Abstract
Background
Optimizing prescribing practices is important due to the substantial clinical and financial costs of polypharmacy and an increasingly aging population. Prior research shows the importance of social relationships in driving prescribing behaviour. Using social network analysis, we examine the relationship between a physician practices’ connectedness to peers and their prescribing performance in two German regions.
Methods
We first mapped physician practice networks using links established between two practices that share 8 or more patients; we calculated network-level (density, average path length) and node-level measures (degree, betweenness, eigenvector). We defined prescribing performance as the total number of inappropriate medications prescribed or appropriate medications not prescribed (PIMs) to senior patients (over the age of 65) during the calendar year 2016. We used FORTA (Fit fOR The Aged) algorithm to classify medication appropriateness. Negative binomial regression models estimate the association between node-level measures and prescribing performance of physician practices controlling for patient comorbidity, provider specialization, percentage of seniors in practice, and region. We conducted two sensitivity analyses to test the robustness of our findings – i) limiting the network mapping to patients younger than 65; ii) limiting the network ties to practices that share more than 25 patients.
Results
We mapped two patient-sharing networks including 436 and 270 physician practices involving 28,508 and 20,935 patients and consisting of 217,126 and 154,274 claims in the two regions respectively. Regression analyses showed a practice’s network connectedness as represented by degree, betweenness, and eigenvector centrality, is significantly negatively associated with prescribing performance (degree—bottom vs. top quartile aRR = 0.04, 95%CI: 0.035,0.045; betweenness—bottom vs. top quartile aRR = 0.063 95%CI: 0.052,0.077; eigenvector—bottom vs. top quartile aRR = 0.039, 95%CI: 0.034,0.044).
Conclusions
Our study provides evidence that physician practice prescribing performance is associated with their peer connections and position within their network. We conclude that practices occupying strategic positions at the edge of networks with advantageous access to novel information are associated with better prescribing outcomes, whereas highly connected practices embedded in insulated information environments are associated with poor prescribing performance.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Introduction
Optimizing prescribing practices is a priority in healthcare due to the substantial clinical and financial costs of drug-related complications. This is particularly a problem among seniors: in Germany, seniors over the age of 65 comprise 20% of the total population, and a regional study estimated that 22% of seniors are prescribed at least one potentially inappropriate medication [1, 2]. In 2009, the direct cost of prescribing potentially inappropriate medications was estimated at €387.8 million in Germany, which does not include other downstream expenditures such as hospitalizations and emergency department visits [3].
Polypharmacy, generally defined as the concomitant and long-term use of five or more medications, is a direct consequence of inappropriate prescribing and is associated with increased hospitalization, mortality, and adverse drug events [4,5,6]. Seniors, typically living with multiple chronic conditions, are at increased risk of polypharmacy due to their multiple interactions with different specialists who may prescribe medications for different conditions without regular medication review [7]. This is further exacerbated by the proliferation of single disease-specific guidelines recommended for use to prescribers, which has been shown to lead to a number of adverse drug interactions [8]. Multiple groups have argued for an interprofessional and team-based approach to reduce polypharmacy, considering patient complexity and multimorbidity [9, 10]. There is a paucity of data, however, on how interprofessional teams collaborate to manage polypharmacy.
The Fit fOR The Aged (FORTA) classification was first developed in 2010 through consensus by German-based experts to support physicians in improving appropriate prescribing for seniors (aged over 65) in the German context [11]. This was created in response to the paucity of evidence-based guidelines for the senior patients combined with the high level of polypharmacy and potentially inappropriate medications (PIMs) observed [11]. FORTA scores provide an assessment of prescribing quality for a patient by cross-referencing prescribed medications with a patient’s diagnosis to detect over- and under-prescribing based on drug-drug interactions. Research has shown that application of FORTA in clinical workflow improved appropriate prescribing among prescribers for seniors (over the age of 65) in Germany [11, 12] and has a comparatively better performance in detecting potentially inappropriate medications (PIMs) [13]; it has subsequently been applied to different settings following expert consensus meetings [14,15,16].
Recent decades have seen the rise of both evidence-based prescribing—prescribing based on the best available, critically appraised evidence—and rational prescribing, wherein judicious prescribing maximizes effectiveness while minimizing harm and avoiding waste [17]. However, social relationships remain an important driver of prescribing decisions [18,19,20,21]: for example, research on diffusion of new medications has shown that prescribers are highly influenced by peer adoption [22] and pharmaceutical manufacturer exposure [23, 24]. Additionally, a growing body of literature in behavioural science demonstrates the applicability of socially-influenced interventions such as peer comparison to improve guideline-concordant practices among prescribers [25]. Influences on prescribing practice are not limited to physicians within the same practice [26, 27], but also among physicians that share patients with one another [28]. This finding may be particularly relevant to seniors who typically have comparatively higher rates of comorbidities; and as a result, require multiple prescribers to be aware of their medication history and coordinate treatment.
Such peer relationships can be studied using network analysis, which considers the interaction and dynamics between individuals within a system. Network analysis can reveal implicit relationships between different actors within a system, who act as communication channels for information exchange and social influence [29]. These relationship structures and the positioning of individual actors within a network has important implications on the behaviour, perceptions, and attitudes for the individual as well as those around them [29]. Network positioning of an individual is associated with performance measures in various sectors including education [30], engineering [31], and innovation [32]. Physician social networks have increasingly been the subject of study as increasing pressure is exerted on health systems to contain costs and improve quality [33].
Time constraints among healthcare providers and low survey response rates are significant barriers to network analysis in healthcare [33]. To overcome these barriers, Barnett and colleagues have used administrative claims data to identify connections between physicians based on shared patients, and showed that physicians who share patients are more likely to personally know each other, exchange information and informal advice [34,35,36]. Physician connectedness to others in their professional network is associated with improvements in patient outcomes including length of stay [37], mortality [38], and patient satisfaction [39]. This method has since been applied widely to characterize relationships among physicians in hospitals [40], and outpatient clinics [41], and at the organizational level in long term care facilities [42] and between hospitals [43].
Physician practices may share patients because of unresolved clinical problems that require complementary expertise, when different specialists are providing team-based care to a patient with complex and chronic disease, or when patients seek second opinions for further clinical information [44]. Apart from the latter patient-driven mechanisms for patient-sharing, the first two scenarios create a motivation for the physician involved to review the patient’s current and past medical history, and to either formally connect with a colleague that is also caring for the patient or to informally consult a colleague for management, ethical or therapeutic advice [45,46,47] as a form of information exchange. Additionally, physician relationships may be reinforced in professional practice settings outside of clinical environments where acquaintance is established through shared patients.
Here we identify a patient-sharing provider network using German regional healthcare claims data and characterize the association between a physician practice’s level of connectedness to peers and its performance in prescribing quality. We hypothesized that more connected practices are better positioned to exercise their social capital and draw on the knowledge of peers, which may guide them to adopt quality prescribing practice and exhibit better performance. By applying a method previously validated in large medical centers and community hospitals in the United States [34], we demonstrate the transferability to German data, and also to smaller geographic regions, where analytical implications are more relevant for local decision-making.
Methods
Social networks can be used to reveal underlying relationships between different actors in a system. Studying how different actors are connected can lead to insights on the relative social capital individuals have to draw on and how others in the proximity of their network might influence them. Networks consist of nodes and ties that represent how nodes are connected to one another. In our study, each node represents a physician practice, and a tie represents the relationship between two physician practices, inferred using the number of shared patients between the two corresponding practices.
Study design
This is a retrospective, observational study using administrative claims data to examine the relationship between a physician practice’s positioning within a social network and their prescribing performance for senior patients in 2016. We follow the research framework for network analysis using administrative claims data proposed by Uddin following a systematic review of this field of work [48]. We used claims data from two insurance companies in the respective geographic regions in Billstedt-Horn and Werra-Meissner Kreis, which covers roughly 45% and 23% of the patient population in the two regions respectively. The majority of existing work on mapping patient sharing networks using administrative data is based in the United States [33]. While recent research set in the United States estimated differences in network statistic when different sources of insurance claims data is used to map the network [49], we assume that the insuree demographic is similarly distributed between health insurance companies in Germany. This is because in the German healthcare system, individuals have free choice of the health insurance company (i.e., sickness fund), and risk adjustment schemes are in place to minimize incentives for adverse selection by the insurance companies. Thus, any differences between the two subsamples are attributed to regional differences. Practice claims data were obtained through established data-sharing agreements with the regional sickness fund. We used pseudonymized physician practice and patient data in this study and thus our study was exempt from institutional ethics review at the University of Hamburg.
Research setting
This study is set in two areas in Germany: Billstedt-Horn, and Werra-Meißner-Kreis. Billstedt-Horn is a multicultural and urban district with a population of roughly 110,000 in Northern Germany [50]. Werra-Meißner-Kreis is a region in rural central Germany consisting of a little over 100,000 population and have a larger share of elderly population. In our study, we limit the analysis to ambulatory clinics, which include both primary care practices and specialist clinics working as solo or group practice.
Study samples
The population in this study included physician practices who were involved in the treatment and care of patients insured by the insurance companies in the year 2016 in Billstedt-Horn and Werra-Meissner-Kreis respectively. To identify eligible claims for network mapping, we started from the full claims record, and excluded practices whose specialty is not typically responsible for direct patient care and hence coordination (anesthesiology, radiology, pathology, radiotherapy, and nuclear medicine); claims that were categorized as emergency visits; and practices that had fewer than 30 patients in the 2016 calendar year. By filtering out practices with fewer than 30 unique patients that filed claims within the year, we effectively exclude practices that may not have a strong presence in the regional professional social network. As the claims data is based on the patient postal code, practices excluded may be physicians that patients seek out outside of the region of interest in this study; thus, while a large percentage of practices are excluded based on this criterion (Fig. 1), we do not believe that the resulting sample introduces selection bias.
To reach the final analytic sample, we further excluded practices that do not have claims records of patients over the age of 65 due to the outcome variable of interest in this study. The physician practice sample used to map the patient-sharing network and the analytic sample is shown in Fig. 1.
Mapping patient-sharing network and analysis
We map patient-sharing networks between practices in the same geographic region using administrative claims data. This method of mapping affiliation networks [51] was previously validated [34] and has been performed on outpatient clinic data [41]. Affiliation networks show connections between actors based on common events or co-membership, assuming that this indicates an underlying (or potential) social tie [51]. Using this method of network mapping, connections between physician practices are based on linkages established through common patients shared between the two [26, 34, 51]. In this view, shared patients act as a conduit for diffusion of clinical practices between those that provide care.
Patient-sharing networks were mapped separately for the two regions included in this study using a previously validated method developed by Barnett and colleagues [34]. Accounting for practical considerations highlighted by Dugoff and colleagues in network identification [33], we applied the following criteria for inclusion of practices in our study: i) medical specialities whose scope of practice includes patient coordination with others; ii) non-emergency visits; iii) claims within the calendar year 2016; iv) more than 30 patients seen in 2016; v) include patients over the age of 65 years old. Specific details on steps taken to map the network can be found in the additional file (Supplement—S1). Each node within our network represents a physician practice, and an edge implies an information-sharing relationship between two practices based on more than 8 shared patients. Using this method of network mapping and in line with prior research, we infer an information-sharing relationship between two physicians that share a significant number of patients and that the extent to which they are connected is reflected by the number of patients shared.
Network-level and node-level measures
We characterize the degree of network integration using network-level indicators and the structural position of a practice within the network using node-level centrality measures (Table 1). Network-level indicators include density and average path length. Density represents how densely connected the nodes are to all other nodes in each network; average path length captures the average of the shortest paths between any two nodes in the network.
Node-level indicators we used to characterize centrality include degree, betweenness, and eigenvector centrality [35]. These centrality measures were chosen based on our assumptions of how information flows in our shared-patient network [52] and informed by theory [54, 55]. Degree measures the number of other practices the practice of interest is directly connected to through shared patients with each practice weighted equally. This measure implicates the level of available intangible resources a practice may draw upon and represents a communication focal point for those to whom this node is connected. Betweenness measures the number of times a practice is located on the intermediary step of all shortest paths linking two other practices in the network. Graphically, practices with a high betweenness centrality will be positioned in the middle of a network rather than on the periphery. Practices with a high betweenness score can be interpreted as having great influence on their network peers and having greater access to the information flow among practices within the network. Eigenvector centrality is a measure of the practice’s influence on the overall practice network over the long term, whereby a high eigenvector value indicates that the practice of interest is linked to other practices that are themselves highly connected.
Study variables
Outcome prescribing quality measure
To assess practice prescribing quality for senior patients, we used the FORTA tool that classifies prescriptions as a PIM using an evidence-based algorithm [11]. The FORTA tool provides a numeric score of PIMs received for each patient (over the age of 65) by cross-referencing the patient’s diagnosis, history, and medication profile; this score may reflect repeating PIMs receives over multiple clinical encounters if left unresolved. A high FORTA score for a patient indicates a high number of PIMs prescribed, including both over- and under- prescribing, which may be attributed to all physicians who were responsible for the patient.
As our unit of analysis was at the practice level, the prescribing quality measure is aggregated at the practice level. Specifically, the practice prescribing quality is calculated by summing the cumulative annual FORTA score of each patient in the practice. An increase in one summed FORTA score represents an increase in one PIM prescribed to a patient under the care of the practice during the calendar year. A high summed FORTA score represents poor prescribing quality among the physician practice; and a low summed FORTA score represents good prescribing quality.
Predictors – physician related
Apart from centrality measures as described above, we considered supply-side factors identified in literature that affect prescribing quality. These include practice patient load [56], physician age [56], rural/urban location [57], payment and incentive schemes [58, 59], physician specialization, and physician education [60, 61]. The claims data to which we had access allowed us to control for the percentage of seniors in each practice, the practice specialty, and the practice region. We controlled for the percentage of seniors in each practice as senior patients tend to have more co-morbidities, and thus to have more concurrent medications and opportunities for prescription error. Though it is conceivable that the total number of patients under the care of one practice may affect the prescribing quality, due to its high correlation with the percentage of seniors variable, it was not included in model as a control. Practice specialty was deduced based on service practices predominantly filed for claims within the calendar year of 2016. Primary care providers indicate practices that are categorized as “Hausarzt” or general practice medicine and gynecologists who serve as the first point of contact for most patients and are expected to have more of a role in medication coordination. Additionally, due to the broad scope of practice of general practitioners, specialists’ knowledge of appropriate medications are generally deferred to [62]. We also controlled for the region, where Billstedt Horn represented an urban and moderately deprived region while Werra Meissner Kreis represents a rural and monoethnic community.
Predictors – patient related
We used the Charlson comorbidity index to control for patient comorbidity, as patients with more comorbidities are likely to be prescribed more medications, which may inadvertently drive-up medication errors. Because it would be impractical to include all comorbidity variables in a regression model due to issues of multicollinearity and inadequate degrees of freedom, we chose to use a composite measure. The Charlson comorbidity index considers the severity of specific diseases and the patient’s age in the weighted score; it is widely used as a mechanism to control for patient comorbidity when analyzing administrative data [63]. We transform the patient-level Charlson comorbidity score to a physician practice-level variable by averaging the Charlson comorbidity score of all patients in the practice.
Statistical analysis
Following the mapping of physician practice network, we conducted descriptive analysis in both regions of interest including patient demographics, provider coverage, and network parameters; we also explored the distribution of outcome variable in both the healthcare regions. We then conducted bivariate analyses using Spearman’s rank correlation to describe the strength of association and direction between the centrality measures (degree, betweenness, and eigenvector) and potentially inappropriate prescriptions (summed FORTA score). Spearman’s rank correlation was used as it does not make assumptions regarding the underlying distribution of the data, which did not follow a normal distribution. Variables were assessed for potential multicollinearity by measuring the variance inflation factors (VIF) and balanced with omitted variable bias when constructing the regression model.
We defined quartiles within each region for all network centrality measures (degree, betweenness, and eigenvector) and treated the quartiles as categorical variables. Data from both regions were pooled in the regression analyses to increase the power to determine the effect. Each region was identified using a dummy variable to account for underlying differences. We used a negative binomial regression to estimate the adjusted incidence rate ratio of prescribing a potentially inappropriate prescription due to the overdispersed count outcome variable. It is important to note that the unit of analysis is at the practice level, and thus patient-associated scores were aggregated.
Our model specification is as follows:
where: centrality measure represents degree, closeness, or eigenvector centrality (Table 1); percentage of seniors refer to the percentage of elderly patients (over the age of 65) over the total practice patient panel size; mean Charlson score represents the mean Charlson comorbidity score of all included patients (seniors) belonging to the practice; primary care provider is a dummy variable, where practices specialize in either general internal medicine, family practice, or gynecology were assigned a value of 1, and 0 otherwise; finally, region is also a dummy variable that identifies if the practice is in Billstedt-Horn or Werra-Meissner-Kreis and is used as a proxy for rural or urban context.
Sensitivity analysis
We conducted two separate sensitivity analyses to check for robustness of effects in different subsamples. First, we address the challenge of endogeneity between the network measures and the outcome measure by remapping the social network with only physician practices that shared younger patients (defined as under the age of 65) and deriving practice-level centrality measures. We then retained our original analytical sample (from main analysis) and updated the associated centrality measures derived from physician practices sharing only younger patients. By doing so, we isolate the effects of practice centrality on prescribing quality from the contribution of senior patients who are more likely to have multiple comorbidities and thus simultaneously increasing the number of physician visits (contributing to a higher centrality measure) and increasing the FORTA scores. Second, we tested the stability of effects by remapping the network using an increased threshold of 25 shared patients. By increasing the threshold of shared patients in which ties between practices are drawn, we effectively increase the specificity of linkages (i.e., decreased likelihood of drawling linkages between spurious connections) [34]. In other words, we increase the certainty that linkages between practices detected represent true connections.
All statistical analyses were performed using the R statistical software (version 4.0.3).
Results
The two patient-sharing networks we mapped—Billstedt-Horn and Werra Meissner Kreis—included 436 and 270 practices; 28,508 and 20,935 patients; and consisted of 217,126 and 154,274 claims in the 2016 calendar year, respectively. We further excluded patients under the age of 65 and practices that did not care for patients over the age of 65 to reach the analytical sample consisting in total of 659 practices (Billstedt-Horn: 401; Werra Meissner Kreis: 269) (Fig. 1). Within the analytical sample, the patient population in both regions have similar sex distribution; though in Billstedt-Horn, patients are slightly older (median of 75 years of age, compared to 71 in Werra Meissner Kreis) and have a higher comorbidity score (Charlson index of 4.25, compared to 4 in Werra Meissner Kreis) (Table 2). Physician practices have larger patients under their care in Werra Meissner Kreis (median of 128 patients, IQR = 382), as compared to Billstedt-Horn (median of 95 patients, IQR = 203); and Billstedt-Horn have a larger percentage of primary care providers among all practices (Billstedt-Horn 38%; Werra Meissner Kreis 30%). In the two networks included, Werra Meissner Kreis appears to be a more connected network with a higher density (0.16, compared to 0.08 in Billstedt-Horn) and shorter mean distance (1.99, compared to 2.10 in Billstedt-Horn). Both networks have a longer mean distance compared to the average generated over 1000 random graphs with the same number of nodes, which indicates a less connected network compared to what would be expected of an average network with the same number of nodes. While the Billstedt-Horn network had a slightly higher percentage of same-specialty tie (BH: 6.76%; WMK: 4.40%), the proportion that are primary care provider is similarly high across both regions (BH: 82%; WMK: 84%) (Table 3).
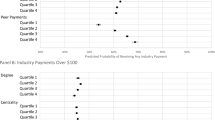
Results from the negative binomial regression are presented in Table 4, where degree, betweenness, and eigenvector centrality are shown separately. For each centrality measure, we present three different models: model 1 contains only the centrality measure; model 2 is a control-only model; and model 3 is the full model with control variables included. For ease of interpretation, we also present results as adjusted incidence rate ratio (IRR) for centrality measures and number of potentially inappropriate prescriptions (Table 5). Our results showed that network centrality measures were significantly associated with PIMs. We found a significant negative relationship between the centrality measures degree, betweenness, and eigenvector and prescribing a PIM (Table 5). Those in the bottom quartile of degree centrality had an incidence rate 0.4 times that of practices in the top quartile (IRR = 0.040, 95%CI (0.035, 0.045)); those in the bottom quartile of betweenness centrality had an incidence rate 0.06 times that of practices in the top quartile (IRR = 0.063, 95%CI (0.052, 0.077)); and those in the bottom quartile of eigenvector centrality had an incidence rate 0.04 times that of practices in the top quartile (IRR = 0.039, 95%CI (0.034, 0.044)).
Across the specified models, adjusted results show that many practice-level variables were consistently associated with PIMs (Table 4). We found that increases in mean Charlson score to be significantly associated with increased PIMs across all centrality measures. We found percentage of senior patients to have a significant positive association with PIMs across all centrality measures. We did not find a consistent association between primary care provider and PIM, however among models encompassing betweenness centrality, we observed a significant positive relationship. Lastly, we observed a consistent relationship between region and PIMs with Werra-Meissner-Kreis to have a significantly higher PIM as compared to Billstedt Horn.
We examined the consistency of the association between centrality measures and PIM by remapping the network with two alternative methods: i) including only clinical encounters involving younger patients (less than 65 years old); ii) increasing the threshold of shared patients that defines a tie between two practices from 8 to 25 patients. In both sensitivity analyses conducted, the results were in line with main analysis with slight changes in effect size, same directionality of association and significance (Table 6). From the first sensitivity analysis, we found that as degree of connectedness decreases, the incidence of a PIM also decreases (network including clinical encounter with young patients: adjusted IRR for practices in the bottom quartile had an incidence rate of 0.044 times that of practices in the top quartile (95%CI: 0.039, 0.050); network including only ties established by more than 25 shared patients: adjusted IRR for practices in the bottom quartile had an incidence rate of 0.059 times that of practices in the top quartile (95%CI: 0.050, 0.069)) (Table 6). We also found a similar negative association between PIM and betweenness centrality as well as eigenvector centrality (Table 6).Similar results were found for the second sensitivity analysis, where a positive association was observed between centrality measures degree, betweenness, and eigenvector centrality of practices and PIMs (Table 6). We found that results from both sensitivity analyses confirm the robustness of our findings. The models for all three centrality measures resulted in slight changes in effect size, but the same directionality and level of significance. Results from both sensitivity analyses confirm the robustness of our findings.
Discussion
Our research examines the structure and composition of shared-patient networks mapped between practices in two German healthcare areas using insurance claims data. We find that practice centrality is associated with prescribing quality for elderly patients when controlling for confounding factors; contrary to our hypothesis, the association is in the negative direction (i.e., more well-connected practices are associated with poorer prescribing quality). Research mapping social networks using administrative data has increased steadily in recent decades; while most studies are set in the US and Australia [33], studies in France [64] and Germany [65, 66] has been published in the last couple years. To our knowledge, this research is the first to apply this novel method to study prescribing performance for elderly patients in the German health system context. Prior research mainly focused on organizational, physician, and patient determinants of prescribing quality [67]. This study elucidates the influence of information-sharing relationships and the structural position within one’s network on prescribing quality outcome. By applying a replicable method leveraging administrative data, our research provides an additional tool for regional change management in designing quality improvement strategies.
In this study, we find practices that are well-connected to others (high degree centrality), well-positioned to broker information between network practices (high betweenness centrality) and connected to well-connected others (high eigenvector centrality), were more likely to be associated with an overall increased over- and under- prescribing scores (i.e., to have worse prescribing quality). We interpret this finding by expanding on the following: i) occupying positions on the network fringes may confer advantage in accessing novel information; ii) highly connected practices may be subject to an insulated information environment; iii) negative resource exchanges in professional networks.
This work draws upon social network theory, which posits that the embeddedness of each individual’s positioning within a network, and their connectedness to others, confer both advantages and drawbacks [68]. In turn, social capital theory can provide a foundation for describing and characterizing the observed results. Social capital, as first defined by Boudieu [69] and expanded upon by Burt [70], Coleman [71], and others, is the ability of actors to secure benefits such as information through membership in social networks or other structures [72]. Within the context of network structures, social capital theory can be conceived as two mutually exclusive yet reinforcing mechanisms—structural holes and social cohesion [73].
Access and exchange of knowledge and information is particularly salient in our study of prescribing quality. While clinical knowledge is typically imparted through textbooks, clinical guidelines and journal articles [19], research shows that physicians also learn directly through peer interactions [45,46,47] and may be influenced by their social milieu, particularly under high levels of uncertainty [19,20,21]. Following Burt’s structural hole theory, practices that are less connected in their local networks may act as a bridge to distinct communities, and may have a strategic advantage in accessing diverse information [73, 74]. Conversely, well-connected practices embedded in dense networks may be exposed to redundant information from peers that possess similar knowledge and information. This low information diversity among more central practices may be a barrier to integration of prescribing guidelines or novel clinical information and thereby reflect the higher over- and under-prescribing associated with practices that were highly connected. Others have shown that physicians reporting higher adoption of evidence-based medicine adoption were negatively associated with indicators of network prominence and centrality [75].
The social cohesion perspective proposes that individuals in highly connected networks are likely to demonstrate homogeneous behavior due to the spread of norms, ideas, and practices through social relationships [76, 77]. This phenomenon is further evidenced by the strong and restrictive social control that exists in knowledge-intensive networks [31]. Physicians conforming to local norms, etiquette, and hierarchical structure, even in instances where prescribing decisions should be challenged, is well documented in literature [18, 21]. One manifestation of strong social cohesion is the process of “groupthink” where a premature consensus is achieved based on group judgement, which may reduce the rationality of the decision or behaviour of a group [78]. Closely linked is the concept of “echo” whereby individuals access information through their peers and hence rely on the peers’ filtering and interpretation of information according to their prior knowledge and opinions [79]. Through socialization processes such as echo and groupthink, highly connected physician practices may develop prescribing patterns that depart from best evidence yet are locally endorsed and reinforced.
While a variety of benefits may be derived from social capital, negative exchange relations also exist and have been associated with individual performance [80] and described in healthcare teams as “hindrance networks” [81]. Hindrance networks can be characterized as the exchange of negative resources that may inhibit or hinder the individual or team’s performance, particularly when performance of individuals depends on access to information from others [81]. In a recent study of polypharmacy in Germany, general practitioners expressed concern regarding the infrequent and poor communication of medication changes during care transitions and following specialist appointments [82]. In the context of this study, prescribing quality among well-connected practices may be compromised due to differences in communication channels between prescribers in different settings, and the lack of structures to share patient medication history.
Poor prescribing performance associated with well-connected practices found in our study reflects a fragmented healthcare system where physicians who are positioned to provide coordinated care lack formal structures and incentives to support this practice. In Germany, the lack of electronic patient health records that service providers from different settings can access undermines their ability to provide coordination of care and medication reconciliation [6, 83]. Integrated care initiatives have been introduced in both regions under study in recent years. The focus on optimizing appropriate prescribing practices through integration of the FORTA algorithm in providers’ clinical workflow provides feedback on prescribing quality. Future research may well utilize this analysis to evaluate whether introduction of feedback mechanisms and care management programs improve care coordination within established professional networks.
Implications for practice and policy
Our research generates several implications for practice and policy. As health systems restructure to improve care coordination for the patient, it is critical for system administrators and policy makers to recognize the importance of relationships and positioning within existing networks in shaping behaviour and performance.
Adherence to local prescribing norms creates a closed network where novel information, such as revised prescribing guidelines, fail to reach prescribers. Our findings that well-connected practices are associated with worse prescribing practices may be a result of practices conforming to local prescribing norms that depart from best practice guidelines. Implementing quality circles [84], where physician colleagues gather to discuss locally relevant quality improvement initiatives and exchange ideas, may present an opportunity to introduce information in an insular environment. Poss-Doering and colleagues found regular peer exchanges through quality circles was highly valued by physicians in primary care networks and contributed to improved guideline-concordant antibiotic prescribing [85].
Physicians tap into their peers’ knowledge within their social and professional networks and rely on their community of practice as important sources of information for prescribing practices, even more so than national guidelines [86, 87]. Using social networks analyses, administrators can better identify well-connected practices (i.e., key opinion leaders) and target dissemination of best practices through key opinion leaders. In practice, when administrators are introducing new practice guidelines or implementing decision-support, gaining the confidence and support from well-connected physician practices within a local network may increase their use and spread.
Lastly, social capital has long been recognized as integral in individual and team performance. Previous research shows the association between social capital and the maturity of a hospital’s quality management system [88] and validated measurement tools for social capital within hospital management has subsequently been created [89]. Our research demonstrates the feasibility of using network metrics to monitor dimensions of individual social capital in relation to their embedded networks, and its association with quality outcomes such as prescribing. This provides a low-cost, highly replicable option that can be applied and scaled in a variety of settings due to the widely available administrative data in healthcare.
Limitations
Our study is subject to limitations. First, we derived provider relationships based on the presence of patients shared within a year using claims data from one insurance company in each region, which may have overlooked certain links based on shared patients insured by other companies. We minimized the impact of this risk by using data from health insurance companies that have substantial market share in both regions within our study. Additionally, methods have advanced in recent years to restrict linkages among provider dyads to only those that share patients with one another over one episode of care [90]. While we do not have the data to consider this approach, this clinically intuitive method of identifying information-sharing relationships should be considered in future research.
Second, networks are subject to boundary specification problems. In this study, the boundaries are artificially drawn based on providers sought by residents within the two geographical areas. We assume that healthcare utilization in these two areas is relatively self-contained, which is conceivable as one area is a rural community with a natural boundary and the other is a relatively deprived neighborhood in a larger city. Further considering that patients tend to seek care in their communities that are culturally appropriate, we assume in our study that those living in Billstedt-Horn district typically seek care within the district. Third, while there is no standard gate-keeping system in Germany, general practitioners remain well-positioned to coordinate care for patients. Where more comprehensive data with wider geographic coverage is available, future research should consider a general practitioner-only network to better investigate the relationship between prescribing quality and peer relationships. Fourth, pharmaceutical influence on prescribing is widely substantiated with a study showing 84% of physicians indicating weekly pharmaceutical sales representatives visits in Germany [91]. It is conceivable that physician practices with a higher percentage of senior patients may be targetted more frequently by pharmaceutical sales representatives which inadvertently contributes towards to prescribing quality as higher prescribing volume has previously been associated with poor prescribing quality [92]. Fifth, where multi-year data is available, future research may account for provider-level fixed effects such as practice style, preference, and experience, thus increasing the precision of the estimate. Lastly, like most network research, this study was cross-sectional and thus hinders our ability to determine causality. While our objective was to characterize the association between centrality measures and prescribing performance among practices, it is likely that poorly performing practices have been operating for longer, and hence are further away from updated best practices and prescribe based on experience. Established practices are likely to have an established network of peers and accumulate more patients within the community, which drives up the measured centrality indicators. Future studies with richer data sources may consider longitudinal designs to determine the directionality of this association.
Conclusions
Our study builds on the momentum of research in patient-sharing network analysis in the last decade and finds well-connected practices to be associated with increased over- and under-prescribing for senior patients in two regions in Germany. From this, we conclude that practices occupying strategic positions at the edge of networks with advantageous access to novel information are associated with better prescribing outcomes, whereas highly connected practices embedded in insulated information environments are associated with poor prescribing performance.
Availability of data and materials
The data associated with the paper are not publicly available due to legal restrictions imposed by the health insurance companies providing the data (data contains potentially identifying patient information). The data set supporting the conclusions of this article are owned by German statutory health insurance and are subject to strict data protection rules according to the German social security code. Therefore, the data cannot be made publicly accessible. Data access for this research project was granted to the data processing organization (OptiMedis AG) based on a bilateral contract with the statutory health insurance companies.
The data we accessed is collected by the health insurance fund when health providers bill their services. To fulfill the legal requirements to obtain the data, data users must agree on a contract with the statutory health insurer regarding data access. The licensee is permitted to use the data for the purpose as set out in the contract. Licensees are not allowed to pass the data to a third party. For assistance in obtaining access to the data, please contact the corresponding author (SYW).
Abbreviations
- BH:
-
Billstedt Horn
- FORTA:
-
Fit fOR The Aged
- PIM:
-
Potentially inappropriate medications
- WMK:
-
Werra Meissner Kreis
References
Moßhammer D, Haumann H, Mörike K, Joos S. Polypharmacy—an Upward Trend with Unpredictable Effects. Deutsches Aerzteblatt Online. 2016 Sep 23; Available from: https://www.aerzteblatt.de/https://doi.org/10.3238/arztebl.2016.0627. [cited 2021 Aug 27].
Schubert I, Küpper-Nybelen J, Ihle P, Thürmann P. Prescribing potentially inappropriate medication (PIM) in Germany’s elderly as indicated by the PRISCUS list. An analysis based on regional claims data. Pharmacoepidemiol Drug Saf. 2013;22(7):719–27.
Pohl-Dernick K, Meier F, Maas R, Schöffski O, Emmert M. Potentially inappropriate medication in the elderly in Germany: an economic appraisal of the PRISCUS list. BMC Health Serv Res. 2016;16(1):109.
Black CD, Thavorn K, Coyle D, Bjerre LM. The Health System Costs of Potentially Inappropriate Prescribing: A Population-Based, Retrospective Cohort Study Using Linked Health Administrative Databases in Ontario. Canada Pharmacoecon Open. 2020;4(1):27–36.
Cahir C, Fahey T, Teeling M, Teljeur C, Feely J, Bennett K. Potentially inappropriate prescribing and cost outcomes for older people: a national population study. Br J Clin Pharmacol. 2010;69(5):543–52.
Spinewine A, Schmader KE, Barber N, Hughes C, Lapane KL, Swine C, et al. Appropriate prescribing in elderly people: how well can it be measured and optimised? The Lancet. 2007;370(9582):173–84.
Moriarty F, Hardy C, Bennett K, Smith SM, Fahey T. Trends and interaction of polypharmacy and potentially inappropriate prescribing in primary care over 15 years in Ireland: a repeated cross-sectional study. BMJ Open. 2015;5(9):e008656.
Dumbreck S, Flynn A, Nairn M, Wilson M, Treweek S, Mercer SW, et al. Drug-disease and drug-drug interactions: systematic examination of recommendations in 12 UK national clinical guidelines. BMJ. 2015;11(350):h949.
Steinman MA. Polypharmacy—Time to Get Beyond Numbers. JAMA Intern Med. 2016;176(4):482–3.
Benny Gerard N, Mathers A, Laeer C, Lui E, Kontio T, Patel P, et al. A Descriptive Quantitative Analysis on the Extent of Polypharmacy in Recipients of Ontario Primary Care Team Pharmacist-Led Medication Reviews. Pharmacy. 2020;8(3):110.
The FORTA authors/expert panel members, Kuhn-Thiel AM, Weiß C, Wehling M. Consensus Validation of the FORTA (Fit fOR The Aged) List: A Clinical Tool for Increasing the Appropriateness of Pharmacotherapy in the Elderly. Drugs Aging. 2014;31(2):131–40.
Wehling M, Burkhardt H, Kuhn-Thiel A, Pazan F, Throm C, Weiss C, et al. VALFORTA: a randomised trial to validate the FORTA (Fit fOR The Aged) classification. Age Ageing. 2016;45(2):262–7.
Krüger C, Schäfer I, van den Bussche H, Bickel H, Dreischulte T, Fuchs A, et al. Comparison of FORTA, PRISCUS and EU(7)-PIM lists on identifying potentially inappropriate medication and its impact on cognitive function in multimorbid elderly German people in primary care: a multicentre observational study. BMJ Open. 2021;11(9):e050344.
Pazan F, Weiss C, Wehling M, FORTA. The EURO-FORTA (Fit fOR The Aged) List: International Consensus Validation of a Clinical Tool for Improved Drug Treatment in Older People. Drugs Aging. 2018;35(1):61–71.
Pazan F, Gercke Y, Weiss C, Wehling M, FORTA Raters. The U.S.-FORTA (Fit fOR The Aged) List: Consensus Validation of a Clinical Tool to Improve Drug Therapy in Older Adults. J Am Med Dir Assoc. 2020;21(3):439.e9-439.e13.
Pazan F, Gercke Y, Weiss C, Wehling M, FORTA Raters. The JAPAN-FORTA (Fit fOR The Aged) list: Consensus validation of a clinical tool to improve drug therapy in older adults. Arch Gerontol Geriatr. 2020;91:104217.
Burgers JS, Grol R, Klazinga NS, Mäkelä M, Zaat J, For the AGREE Collaboration. Towards evidence-based clinical practice: an international survey of 18 clinical guideline programs. Int J Qual Health Care. 2003;15(1):31–045.
Charani E, Castro-Sanchez E, Sevdalis N, Kyratsis Y, Drumright L, Shah N, et al. Understanding the Determinants of Antimicrobial Prescribing Within Hospitals: The Role of “Prescribing Etiquette.” Clin Infect Dis. 2013;57(2):188–96.
Malterud K. The art and science of clinical knowledge: evidence beyond measures and numbers. Lancet. 2001;358(9279):397–400.
Mano-Negrin R, Mittman B. Theorising the social within physician decision making. J Manag Med. 2001;15(4–5):259–65.
Lewis PJ, Tully MP. Uncomfortable prescribing decisions in hospitals: the impact of teamwork. J R Soc Med. 2009;102(11):481–8.
Coleman J, Katz E, Menzel H. The Diffusion of an Innovation Among Physicians. Sociometry. 1957;20(4):253–70.
Fleischman W, Agrawal S, King M, Venkatesh AK, Krumholz HM, McKee D, et al. Association between payments from manufacturers of pharmaceuticals to physicians and regional prescribing: cross sectional ecological study. BMJ. 2016;18(354):i4189.
Lublóy Á. Factors affecting the uptake of new medicines: a systematic literature review. BMC Health Serv Res. 2014;14(1):469.
Wang SY, Groene O. The effectiveness of behavioral economics-informed interventions on physician behavioral change: A systematic literature review. Triberti S, editor. PLoS ONE. 2020 Jun 4;15(6):e0234149.
Fattore G, Frosini F, Salvatore D, Tozzi V. Social network analysis in primary care: the impact of interactions on prescribing behaviour. Health Policy. 2009;92(2–3):141–8.
Nair HS, Manchanda P, Bhatia T. Asymmetric Social Interactions in Physician Prescription Behavior: The Role of Opinion Leaders. J Mark Res. 2010;47(5):883–95.
Ong MS, Olson KL, Chadwick L, Liu C, Mandl KD. The Impact of Provider Networks on the Co-Prescriptions of Interacting Drugs: A Claims-Based Analysis. Drug Saf. 2017;40(3):263–72.
West E, Barron DN, Dowsett J, Newton JN. Hierarchies and cliques in the social networks of health care professionals: implications for the design of dissemination strategies. Soc Sci Med. 1999;48(5):633–46.
Abbasi A, Altmann J, Hossain L. Identifying the effects of co-authorship networks on the performance of scholars: A correlation and regression analysis of performance measures and social network analysis measures. J Informet. 2011;5(4):594–607.
Cross R, Cummings J. Tie and Network Correlates of Performance in Knowledge Intensive Work. Acad Manag J. 2004;1(47):928–37.
Wang H, Zhao J, Li Y, Li C. Network centrality, organizational innovation, and performance: A meta-analysis. Can J Adm Sci Rev Can des Sci de l’Administration. 2015;32(3):146–59.
DuGoff EH, Fernandes-Taylor S, Weissman GE, Huntley JH, Pollack CE. A scoping review of patient-sharing network studies using administrative data. Transl Behav Med. 2018;8(4):598–625.
Barnett ML, Landon BE, O’Malley AJ, Keating NL, Christakis NA. Mapping Physician Networks with Self-Reported and Administrative Data. Health Serv Res. 2011;46(5):1592–609.
Barnett ML, Christakis NA, O’Malley J, Onnela JP, Keating NL, Landon BE. Physician Patient-sharing Networks and the Cost and Intensity of Care in US Hospitals. Med Care. 2012;50(2):152–60.
Song Z, Sequist TD, Barnett ML. Patient Referrals: A Linchpin for Increasing the Value of Care. JAMA. 2014;312(6):597.
Cowan MJ, Shapiro M, Hays RD, Afifi A, Vazirani S, Ward CR, et al. The effect of a multidisciplinary hospitalist/physician and advanced practice nurse collaboration on hospital costs. J Nurs Adm. 2006;36(2):79–85.
Baggs JG, Schmitt MH, Mushlin AI, Mitchell PH, Eldredge DH, Oakes D, et al. Association between nurse-physician collaboration and patient outcomes in three intensive care units. Crit Care Med. 1999;27(9):1991–8.
Larrabee JH, Ostrow CL, Withrow ML, Janney MA, Hobbs GR, Burant C. Predictors of patient satisfaction with inpatient hospital nursing care. Res Nurs Health. 2004;27(4):254–68.
Landon BE, Keating NL, Barnett ML, Onnela JP, Paul S, O’Malley AJ, et al. Variation in patient-sharing networks of physicians across the United States. JAMA. 2012;308(3):265–73.
Pollack CE, Weissman GE, Lemke KW, Hussey PS, Weiner JP. Patient Sharing Among Physicians and Costs of Care: A Network Analytic Approach to Care Coordination Using Claims Data. J Gen Intern Med. 2013;28(3):459–65.
Lee BY, Song Y, Bartsch SM, Kim DS, Singh A, Avery TR, et al. Long-Term Care Facilities: Important Participants of the Acute Care Facility Social Network? Morgan D, editor. PLoS ONE. 2011;6(12):e29342.
Lomi A, Mascia D, Vu DQ, Pallotti F, Conaldi G, Iwashyna TJ. Quality of care and interhospital collaboration: a study of patient transfers in Italy. Med Care. 2014;52(5):407–14.
Hirsch O, Träger S, Bösner S, Ilhan M, Becker A, Baum E, et al. Referral from primary to secondary care in Germany: Developing a taxonomy based on cluster analysis. Scand J Public Health. 2012;40(6):571–8.
Kuo D, Gifford DR, Stein MD. Curbside Consultation Practices and Attitudes Among Primary Care Physicians and Medical Subspecialists. JAMA. 1998;280(10):905–9.
Keating NL, Zaslavsky AM, Ayanian JZ. Physicians’ experiences and beliefs regarding informal consultation. JAMA. 1998;280(10):900–4.
Gabbay J, le May A. Evidence based guidelines or collectively constructed “mindlines?” Ethnographic study of knowledge management in primary care. BMJ. 2004;329(7473):1013.
Uddin S, Kelaher M, Srinivasan U. A framework for administrative claim data to explore healthcare coordination and collaboration. Aust Health Rev. 2016;40(5):500–10.
Trogdon JG, Weir WH, Shai S, Mucha PJ, Kuo TM, Meyer AM, et al. Comparing Shared Patient Networks Across Payers. J Gen Intern Med. 2019;34(10):2014–20.
Golubinski V, Wild EM, Winter V, Schreyögg J. Once is rarely enough: can social prescribing facilitate adherence to non-clinical community and voluntary sector health services? Empirical evidence from Germany. BMC Public Health. 2020;20(1):1827.
Borgatti S, Halgin D. Analyzing Affiliation Networks. The Sage Handbook of Social Network Analysis. 2011 Jan 1;1
Borgatti SP. Centrality and network flow. Soc Netw. 2005;27(1):55–71.
Brandes U, Borgatti SP, Freeman LC. Maintaining the duality of closeness and betweenness centrality. Soc Netw. 2016;1(44):153–9.
Freeman LC. Centrality in social networks conceptual clarification. Soc Netw. 1978;1(3):215–39.
Bavelas A. Communication patterns in task-oriented groups. J Acoust Soc Am. 1950;22(6):725–30.
Davidson W, Molloy DW, Bédard M. Physician characteristics and prescribing for elderly people in New Brunswick: relation to patient outcomes. CMAJ. 1995;152(8):1227–34.
Clark AW, Durkin MJ, Olsen MA, Keller M, Ma Y, O’Neil CA, et al. Rural–urban differences in antibiotic prescribing for uncomplicated urinary tract infection. Infect Control Hosp Epidemiol. 2021;42(12):1437–44.
Wettermark B, Pehrsson A, Juhasz-Haverinen M, Veg A, Edlert M, Törnwall-Bergendahl G, et al. Financial incentives linked to self-assessment of prescribing patterns: a new approach for quality improvement of drug prescribing in primary care. Qual Prim Care. 2009;17(3):179–89.
Crowe S, Tully MP, Cantrill JA. The prescribing of specialist medicines: what factors influence GPs’ decision making? Fam Pract. 2009;26(4):301–8.
Figueiras A, Caamaño F, Gestal-Otero JJ. Influence of physician’s education, drug information and medical-care settings on the quality of drugs prescribed. Eur J Clin Pharmacol. 2000;56(9–10):747–53.
Beers MH, Fingold SF, Ouslander JG, Reuben DB, Morgenstern H, Beck JC. Characteristics and Quality of Prescribing by Doctors Practicing in Nursing Homes. J Am Geriatr Soc. 1993;41(8):802–7.
Pohontsch NJ, Heser K, Löffler A, Haenisch B, Parker D, Luck T, et al. General practitioners’ views on (long-term) prescription and use of problematic and potentially inappropriate medication for oldest-old patients—A qualitative interview study with GPs (CIM-TRIAD study). BMC Fam Pract. 2017;17(18):22.
Southern DA, Quan H, Ghali WA. Comparison of the Elixhauser and Charlson/Deyo methods of comorbidity measurement in administrative data. Med Care. 2004;42(4):355–60.
Gandré C, Beauguitte L, Lolivier A, Coldefy M. Care coordination for severe mental health disorders: an analysis of healthcare provider patient-sharing networks and their association with quality of care in a French region. BMC Health Serv Res. 2020;20(1):548.
Flemming R, Schüttig W, Ng F, Leve V, Sundmacher L. Using social network analysis methods to identify networks of physicians responsible for the care of specific patient populations. BMC Health Serv Res. 2022;22(1):462.
Arnold C, Koetsenruijter J, Forstner J, Peters-Klimm F, Wensing M. Influence of physician networks on prescribing a new ingredient combination in heart failure: a longitudinal claim data-based study. Implement Sci. 2021;16(1):84.
Soumerai SB, Avorn J. Predictors of Physician Prescribing Change in an Educational Experiment to Improve Medication Use. Med Care. 1987;25(3):210–21.
Granovetter M. Economic Action and Social Structure: The Problem of Embeddedness. Am J Sociol. 1985;91(3):481–510.
Granovetter MS, Swedberg R. The sociology of economic life. 2018. Available from: http://search.ebscohost.com/login.aspx?direct=true&scope=site&db=nlebk&db=nlabk&AN=1815212. [cited 2021 Sep 29].
BURT RS. Structural Holes: The Social Structure of Competition. Harvard University Press; 1992. Available from: https://www.jstor.org/stable/j.ctv1kz4h78. [cited 2021 Sep 29].
Coleman JS. Social Capital in the Creation of Human Capital. Am J Sociol. 1988;94:S95-120.
Portes A. Social Capital: Its Origins and Applications in Modern Sociology. Ann Rev Sociol. 1998;24:1–24.
Burt RS. Toward a structural theory of action: network models of social structure, perception, and action. New York: Academic Press; 1982. 381 p. (Quantitative studies in social relations).
Sparrowe R, Liden R. Process and Structure in Leader-Member Exchange. Acad Manag Rev. 1997;1(22):522.
Mascia D, Cicchetti A, Damiani G. “Us and Them”: a social network analysis of physicians’ professional networks and their attitudes towards EBM. BMC Health Serv Res. 2013;13(1):429.
Burt RS. Social Contagion and Innovation: Cohesion versus Structural Equivalence. Am J Sociol. 1987;92(6):1287–335.
Christakis NA, Fowler JH. Social contagion theory: examining dynamic social networks and human behavior. Stat Med. 2013 Feb 20;32(4). Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3830455/. [cited 2021 Jan 6].
Mohamed AA, Wiebe FA. Toward a Process Theory of Groupthink. Small Group Res. 1996;27(3):416–30.
Burt RS. Brokerage and closure: an introduction to social capital. 1. publ. in paperback. Oxford: Oxford Univ. Press; 2007. 279 p.
Sparrowe RT, Liden RC, Wayne SJ, Kraimer ML. Social Networks and the Performance of Individuals and Groups. Acad Manag J. 2001;44(2):316–25.
Scheuer CL, Voltan A, Kumanan K, Chakraborty S. Exploring the impact of decentralized leadership on knowledge sharing and work hindrance networks in healthcare teams. J Manag Organ. 2021;12:1–20.
Straßner C, Steinhäuser J, Freund T, Szecsenyi J, Wensing M. German healthcare professionals’ perspective on implementing recommendations about polypharmacy in general practice: a qualitative study. Fam Pract. 2018;35(4):503–10.
Grote-Westrick M. New German digital project paves the way for online access to personal electronic health records. The BMJ. 2021 Feb 18; Available from: https://blogs.bmj.com/bmj/2021/02/18/new-german-digital-project-paves-the-way-for-online-access-to-personal-electronic-health-records/. [cited 2022 Apr 6].
Wensing M, Broge B, Riens B, Kaufmann-Kolle P, Akkermans R, Grol R, et al. Quality circles to improve prescribing of primary care physicians Three comparative studies. Pharmacoepidem Drug Safe. 2009;18(9):763–9.
Poss-Doering R, Kamradt M, Glassen K, Andres E, Kaufmann-Kolle P, Wensing M. Promoting rational antibiotic prescribing for non-complicated infections: understanding social influence in primary care networks in Germany. BMC Fam Pract. 2020;21(1):51.
McGettigan P, Golden J, Fryer J, Chan R, Feely J. Prescribers prefer people: The sources of information used by doctors for prescribing suggest that the medium is more important than the message. Br J Clin Pharmacol. 2001;51(2):184–9.
May L, Gudger G, Armstrong P, Brooks G, Hinds P, Bhat R, et al. Multisite Exploration of Clinical Decision Making for Antibiotic Use by Emergency Medicine Providers Using Quantitative and Qualitative Methods. Infect Control Hosp Epidemiol. 2014;35(9):1114–25.
Hammer A, Arah OA, DerSarkissian M, Thompson CA, Mannion R, Wagner C, et al. The Relationship between Social Capital and Quality Management Systems in European Hospitals: A Quantitative Study. PLoS ONE. 2013;8(12):e85662.
Hammer A, Arah OA, Mannion R, Groene O, Sunol R, Pfaff H, et al. Measuring social capital of hospital management boards in European hospitals: A validation study on psychometric properties of a questionnaire for Chief Executive Officers. BMC Health Serv Res. 2021;21(1):1036.
Onnela JP, O’Malley AJ, Keating NL, Landon BE. Comparison of physician networks constructed from thresholded ties versus shared clinical episodes. Appl Netw Sci. 2018;3(1):28.
Lieb K, Scheurich A. Contact between Doctors and the Pharmaceutical Industry, Their Perceptions, and the Effects on Prescribing Habits. PLoS ONE. 2014;9(10):e110130.
Spurling GK, Mansfield PR, Montgomery BD, Lexchin J, Doust J, Othman N, et al. Information from pharmaceutical companies and the quality, quantity, and cost of physicians’ prescribing: a systematic review. PLoS Med. 2010;7(10):e1000352.
Acknowledgements
The authors would like to thank Dr. Derek van Pel for their constructive feedback in advancing this work. Further, we would like to thank Pascal Wendel and Andre Rabenberg for their support in data preparation and handling. We acknowledge financial support from the Open Access Publication Fund of Universität Hamburg.
Funding
Open Access funding enabled and organized by Projekt DEAL. Financial support for this study was provided entirely by the European Union’s Horizon 2020 research and innovation programme under grant agreement 765141. This presentation reflects only the authors’ view, and the European commission is not responsible for any use that may be made of the information it contains. The funding agreement ensured the authors’ independence in designing the study, interpreting the data, writing, and publishing the report.
Author information
Authors and Affiliations
Contributions
SYW conducted the initial literature review, study conception, study design, analysis, and drafted the manuscript. NL and OG provided critical appraisal of analysis and interpretation of study results. All authors critically read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This manuscript does not contain studies involving human participants, or animals. Thus, informed consent for human participation is not required.
In Germany, the use of social health insurance claims data for scientific purposes is regulated by Code of Social Law (Sozialgesetzbuch or SGB) ensuring compliance with data protection laws including the General Data Protection Regulation (GDPR) and Germany’s Federal Data Protection Act (BDSG). Specifically, the SGB V (Fifth Book of Social Code) includes provisions for health insurance data to be used for scientific purposes when pseudonymized or anonymized. Data acquisition, use, and subsequent analysis was performed within the framework of a contractual agreement between OptiMedis AG and the respective health insurance funds with approval from the data protection officer.
The principles outlined in the Declaration of Helsinki was strictly observed.
All study procedures were conducted in accordance with Sect. 67b-3 of Book X of the German Code of Social Law (§67b SGB X). Access to databases used in this project was authorized by a data use and sharing agreement with the relevant social health insurance. As data used in this research to identify networks was pseudonymized, patients and office-based physicians do not need to be asked for written and informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, S.Y., Larrain, N. & Groene, O. Can peer effects explain prescribing appropriateness? a social network analysis. BMC Med Res Methodol 23, 252 (2023). https://doi.org/10.1186/s12874-023-02048-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12874-023-02048-7