers have also been included in pathology report for resected GaC in China. The association of Her2 and Ki-67 expression with clinicopathologic factors including tumor pathological tumor-node-metastasis (pTNM) and individual pathological tumor (pT) or pathological node (pN) stages and differentiation and their prognostic roles in non-metastatic GaC remains controversial [13, 14]. This study aimed at investigating the expression of Her2 and Ki-67 in resected GaC without distant metastasis, and at revealing their clinicopathologic and prognostic values.
Methods
Patients and specimens
A total of 210 GaC patients undergoing surgical resection between October 2013 and December 2014 in the First Affiliated Hospital of Anhui Medical University where yearly the greatest number of GaC patients receive resection [15] were potential candidates for this study. The inclusion criteria were: (1) the patient had not received any pre-surgical chemo-, radio-, or bio-therapy; (2) diagnosis of GaC was confirmed by postsurgical pathological examination of resected specimens by at least two experienced pathologists, which was consistent with preoperational pathology; (3) the patient underwent open radical total or partial D2 gastrectomy as appropriate; and (4) all cancers were adenocarcinomas. The exclusion criteria included: (1) the patient had serious comorbidities; (2) the patient had other malignancies simultaneously; (3) the pathological diagnosis was controversial; (4) the patient had previous gastric malignancies; and (5) the patient had received chemotherapy or radiotherapy before resection. In our institution in China, it is not the clinical routine that patients with gastric or cardia cancers receive preoperative treatment. Finally 195 GaC patients were eligible for investigation (Table 1). The resected GaC specimens were collected after resection. Basic patient characteristics were recorded. The clinicopatholgic data were obtained from the surgery and pathology records. Disease stage was classified or recoded according to the American Joint Committee on Cancer/Union for International Cancer Control TNM staging system, seventh edition [16]. Written informed consent was obtained from each participating individual, and our study was approved by the local Institutional Review Board and carried out following the Declaration of Helsinki [17] and Good Clinical Practice guidelines [18].
Reagents
Anti-Her2 monoclonal antibody was purchased from the Fuzhou New Biotechnology Development company. The other primary antibodies including the mouse-anti-human Ki-67 monoclonal antibody working solution (MAB-0129), the ready-to-use non-biotin immunohistochemistry streptavidin-peroxidase detection kit (KIT-9902), the diaminobenzidine chromogenic reagent (DAB-2301), the citrate tissue-antigen recovery solution (MVS-0100), and the neutral gum were all obtained from the Maixin Biotechnology Co. Ltd., Fuzhou. The reagents were all stored at 4 °C before use.
Immunohistochemistry
The obtained tissues were first hematoxylin–eosin-stained to be confirmed as GaC tissues. The protein marker expression in GaC tissues was detected using immunohistochemistry (streptavidin-peroxidase). As standard, automated platform for immunohistochemistry was used. The staining method has been previously described [19], and the major procedures were: (1) the slides were immersed in 10% silver nitrate solution for 24 h, and then dehydrated in 95% ethanol, followed by water rinsing and air drying. Then they were processed by 2% APES acetone for 30 s, followed by wash with pure acetone and airing; (2) the specimens were fixed in 10% formaldehyde, embedded in paraffin, serially sectioned into 3 μm-thick slices, attached to slides, dewaxed by xylene, and hydrated by gradient ethanol, followed by phosphate-buffered saline (PBS) wash and airing; (3) antigen recovery was conducted using citrate with high temperature and pressure, followed by cooling and water and PBS wash. Then the sections were incubated in 3% hydrogen peroxide deionized water for 5–10 min to inactivate the endogenous peroxidase, followed by water and PBS wash; (4) the Her2 or Ki-67 (working solution) monoclonal antibodies (50 μL) was added to the specimens respectively, followed by incubation at 4° C overnight or at room temperature for 1 h and then PBS wash; (5) the polymer enhancer (50 μL) was added followed by incubation at room temperature for 20 min and then PBS wash. Then the general-type Immunoglobulin G antibody-Horseradish Peroxidase polymer (anti-mouse/rabbit, 50 μL) was added followed by incubation at room temperature for 15–30 min and then PBS wash; (6) the sections underwent color-developing in diaminobenzidine solution (100 μL/section) for 2–8 min with microscopic observation, followed by distilled water rinse for 10 min to stop the reaction and then hematoxylin counterstain for 3 min plus water wash; and (7) the sections were acidized using 1% hydrochloric acid and processed by saturated lithium carbonate solution, followed by rinse. Then the sections were gradient alcohol-hydrated, xylene-hyalinized, and neutral gum-sealed, followed by microscopic observation. The positive tissue section in the pre-experiment set was selected as the positive control, and the negative control was obtained by replacing the primary antibody with PBS.
Assessment criteria
The stained sections were evaluated by 2 experienced pathologists in a blind way. Observed in high power field (× 400), randomly 5 fields were selected and examined for each section, and the number and intensity of positively-stained cells were recorded and averaged, with 100 cells in each field counted respectively. In case of a discrepancy, a third senior investigator was consulted and agreement was reached by consensus. The positivity level of Her2 expression follows the National Comprehensive Cancer Network (NCCN) Guidelines (https://www.nccn.org): − means “no reactivity or membranous reactivity in < 10% of cancer cells”; + means “faint or barely perceptible membranous reactivity in ≥ 10% of cancer cells; cells are reactive only in part of their membrane”; ++ means “weak to moderate complete, basolateral or lateral membranous reactivity in ≥ 10% of cancer cells”; +++ means “strong complete, basolateral, or lateral membranous reactivity in ≥ 10% of cancer cells”. – or + expression indicates a negative result, and +++ expression suggests a positive finding. In case of ++ expression by immunohistochemistry, in situ hybridization was further performed, and cases with an average Her2 copy number ≥ 6.0 signals/cell were considered positive. For Ki-67, the results were assessed according to the percentage of positive cells: − means very low proliferation activity with proportion of Ki-67-positive cells < 25%; + means low proliferation activity with proportion of Ki-67-positive cells 25–50%; ++ means moderate proliferation activity with proportion of Ki-67-positive cells 50–75%; +++ means high proliferation activity with proportion of Ki-67-positive cells > 75%. A section was regarded negative in Ki-67 expression with < 50% positive cells, and positive with ≥ 50% positive cells following relevant reports published recently [20,21,22,23,24]. Cancers were further categorized into 4 groups according to combined expression of both markers: double negative, Ki-67 positive and Her2 negative, Her2 positive and Ki-67 negative, and double positive.
Statistical analysis
Continuous variables were presented as mean ± standard deviation; median (interquartile range), and categorical variables as count (percentage [%]). Clinicopathologic factors in different subgroups by the combined expression of Her2 and Ki-67 were compared using the χ2 or analysis of variance test according to data type. Logistic regression was used to determine the association of marker expression with other clinicopathologic factors. Kaplan–Meier survival curves stratified by marker expression were plotted, and prognostic differences across groups were examined using the log-rank test. The association of marker expression with survival was further examined using univariable and multivariable Cox proportional hazards regression. Data were analyzed using the R (v. 3.4.0, Vienna, Austria) statistical software (https://www.r-project.org/). A finding was statistically significant with a 2-sided P value < 0.05.
Results
Patient characteristics
For the 195 analyzed patients (Table 1), the mean age was 64 years, and males took up 76%. Most of the tumors were located at gastric cardia and/or fundus (52%), and were poorly-differentiated or undifferentiated (75%). The mean tumor length was 4.8 cm. The mean metastatic lymph node number was 4 based on an average harvest of 19 lymph nodes per patient, and the mean positive-harvested lymph node ratio was 0.20.
Expression of Her2 and Ki-67 and their association with clinicopathologic factors in GaC
Both Her2 and Ki-67 were presented as brown or yellow granules in GaC. Her2 was majorly expressed in cell membrane and the positive expression proportion was 28% (Fig. 1). Ki-67 was located at cell nucleus, and the positive proportion was 64% (Fig. 2). Stronger Her2 expression was associated with poorer tumor differentiation, neurovascular invasion, earlier pT stage, and more advanced pN stage; however, expression of Ki-67 was not significantly associated with the investigated clinicopathologic variables (Table 2). Expression of Her2 or Ki-67 was not significantly associated with overall pTNM stage (Table 3).
Expression of Her2. a pTNM stage I gastric adenocarcinoma; b + from pTNM stage III gastric adenocarcinoma; c ++ from pTNM stage II gastric adenocarcinoma; d +++ from pTNM stage II gastric adenocarcinoma. The positivity degree of Her2 expression follows the National Comprehensive Cancer Network guidelines (https://www.nccn.org): − means “no reactivity or membranous reactivity in < 10% of cancer cells”; + means “faint or barely perceptible membranous reactivity in ≥ 10% of cancer cells; cells are reactive only in part of their membrane”; ++ means “weak to moderate complete, basolateral or lateral membranous reactivity in ≥ 10% of cancer cells”; +++ means “strong complete, basolateral, or lateral membranous reactivity in ≥ 10% of cancer cells”. Her2, human epidermal growth factor receptor 2
Expression of Ki-67. a from pTNM stage I gastric adenocarcinoma; b from pTNM stage II gastric adenocarcinoma; c ++ from pTNM stage I gastric adenocarcinoma; d +++ from pTNM stage III gastric adenocarcinoma. For Ki-67, − means very low proliferation activity with proportion of Ki-67-positive cells < 25%; + means low proliferation activity with proportion of Ki-67-positive cells 25–50%; ++ means moderate proliferation activity with proportion of Ki-67-positive cells 50–75%; +++ means high proliferation activity with proportion of Ki-67-positive cells > 75%
Association of combined expression of both markers with clinicopathologic factors in GaC
Based on the combined expression of both markers, cancers were categorized into four groups (Table 4): double negative (n = 49, 25%), Ki-67 positive and Her2 negative (n = 91, 47%), Her2 positive and Ki-67 negative (n = 21, 11%), and double positive (n = 34, 17%). While no significant differences in patient age, sex, tumor location, size, metastatic lymph node number, lymph node ratio, neurovascular invasion, cancer embolus, or pN stage were observed between the four groups, there existed significant inter-group differences in tumor differentiation (P = 0.001), pT stage (P = 0.009), and pTNM stage (P = 0.022). Patients with negative expression of both markers had highest proportions of poorly-differentiated/undifferentiated tumors (86%), pT stage 3–4 (94%), and pTNM stage III (69%).
The above findings appeared contradictory to the well-known knowledge that strong expressions of Her2 and Ki-67 are both predictors of poor survival, and we further explored the association of the combined expression with pTNM stage in overall, node-negative, and node-positive GaCs and with pT and pN stages using logistic regression. Interestingly, in node-negative disease, negative expression of both markers was significantly associated with the most advanced pTNM stage; however, in overall or node-positive GaC, no significant association of combined expression of the two markers with pTNM stage was observed (Table 5). We further investigated the association of the combined expression of both markers with individual pT and pN stages (Table 6), and performed univariable analysis and 2 sets of multivariable analyses (one with pN and pT stage excluded from covariates, and the other with pN or pT stage included). All models revealed that cancers with negative expression of both markers had the most advanced pT stage; while the combined expression was not significantly associated with pN stage.
The prognostic significance of Her2 and Ki-67
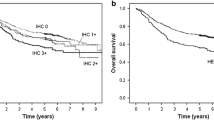
Kaplan–Meier analysis showed no significantly different survival across the four groups by Ki-67 (P = 0.17), Her2 (P = 0.62), or combined expression of Her2 and Ki-67 (P = 0.24; Fig. 3). Using Cox proportional hazards regression (Table 7), neither univariable nor multivariable-adjusted analysis showed a significant association of Ki-67 expression with survival; a strongly positive (+++) Her2 expression was significantly associated with poorer survival compared to − expression in multivariable analysis (hazard ratio = 2.17, 95% confidence interval = 1.01–4.68). Using the Cox model, neither univariable nor multivariable-adjusted analysis showed a significant association of combined Her2 and Ki-67 expression with survival (Table 7).
Kaplan–Meier survival curves according to Ki-67 (a), Her2 (b), and combined expression (c). Prognostic differences across groups were examined using the log-rank test. Her2, human epidermal growth factor receptor 2; H–K−, Her2 negative and Ki-67 negative; H–K+, Her2 negative and Ki-67 positive; H+K−, Her2 positive and Ki-67 negative; H+K+, Her2 positive and Ki-67 positive
Discussion
Given that Her2 and Ki-67 might both exert functions in discrepant phases during cell proliferation, the combined expression of both markers were examined in our study. We found that in non-metastatic GaC, stronger expression of the receptor tyrosine kinase Her2 alone was significantly associated with better tumor differentiation, neurovascular invasion, less advanced pT stage, and more advanced pN stage; while the expression of the nucleus-associated antigen Ki-67 alone was not significantly associated with any investigated clinicopathologic factors. Patients with both negative Her2 and negative Ki-67 expression had poorer tumor differentiation, and more advanced pT and pTNM stages; the association with pT and pTNM stages were further confirmed by multivariable analyses, especially in node-negative disease. Her2 or Ki-67 alone was not significantly associated with pTNM stage. A strongly positive (+++) Her2 expression was associated with poor survival; while Ki-67 or combined expression was not significantly associated with prognosis.
The human Her2 gene located in chromosome 17q12-21.23 can code a transmembrane receptor tyrosine kinase, and the interaction of the receptor with their ligands can modulate cell survival, growth, differentiation, and proliferation through altering cell signaling [25]. We found that it was majorly expressed in GaC cell membrane. Studies have indicated a role of Her2 in the development of various types of human cancers. The proportion of positive expression is about 10–20% in breast carcinoma [26] and is approximately 20% in GaC, where a high expression of Her2 is associated with poor prognosis [27]. A phase III randomized trial [9] showed that trastuzumab (an anti-Her2 agent) in combination with conventional chemotherapy is superior to conventional chemotherapy alone in the treatment of Her2/neu-positive advanced GaC.
In our study, stronger Her2 expression was associated with better tumor differentiation but with neurovascular invasion. Interestingly, higher Her2 expression was associated with earlier pT stage, but with more advanced pN stage. In a pooled analysis, while consistently, Her2 expression was found to be positively associated with lymph node metastasis, it was not significantly associated with cancer invasion depth or TNM stage [28]. A study [29] has shown that the expression of Her2 is not the result of the development and progression of malignant tumor, but rather the initiating carcinogenic factor of the disease. This might partly explain the observed association with the primary tumor stage. The positive association between Her2 expression and involved lymph node number suggests that Her2 may play an important role in the nodal metastasis of GaC. The role of Her2 in the progression of GaC warrants further exploration. It is well known that in breast cancer Her2 was found to be a negative prognostic factor [30], but for GaC there still seems to be no consensus, despite the fact that the first studies demonstrated an association between positive Her2 status and poor prognosis. The majority of previous publications showed that Her2-postive status provided additional prognostic information and was associated with relevant clinicopathologic characteristics, such as serosal invasion and lymph node metastasis [31,32,33]. We found that Her2 expression was significantly associated with tumor pT and pN stages, and differentiation with some seemingly contradictory patterns, and that only a strongly positive (+++) expression of Her2 was marginally significantly associated with poorer survival.
Ki-67 is receiving increasing attention as an important tumor proliferation marker since firstly revealed as a non-histone protein in 1991 by Gerdes et al. [34], and is highly associated with tumor development, progression, invasion, metastasis, and prognosis [35]. It is only expressed in the nucleus of proliferating cells during the proliferation and synthesis phases of the cell cycle, and could be detected from the G1 through the M phase, but not in the resting G0 phase. It has been used as a proliferation marker in cancers [36]. Some studies screening potential tumor markers and investigating their correlation with clinicopathologic factors have shown that Ki-67 is associated with tumor invasion and metastasis in various cancer types [10,11,12]. A study [37] suggests that Ki-67 is an independent prognostic factor for breast cancer patients with positive sentinel lymph nodes who have received adjuvant chemotherapy.
This study revealed that the expression of Ki-67 alone was not significantly associated with any investigated clinicopathologic factors. Studies on the association between Ki-67 expression and clinicopathologic factors revealed controversial results [38,39,40]. Notably, a recent meta-analysis showed that high Ki-67 expression was not significantly associated with lymph node metastasis, tumor stage, or differentiation, but that it could serve as a predictive biomarker for poor prognosis in GaC patients [41]. However, our study further showed that Ki-67 expression was also not significantly associated with postsurgical survival.
Her2 or Ki-67 alone has ambiguous clinicopathologic significance. We here for the first time revealed the significance of the combined examination of both markers in GaC. Based on the significance of individual markers [25, 29, 35, 36], it is hypothesized that in non-metastatic GaC, cancers with negative expression of both markers may be relatively weak in both local growth and nodal metastasis capacities, cancers with positive Ki-67 expression but negative Her2 expression may have a stronger local growth potential, tumors with positive Her2 expression but negative Ki-67 expression may be more prone to invasion and nodal metastasis, and tumors with positive expression of both markers may be strong in both capacities. GaC negative in both marker expression can be more heterogeneous and can also include quite a proportion of more advanced cancers where the biological role of local growth and nodal involvement has been relatively weakened during the specific phase of malignant progression. The biological relevance needs to be further clarified in translational or basic studies. In our study, patients with both negative Her2 and Ki-67 expression had poorer tumor differentiation, and more advanced pT and pTNM stages, especially in node-negative disease. The combined expression of both markers was not significantly associated with pN stage. The discrepancy in the associations of the marker expression with tumor local invasion and with lymph node metastasis and the seemingly contradictory findings between the associations of Her2 and Ki-67 expression with cliniopathologic parameters and with survival should be better clarified in future studies. Notably, the assessment of Ki-67 in archived tissue samples is unsuitable as a prognostic biomarker for GaC. Ki-67 expression may be complicated by tumor heterogeneity, and future studies should pay special attention to standardized evaluation and appropriate and representative tissue sampling [23]. In this study the tissue samples were examined soon after resection.
This study is first limited by its observational nature. Possibly due to difference in cancer entity, proportion of positive Ki-67 expression was as high as 95% when we followed the recommendations by the International Ki-67 in Breast Cancer working group [42], which we considered not suitable for classifying Ki-67 expression in GaC. Different cutoff values for Ki-67 in GaC were adopted in previous studies [41], and there lacks a standard or a recommendation. While in this report we used the cutoff value following recent publications [20,21,22,23,24], the optimal one needs to be further determined. In this study, multivariable regression models were fitted when patient age and tumor length were assessed as continuous covariates to avoid arbitrary selection of cut-off points for continuous variables.
The novelty of this work lies in the fact that the combined expression of Her2 and Ki-67 was examined, in addition to either individual marker. While there have been investigations on the clinical significance of Her2 expression in patients with gastric cancer, they mostly investigated Her2 alone, and a large proportion of the studies were on advanced unresectable gastric cancer; furthermore, the associations of Her2 expression with clinicopathologic factors (especially tumor pTNM stage, pT stage, pN stage, and differentiation) and prognosis remain largely controversial, and the quality of statistics in previous studies varied [13, 28]. In this study, we investigated the significance of Her2 expression in resected gastric adenocarcinoma without distant metastasis in a cohort study design, both alone and in comparison to and in combination with Ki-67 expression, using both univariable and multivariable analyses. We performed careful analyses of the association of Her2 expression with various clinicopathologic factors especially cancer stages both overall and with careful subgroup analyses stratified by lymph node metastasis status. The findings also aid to personalized medicine and provide important hints for further investigation. Cancer genesis and progression are both dependent on cell proliferation, and Her2 and Ki-67 are expressed in proliferating cells during various phases. Both play vital regulatory roles in cell proliferation as major proliferation indexes [43]. Other strengths of our study included the strict inclusion and exclusion criteria, the careful cohort study design, and the careful statistical and subgroup analyses.
Conclusions
In non-metastatic GaC, Her2 expression and combined expression of Her2 and Ki-67 were associated with several clinicopathologic factors including tumor differentiation and stage, and only a +++ Her2 expression was associated with poorer prognosis in multivariable analysis with marginal significance in this study; while Ki-67 alone had both limited clinicopathologic and prognostic values.
Availability of data and materials
Restrictions apply to the availability of the data that support the findings of this study, which were used under license for the current study, and so are not publicly available. However, the data used/generated by our study is available from the corresponding author upon reasonable request, and formal study proposal and variable sheet and approval by the authors’ institution are needed.
Abbreviations
- GaC:
-
Gastric adenocarcinoma
- TNM stage:
-
Tumor-node-metastasis stage
- pTNM stage:
-
Pathological tumor-node-metastasis stage
- pT stage:
-
Pathological tumor stage
- pN:
-
Pathological node stage
- Her2:
-
Human epidermal growth factor receptor 2
References
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108.
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359-386.
Wang W, Li YF, Sun XW, Chen YB, Li W, Xu DZ, Guan XX, Huang CY, Zhan YQ, Zhou ZW. Prognosis of 980 patients with gastric cancer after surgical resection. Chin J Cancer. 2010;29(11):923–30.
Zhang JW, Huang L, Xu AM. Preoperative monocyte-lymphocyte and neutrophil-lymphocyte but not platelet-lymphocyte ratios are predictive of clinical outcomes in resected patients with non-metastatic Siewert type II/III adenocarcinoma of esophagogastric junction: a prospective cohort study (the AMONP corhort). Oncotarget. 2017;8(34):57516–27.
Huang L, Xu AM. Adenocarcinoma of esophagogastric junction: controversial classification, surgical management, and clinicopathology. Chin J Cancer Res. 2014;26(3):226–30.
Huang L, Wu RL, Xu AM. Epithelial-mesenchymal transition in gastric cancer. Am J Transl Res. 2015;7(11):2141–58.
Xu AM, Huang L, Zhu L, Wei ZJ. Significance of peripheral neutrophil-lymphocyte ratio among gastric cancer patients and construction of a treatment-predictive model: a study based on 1131 cases. Am J Cancer Res. 2014;4(2):189–95.
Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344(11):783–92.
Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376(9742):687–97.
Guzinska-Ustymowicz K, Stepien E, Kemona A. MCM-2, Ki-67 and PCNA protein expressions in pT3G2 colorectal cancer indicated lymph node involvement. Anticancer Res. 2008;28(1B):451–7.
Stroescu C, Dragnea A, Ivanov B, Pechianu C, Herlea V, Sgarbura O, Popescu A, Popescu I. Expression of p53, Bcl-2, VEGF, Ki67 and PCNA and prognostic significance in hepatocellular carcinoma. J Gastrointest Liver Dis. 2008;17(4):411–7.
Lyzogubov V, Khozhaenko Y, Usenko V, Antonjuk S, Ovcharenko G, Tikhonkova I, Filonenko V. Immunohistochemical analysis of Ki-67, PCNA and S6K1/2 expression in human breast cancer. Exp Oncol. 2005;27(2):141–4.
Lei YY, Huang JY, Zhao QR, Jiang N, Xu HM, Wang ZN, Li HQ, Zhang SB, Sun Z. The clinicopathological parameters and prognostic significance of HER2 expression in gastric cancer patients: a meta-analysis of literature. World J Surg Oncol. 2017;15(1):68.
Liu G, Xiong D, Zeng J, Chen B, Huang Z. Clinicopathological and prognostic significance of Ki-67 immunohistochemical expression in gastric cancer: a systematic review and meta-analysis. Onco Targets Ther. 2017;10:4321–8.
Huang L, Wei ZJ, Li TJ, Jiang YM, Xu AM. A prospective appraisal of preoperative body mass index in D2-resected patients with non-metastatic gastric carcinoma and Siewert type II/III adenocarcinoma of esophagogastric junction: results from a large-scale cohort. Oncotarget. 2017;8:68165.
Washington K. 7th edition of the AJCC cancer staging manual: stomach. Ann Surg Oncol. 2010;17(12):3077–9.
World Medical A. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–4.
Grimes DA, Hubacher D, Nanda K, Schulz KF, Moher D, Altman DG. The Good Clinical Practice guideline: a bronze standard for clinical research. Lancet. 2005;366(9480):172–4.
Huang L, Xu AM, Peng Q. CD147 and MMP-9 expressions in type II/III adenocarcinoma of esophagogastric junction and their clinicopathological significances. Int J Clin Exp Pathol. 2015;8(2):1929–37.
Giaginis C, Giagini A, Tsourouflis G, Gatzidou E, Agapitos E, Kouraklis G, Theocharis S. MCM-2 and MCM-5 expression in gastric adenocarcinoma: clinical significance and comparison with Ki-67 proliferative marker. Dig Dis Sci. 2011;56(3):777–85.
Liu M, Li JS, Tian DP, Huang B, Rosqvist S, Su M. MCM2 expression levels predict diagnosis and prognosis in gastric cardiac cancer. Histol Histopathol. 2013;28(4):481–92.
Li N, Deng W, Ma J, Wei B, Guo K, Shen W, Zhang Y, Luo S. Prognostic evaluation of Nanog, Oct4, Sox2, PCNA, Ki67 and E-cadherin expression in gastric cancer. Med Oncol. 2015;32(1):433.
Boger C, Behrens HM, Rocken C. Ki67—an unsuitable marker of gastric cancer prognosis unmasks intratumoral heterogeneity. J Surg Oncol. 2016;113(1):46–54.
Huang G, Chen S, Wang D, Wang R, Lin L, Chen S, Wang L, Huang Q. High Ki67 expression has prognostic value in surgically-resected T3 gastric adenocarcinoma. Clin Lab. 2016;62(1–2):141–53.
Jelovac D, Emens LA. HER2-directed therapy for metastatic breast cancer. Oncology (Williston Park). 2013;27(3):166–75.
Moelans CB, de Weger RA, van Diest PJ. Multiplex ligation-dependent probe amplification to detect HER2 amplification in breast cancer: new insights in optimal cut-off value. Cell Oncol. 2010;32(4):311–2.
Jorgensen JT. Targeted HER2 treatment in advanced gastric cancer. Oncology. 2010;78(1):26–33.
Liang JW, Zhang JJ, Zhang T, Zheng ZC. Clinicopathological and prognostic significance of HER2 overexpression in gastric cancer: a meta-analysis of the literature. Tumour Biol. 2014;35(5):4849–58.
Hu Y, Bandla S, Godfrey TE, Tan D, Luketich JD, Pennathur A, Qiu X, Hicks DG, Peters JH, Zhou Z. HER2 amplification, overexpression and score criteria in esophageal adenocarcinoma. Mod Pathol. 2011;24(7):899–907.
Loibl S, Gianni L. HER2-positive breast cancer. Lancet. 2017;389(10087):2415–29.
Gravalos C, Jimeno A. HER2 in gastric cancer: a new prognostic factor and a novel therapeutic target. Ann Oncol. 2008;19(9):1523–9.
De Vita F, Giuliani F, Silvestris N, Catalano G, Ciardiello F, Orditura M. Human epidermal growth factor receptor 2 (HER2) in gastric cancer: a new therapeutic target. Cancer Treat Rev. 2010;36(Suppl 3):S11-15.
Bartley AN, Washington MK, Colasacco C, Ventura CB, Ismaila N, Benson AB 3rd, Carrato A, Gulley ML, Jain D, Kakar S, et al. HER2 Testing and Clinical Decision Making in Gastroesophageal Adenocarcinoma: guideline from the College of American Pathologists, American Society for Clinical Pathology, and the American Society of Clinical Oncology. J Clin Oncol. 2017;35(4):446–64.
Gerdes J, Li L, Schlueter C, Duchrow M, Wohlenberg C, Gerlach C, Stahmer I, Kloth S, Brandt E, Flad HD. Immunobiochemical and molecular biologic characterization of the cell proliferation-associated nuclear antigen that is defined by monoclonal antibody Ki-67. Am J Pathol. 1991;138(4):867–73.
Penault-Llorca F, Andre F, Sagan C, Lacroix-Triki M, Denoux Y, Verriele V, Jacquemier J, Baranzelli MC, Bibeau F, Antoine M, et al. Ki67 expression and docetaxel efficacy in patients with estrogen receptor-positive breast cancer. J Clin Oncol. 2009;27(17):2809–15.
Dziegiel P, Salwa-Zurawska W, Zurawski J, Wojnar A, Zabel M. Prognostic significance of augmented metallothionein (MT) expression correlated with Ki-67 antigen expression in selected soft tissue sarcomas. Histol Histopathol. 2005;20(1):83–9.
Dumontet C, Krajewska M, Treilleux I, Mackey JR, Martin M, Rupin M, Lafanechere L, Reed JC. BCIRG 001 molecular analysis: prognostic factors in node-positive breast cancer patients receiving adjuvant chemotherapy. Clin Cancer Res. 2010;16(15):3988–97.
Zhou Y, Li Y, Zheng J, Liu K, Zhang H. Detecting of gastric cancer by Bcl-2 and Ki67. Int J Clin Exp Pathol. 2015;8(6):7287–90.
Chen L, Li X, Wang GL, Wang Y, Zhu YY, Zhu J. Clinicopathological significance of overexpression of TSPAN1, Ki67 and CD34 in gastric carcinoma. Tumori. 2008;94(4):531–8.
Al-Moundhri MS, Nirmala V, Al-Hadabi I, Al-Mawaly K, Burney I, Al-Nabhani M, Thomas V, Ganguly SS, Grant C. The prognostic significance of p53, p27 kip1, p21 waf1, HER-2/neu, and Ki67 proteins expression in gastric cancer: a clinicopathological and immunohistochemical study of 121 Arab patients. J Surg Oncol. 2005;91(4):243–52.
Luo G, Hu Y, Zhang Z, Wang P, Luo Z, Lin J, Cheng C, Yang Y. Clinicopathologic significance and prognostic value of Ki-67 expression in patients with gastric cancer: a meta-analysis. Oncotarget. 2017;8:50273.
Dowsett M, Nielsen TO, A’Hern R, Bartlett J, Coombes RC, Cuzick J, Ellis M, Henry NL, Hugh JC, Lively T, et al. Assessment of Ki67 in breast cancer: recommendations from the International Ki67 in Breast Cancer working group. J Natl Cancer Inst. 2011;103(22):1656–64.
Maga G, Hubscher U. Proliferating cell nuclear antigen (PCNA): a dancer with many partners. J Cell Sci. 2003;116(Pt 15):3051–60.
Acknowledgements
The authors would most sincerely thank the reviewers and editors for critically reviewing this paper and for the constructive and thoughtful comments and suggestions.
Funding
This work was supported by National Natural Science Foundation of China (Grant No.: 81572350). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
Conception or design of the work: ZW, LH, XZ, and AX. Acquisition, analysis, or interpretation of data: ZW, LH, and XZ. Drafting of the work: ZW, LH, and XZ. Substantive revision of the work: AX. All authors have read and approved the submitted version and any substantially modified version that involves the author's contribution to the study, and have agreed both to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Institutional Review Board of First Affiliated Hospital of Anhui Medical University. Written informed consent was obtained from each investigated individual. No individual patient data were reported.
Consent to publish
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wei, Z., Huang, L., Zhang, X. et al. Expression and significance of Her2 and Ki-67 in gastric adenocarcinoma without distant metastasis: a cohort study. BMC Gastroenterol 20, 343 (2020). https://doi.org/10.1186/s12876-020-01484-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12876-020-01484-9