Abstract
Background
Ischemia/reperfusion injury contributes to periprocedural myocardial injury (PMI) in patients undergoing percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG). PMI can be estimated by the elevation of troponin (Tn) and creatine kinase-MB (CKMB) plasma levels, and it is associated with increased risk of cardiovascular events and mortality. Vitamin C might have a beneficial effect on PMI by improving endothelial function, improving myocardial perfusion, and by reducing oxidative stress generated during/after reperfusion. In several small animal models of cardiac stress, vitamin C reduced the increase in Tn and CKMB levels. The aim of this meta-analysis was to investigate whether vitamin C administration may have an effect on Tn and CKMB levels in patients undergoing PCI or CABG.
Methods
We searched PubMed, Cochrane, Embase and Scopus databases for controlled clinical trials reporting on Tn and CKMB levels in adult patients who underwent PCI or CABG and received vitamin C. As secondary outcomes we collected data on biomarkers of oxidative stress in the included trials. In our meta-analysis, we used the relative scale and estimated the effect as the ratio of means.
Results
We found seven controlled trials which included 872 patients. All included trials administered vitamin C intravenously, with a range from 1 to 16 g/day, and all initiated vitamin administration prior to the procedure. Vitamin C decreased peak Tn plasma levels in four trials on average by 43% (95% CI: 13 to 63%, p = 0.01) and peak CKMB plasma levels in five trials by 14% (95% CI: 8 to 21%, p < 0.001). Vitamin C also significantly decreased the biomarkers of oxidative stress.
Conclusions
Vitamin C may decrease cardiac enzyme levels in patients undergoing elective PCI or CABG. This may be explained partially by its antioxidant effects. Our findings encourage further research on vitamin C administration during cardiac procedures and in other clinical contexts that increase the level of cardiac enzymes. Future studies should search for an optimal dosing regimen, taking baseline and follow-up plasma vitamin C levels into account.
Similar content being viewed by others
Introduction
In patients with acute myocardial infarction (MI) or angina pectoris treated with percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG), ischemia/reperfusion injury contributes to periprocedural myocardial injury (PMI). It has been estimated that PMI occurs in up to 30–40% of the patients treated with PCI [1,2,3] and CABG [4], and is associated with increased risk of cardiovascular events and mortality [2,3,4,5,6,7]. The extent of PMI can be estimated by the elevation of the levels of cardiac enzymes such as creatine kinase-MB (CKMB), though troponin (Tn) is nowadays preferable as it is more sensitive and specific [2,3,4].
In several small animal models of cardiac toxicity, vitamin C administration attenuated the rise of troponin and CKMB [8,9,10]. In addition, vitamin C reduced infarct size in animal studies when administered alone [11], in its oxidized form, as dehydroascorbic acid [12], and when combined with vitamin E [13]. These pre-clinical findings suggest that in cardiac stress vitamin C might also have protective effects on the myocardium in humans. Vitamin C plays an important role in preserving endothelial function [14]. As an indication of clinical relevance of such biochemical effects, vitamin C improved endothelial function in atherosclerotic and heart failure patients [15]. Moreover, vitamin C improved the perfusion of the myocardium in patients undergoing PCI and people exposed to hyperoxia [16,17,18]. Recent meta-analyses found that vitamin C improved left ventricular ejection fraction (LVEF) in cardiac and non-cardiac patients [19], and prevented post-operative atrial fibrillation (POAF) in high risk patients in trials carried out outside of the USA [20].
One of the factors that contributes to ischemia/reperfusion injury is the increase in reactive oxygen species (ROS) [21], mostly arising directly after reperfusion [22]. Oxidative stress may cause reversible or irreversible injury to proteins, lipids and DNA [23]. Decreased plasma vitamin C concentrations are often reported after MI and cardiac surgery, accompanying the increase in oxidative stress [24,25,26], which indicates that the consumption of vitamin C is increased. As a result, the decreased vitamin C levels might impair its pleiotropic effects [27] and may further be harmful as the protection against the remaining oxidative stress can become inadequate. The potential antioxidant effects on PMI should be investigated further [28].
The aim of this meta-analysis was to investigate whether vitamin C administration may have an effect on Tn and CKMB levels in patients undergoing PCI or CABG.
Methods
Selection criteria for studies
We included controlled clinical trials investigating the effect of vitamin C on Tn and CKMB levels in adult patients who underwent PCI or CABG. We did not restrict to randomized trials. We restricted to trials in which vitamin C was the only difference between the trial groups. Administration of placebo to the control group was not required. We included both oral and intravenous vitamin C administration. We did not set limits on the duration of vitamin C administration.
Search strategy
PubMed, Cochrane, Embase and Scopus were searched for eligible studies published up to August 9th 2023. The search consisted of the terms vitamin C, troponin and CKMB (see Additional File 1 for the search terms). Moreover, the references of included studies and relevant reviews were checked for additional studies.
Outcomes
The primary outcomes of our analysis are peak plasma levels of Tn and CKMB. As secondary outcomes we collected data about biomarkers of oxidative stress.
Selection of studies and data extraction
We excluded duplicates through Rayyan software. Two independent investigators (SR and MvB) screened each study for eligibility against the inclusion criteria. When title or abstract suggested a paper might meet inclusion criteria, the full text of these potentially eligible studies was retrieved and reviewed. Any disagreement concerning the eligibility of particular studies was discussed with a third reviewer (AdM).
Quality assessment of the trials
The quality of the included studies was evaluated by two reviewers (SR and HH) using the criteria outlined in the Cochrane Handbook for Systemic Reviews of Interventions [29]. The following criteria were assessed: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data and selective reporting. Disagreements were resolved by consensus. Each quality entry was judged as low, unclear or high risk of bias.
Statistical methods
In our statistical analysis, we used the relative scale and calculated the ratio of means (RoM) as the measure of effect [30]. There was substantial variation in the timing of Tn and CKMB measurements between the studies, see Table 1. If several time points were available, we chose the peak levels in both groups, regardless of the time point at which the peak level was measured. If vitamin C has an effect on the cardiac enzyme levels, it is possible that the timing of peak is changed together with the height of the peak levels. Therefore, selecting the same time point from curves may not be the best comparison.
Additional file 2 (spreadsheet) shows our calculations. Two trials that were included in the meta-analysis did not report the mean and standard deviation (SD) values [16, 31]. We estimated the mean from the reported median and interquartile range [IQR] [32], see Additional file 1 and 2. We used the reported p-values to calculate the standard error (SE) for the log(RoM) for these two trials [16, 31]. In the Oktar trial [33] there were 2 different groups of patients receiving vitamin C. In group II, vitamin C was administered intravenously prior to the induction of anesthesia and in group III, vitamin C was added to the cardioplegic solution. In our analysis, we used the patients receiving intravenous vitamin C (group II) as the intervention group, because vitamin C was initiated earlier and there was more time for the vitamin to distribute in the tissues before the operation. As a sensitivity analysis, we also carried out the meta-analysis with the vitamin C group III, but the result did not differ considerably, see Additional file 1. The CKMB plasma levels of the Dingchao trial [34] were measured from a published figure by using a graphics program; see Additional File 2. In our analyses, we concluded that the dispersion parameter of the CKMB levels in the Oktar and Dingchao trial was SE, and not SD as was reported, see Additional file 1 and 2.
We pooled the included trials with the metagen function of the R package meta [35, 36]. We used the inverse variance fixed effect options. Our calculations are shown in Additional file 1.
Results
Description of the included trials
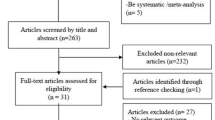
We found 11 potentially eligible trials on the effect of vitamin C on plasma levels of Tn and/or CKMB in patients at risk for PMI (Fig. 1). Four trials were excluded due to suspected data duplication [37, 38], unclear troponin-related outcome [39] and poor data reporting in general [40,41,42,43]. Seven trials were included in the meta-analysis of which four trials reported the changes of TnI and five of CKMB (Table 1). A detailed description of all included trials and a summary of the problems of the excluded trials is available in Additional file 1.
PRISMA flow diagram of the searches. Search terms are described in Additional file 1
In total 872 patients were included in the seven trials. In six trials patients underwent elective PCI (N = 588) or CABG (N = 199). In one trial, the type of cardiac surgery was unknown of 85 patients who underwent cardiopulmonary bypass (CPB) [34]. The mean age of the patients ranged from 56 to 67 years. The doses of vitamin C ranged from 1 to 16 g/day. All seven included trials administered vitamin C intravenously (i.v.) and vitamin C administration was initiated prior to the procedure. The duration of vitamin C administration was one day in six trials, and six days in one trial [44]. The first day was the day of the procedure. The timing of measuring plasma levels of troponin and CKMB was substantially different among the trials (Table 1). Two trials measured cardiac enzymes at more time points than were reported [16, 31].
The risk of bias assessment of the included studies is shown in Fig. 2. Five trials were randomized. One randomized trial [45] and two other trials [33, 34] did not describe the method of allocation. However, Oktar [33] and Dingchao [34] both showed that the groups were balanced for baseline characteristics, and for baseline CKMB and malondialdehyde (MDA) levels. In these three trials there was no information about the blinding of allocation stage, treatment and outcome assessment [33, 34, 45]. However, since our primary outcome is a laboratory measure, we judged that there is low risk of performance and detection bias caused by possible lack of blinding [46].
Risk of bias summary. Review authors’ judgements about each risk of bias item for each included study. A green plus sign (+) indicates ‘low risk’ for bias. A question mark (?) indicates ‘some concerns’ for bias or that conclusions are unable to be drawn regarding potential bias. The reference numbers to the trials are shown in Table 1. In Additional File 1 the support for the assessments can be found
Effect of vitamin C on cardiac enzyme levels
In our statistical analysis, we used the relative scale and calculated the ratio of means (RoM) [30] as the measure of effect. For example, in the vitamin C group of the Oktar trial [33], the peak CKMB level in the vitamin C group was 64 U/L after CABG, and in the control group 89.33 U/L. As a result, the RoM is 0.72 (64/89.33), which corresponds to a 28% lower peak level of CKMB in the vitamin C group.
In a group of four trials, vitamin C significantly decreased peak TnI plasma levels on average by 43% (95% CI: 13 to 63%, p = 0.01), see Fig. 3. One of the troponin trials found a significant effect within the trial. There was no substantial heterogeneity between the trials (p = 0.2). In a group of five trials, vitamin C significantly decreased CKMB plasma levels on average by 14% (95% CI: 8 to 21%, p < 0.001). Three of the CKMB studies found a significant effect within the trial. There was no substantial heterogeneity between the trials also for this outcome (p = 0.2). The Wang trial has by far the greatest weight in the CKMB analysis since it has 532 participants. In contrast, the total number of participants in the four other CKMB trials in Fig. 3 is only 179. Therefore, we carried out a sensitivity analysis in which we excluded the Wang trial. The pooled effect of vitamin C on CKMB level remained significant: p = 0.0085 (Additional File 1). The diagnosis in the Dingchao trial was not described and we carried out a sensitivity analysis in which we excluded that trial. The pooled effect of vitamin C on CKMB level remained significant: p = 0.0001 (Additional File 1).
Effect of vitamin C on cardiac enzyme levels. The upper model shows the effect of vitamin C on Tn and the lower model shows the effect of vitamin C on CKMB. The effect of vitamin C is presented as the RoM with 95% CI. The horizontal lines indicate the 95% CI ranges and the blue squares indicate the point estimate of the effect in that particular trial. Treatment effect (TE) indicates ln(RoM). The size of the blue square reflects the weight of the trial in the meta-analysis. The diamond indicates the pooled effect and 95% CI for the Tn and CKMB subgroup. See Additional File 1 for the description of the trials and calculations
Comparison of timing and vitamin C plasma levels in the Oktar trial
Oktar reported two groups of patients to whom vitamin C was administered using different protocols. Group II received intravenous vitamin C just before the induction of anesthesia, and group III received the vitamin in the cardioplegic solution. The achieved peak plasma vitamin C concentrations were quite similar but the timing of the vitamin C peak was later in group III [33].
We analyzed the time pattern of the CKMB levels in the Oktar et al. trial (Fig. 4). Compared with the control group, the CKMB levels in the two vitamin C groups are consistently lower over the follow-up period (Fig. 4A). In the vitamin C group II, who received vitamin C earlier, the peak CKMB plasma level was lower and at a later timepoint compared with vitamin C group III. We also compared both vitamin C groups against the control group. In the early-vitamin C group II, there were four time points after the operation at which the CKMB levels were significantly lower compared with the control group (Fig. 4B). In the late vitamin C group III, there was only one time point after the operation at which the CKMB levels were significantly lower than the control group (Fig. 4C). Although this indicates that there is stronger evidence of benefit from the earlier vitamin C administration, the confidence intervals of the two vitamin C groups are extensively overlapping and thus the difference between the two administration methods has not been demonstrated. Nevertheless, the difference gives more motivation for further research on the effects of timing.
Effect of vitamin C on CKMB levels in two vitamin C groups in Oktar et al. [33]. A. Course of CKMB plasma level in early-vitamin C group II (red dashed line), late-vitamin C group III (blue dotted line) and control (black continuous line). Effect of vitamin C on CKMB plasma levels in group II (B.) and group III (C.) versus control. Abbreviations: I: Induction; Pr: PreCPB; A: After declamping; Po: PostCPB; S: Skin closure
Effect of vitamin C on biomarkers of oxidative stress
Basili et al. [16] reported that 1 h after balloon inflation the serum level of 8-OHdg, an oxidative stress marker, was decreased in the vitamin C group by 38% (95% CI 26–48%) (Table 2). Wang et al. [31] reported that at 6–8 h after PCI, the 8-OHdG serum level was decreased in the vitamin C group by 41% (95% CI 38–45%) (Table 2). Basili also reported a 63% (95% CI 39–78%) decrease in the level of another oxidative stress marker, 8-iso-PGF2α.
A third marker for oxidative stress that has been measured in the included trials is MDA. Dingchao et al. [34], Oktar [33] and Demirag [45] found significantly lower levels of MDA after the operation in the vitamin C group compared to the respective control group. Dingchao [34] and Oktar [33] measured the MDA levels at multiple time points after the procedure and found significantly lower MDA levels in the vitamin C group until 9 h and 1 day postoperative, respectively.
Discussion
In this meta-analysis we found that on average vitamin C reduced post-operative Tn plasma levels by 43% and CKMB plasma levels by 14% in patients who underwent elective PCI or CABG. The significant reduction in cardiac enzyme levels indicate a parallel reduction in PMI.
There are multiple ways in which vitamin C may exert cardioprotective effects [27]. Vitamin C can directly scavenge harmful oxidative stress, restore other antioxidants and antioxidant enzymes, and decrease ROS production [47]. Vitamin C significantly lowered biomarkers of oxidative stress in all included trials that reported them [16, 31, 33, 34, 45], but further research is needed on oxidative stress biomarkers and PMI [28]. Vitamin C is consumed when there is overwhelming oxidative stress. Furthermore, when recycling of vitamin C is insufficient, its level may decrease systemically or locally, thereby impairing the pleotropic effects of vitamin C [27]. Vitamin C may further exert a cardioprotective effect by preserving endothelial function as has been found in multiple clinical contexts [14, 15, 48,49,50]. There is also evidence suggesting that vitamin C can improve myocardial perfusion [16,17,18].
Some reports in the older literature suggested that vitamin C deficiency, scurvy, may be associated with chest pain and abnormal ECG changes, which were reversed after vitamin C administration [51,52,53]. These early observations also indicate that vitamin C has effects on heart functions. In a further early study, vitamin C was effective in reducing creatine kinase levels in patients undergoing CABG with > 50 min ischemic time, whereas the vitamin had no effect on patients with < 50 min ischemic time [54].
In type II diabetic patients with cardiovascular risk and in surgical patients with limb ischemia/reperfusion injury vitamin C administration significantly reduced Tn levels compared to control [55, 56]. A recent trial, however, found no benefit of vitamin C and N-acetylcysteine versus placebo on post-operative myocardial injury in patients undergoing major non-cardiac surgery [57]. Importantly, median Tn level was low in the placebo group. Thus, the lack of effect of vitamin C in this non-cardiac trial might be explained simply by the very low level of cardiac stress. In our study, we investigated the effects of vitamin C in the setting of PCI and CABG, two conditions that uniformly cause cardiac stress and substantial increase cardiac enzyme levels.
In the large PHS-II trial, long-term daily supplementation of 0.5 g/day vitamin C did not reduce the risk of major cardiovascular events [58]. However, the population consisted of well-nourished physicians without prevalent cardiovascular disease. Therefore, the context was very different from the trials included in our analysis. The findings of the PHS-II trial are not a relevant comparison for findings with PCI and CABG patients.
Previous studies have shown that PMI is associated with the incidence of adverse outcomes and the risk of death after PCI and CABG [2,3,4,5,6,7, 59]. Therefore, reducing prognostically important PMI, especially in high risk patients, might improve patient outcome, e.g. by reducing Type 4–5 myocardial infarction or death [2,3,4]. Three of the included trials also reported beneficial effects of vitamin C on cardiac function [16, 34, 60]. In addition, a recent meta-analysis found a beneficial effect of vitamin C on LVEF in cardiac and non-cardiac patients [19]. A meta-regression analysis in that study found a highly significant relationship between the baseline LVEF level and the size of effect by vitamin C [19]. Vitamin C had no effect for people with high baseline LVEF level, while the effect was progressively larger with lower LVEF levels. Another meta-analysis in cardiac surgery patients found that vitamin C shortened the length of hospital stay, ICU stay and the occurrence of POAF [20]. However, there was significant heterogeneity in the findings as the benefits were only found in non-United States trials [20]. In our current analysis, all included trials were carried out outside of the USA, and therefore we could not carry out a similar comparison.
The effect of vitamin C may depend on dose, timing, route of administration and the total duration of treatment. In the included trials vitamin C dose ranged from 1 to 16 g at the day of surgery, but no dose-effect was evident. All trials administered vitamin C prior to, and some during, the procedure. One trial had a prolonged treatment for 6 days, however TnI was measured at 18 h [44]. The data from the trial by Oktar et al. suggests that early administration of vitamin C, prior to the procedure, is more beneficial compared to during the procedure (Fig. 4), though this requires further study. Also the optimal duration of vitamin C therapy remains to be investigated as it appears that oxidative stress is increased for multiple days post-operatively [26]. None of the trials administered vitamin C orally, so we could not compare oral and intravenous administration. Absorption of oral doses become saturated at about 3 g/day, whereas i.v. administration can lead to over 100 times higher plasma concentrations [61, 62]. Nevertheless, in the context of PCI and CABG, intravenous administration is convenient as all patients have i.v. routes available because of the procedures. Finally, measuring plasma vitamin C concentrations would add valuable information about size of effect by baseline and follow-up vitamin C status [63, 64].
In addition to differences in dosing regimens, treatment/clinical settings also differed among the included trials. In two trials patients were scheduled for PCI, whereas in five trials patients underwent cardiac surgery. Cardiac surgery may generate higher levels of ROS due to extracorporeal circulation compared to PCI. The size of the effect by vitamin C may differ between the two clinical contexts, but the included trials were too small to compare the two contexts. Oxidative stress parameters such as the static oxidation-reduction potential (sORP) can be measured quickly to quantify the amount of oxidative stress that is generated in different settings [64, 65], and may be used in future research to explore the relation between oxidative stress and PMI [28].
No side effects of vitamin C administration were reported in the included trials. Previous evidence also indicates that both oral and intravenous vitamin C administrations are remarkably safe [66, 67]. The US nutritional recommendations monograph considers various proposed harms (e.g. kidney stone formation, increased oxygen demand and pro-oxidant effects) of high doses of vitamin C, but concluded that the great majority of them are unsubstantiated [66]. Nevertheless, high dose vitamin C may be harmful for the kidneys, but only when administered for a longer period of time or at an extremely high dose. The US recommendations stated that 2 g/day is safe for ordinary people, yet encourages research of higher doses in the contexts of controlled trials [66].
The recently published LOVIT-trial investigated the effect of 4-day vitamin C administration in septic patients [68]. Persistent organ dysfunction and mortality were increased in the vitamin C group. However, the harm occurred after vitamin C was stopped, and not during the vitamin C administration. Therefore, the harm is explained by the rebound effect, and does not indicate harm of ongoing vitamin C administration [69].
Limitations
The diverse dosing regimens and differences in treatment/clinical settings among the included trials impact the generalizability of the overall conclusions. In addition, the number of included trials is small. Therefore, the evidence we provide does not allow practical conclusions at clinical level. Our goal was to figure out whether the limited number of trials encourage further research and our positive conclusion is not limited by the heterogeneity in dosing regimens and clinical contexts. The blinding of treatment and outcome measures was not described in three included trials [33, 34, 45], but we do not consider that such objective laboratory measures as Tn and CKMB could be meaningfully influenced by the knowledge about the intervention [46]. Therefore, we judged that performance and detection bias were low also for these three trials. Finally, concerns about blinding do not influence our conclusion that this topic should be investigated further. The number of studies and participants was small; however, small size can lead to a false negative finding, but cannot lead to a false positive finding.
Conclusion
Our study indicates that vitamin C may decrease cardiac enzyme levels in patients undergoing elective PCI or cardiac surgery. Biomarkers of oxidative stress were decreased by vitamin C, suggesting that the effect of vitamin C in the cardiac tissue may be explained, at least in part, through its effect as an antioxidant. The effects of vitamin C administration should be further investigated in cardiac procedures and other clinical conditions in which the levels of cardiac enzymes are increased. Future studies should also search for an optimal dosing regimen, taking baseline and follow-up plasma vitamin C levels into account.
Data availability
All extracted data and calculations are published in the Additional files.
Abbreviations
- 8-iso-PGF2α :
-
8-iso-prostaglandin F(2alpha)
- 8-OHdG:
-
8-hydroxy-2’-deoxyguanosine
- CABG:
-
coronary artery bypass graft
- CKMB:
-
creatine-kinase MB
- CPB:
-
cardiopulmonary bypass
- i.v.:
-
intravenously
- IQR:
-
interquartile range
- LVEF:
-
left ventricular ejection fraction
- MDA:
-
malondialdehyde
- MI:
-
myocardial infarction
- PCI:
-
percutaneous coronary intervention
- PMI:
-
periprocedural myocardial injury
- POAF:
-
post-operative atrial fibrillation
- RoM:
-
ratio of means
- ROS:
-
reactive oxygen species
- SD:
-
standard deviation
- SE:
-
standard error
- sORP:
-
static oxidation-reduction potential
- Tn:
-
troponin
References
Zhou Y, Chen Z, Ma J, Chen A, Lu D, Wu Y, et al. Incidence, predictors and clinical significance of periprocedural myocardial injury in patients undergoing elective percutaneous coronary intervention. J Cardiol. 2020;76(3):309–16.
Zeitouni M, Silvain J, Guedeney P, Kerneis M, Yan Y, Overtchouk P, et al. Periprocedural myocardial infarction and injury in elective coronary stenting. Eur Heart J. 2018;39(13):1100–9.
Bulluck H, Paradies V, Barbato E, Baumbach A, Bøtker HE, Capodanno D, et al. Prognostically relevant periprocedural myocardial injury and infarction associated with percutaneous coronary interventions: a Consensus Document of the ESC Working Group on Cellular Biology of the Heart and European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J. 2021;42(27):2630–42.
Thielmann M, Sharma V, Al-Attar N, Bulluck H, Bisleri G, Bunge J, et al. ESC Joint Working Groups on Cardiovascular surgery and the Cellular Biology of the heart position paper: Perioperative myocardial injury and infarction in patients undergoing coronary artery bypass graft surgery. Eur Heart J. 2017;38(31):2392–407.
Prasad A, Herrmann J. Myocardial infarction due to percutaneous coronary intervention. N Engl J Med. 2011;364(5):453–64.
Tardiff BE, Califf RM, Tcheng JE, Lincoff AM, Sigmon KN, Harrington RA, et al. Clinical outcomes after detection of elevated cardiac enzymes in patients undergoing percutaneous intervention. IMPACT-II investigators. Integrilin (eptifibatide) to minimize platelet aggregation and coronary Thrombosis-II. J Am Coll Cardiol. 1999;33(1):88–96.
Roe MT, Mahaffey KW, Kilaru R, Alexander JH, Akkerhuis KM, Simoons ML, et al. Creatine kinase-MB elevation after percutaneous coronary intervention predicts adverse outcomes in patients with acute coronary syndromes. Eur Heart J. 2004;25(4):313–21.
Asci H, Saygin M, Yesilot S, Topsakal S, Cankara FN, Ozmen O, et al. Protective effects of aspirin and vitamin C against corn syrup consumption-induced cardiac damage through sirtuin-1 and HIF-1alpha pathway. Anatol J Cardiol. 2016;16(9):648–54.
Wahdan A, Shareef M. Study of the Protective effect of vitamin C on Monosodium Glutamate Induced Cardiotoxicity in Adult male albino rats. Ain Shams Journal of Forensic Medicine and Clinical Toxicology. 2016;27(2):49–56.
Abdel-Daim MM, Ghazy EW, Fayez M. Synergistic protective role of mirazid (Commiphora molmol) and ascorbic acid against tilmicosin-induced cardiotoxicity in mice. Can J Physiol Pharmacol. 2015;93(1):45–51.
Okazaki T, Otani H, Shimazu T, Yoshioka K, Fujita M, Iwasaka T. Ascorbic acid and N-acetyl cysteine prevent uncoupling of nitric oxide synthase and increase tolerance to ischemia/reperfusion injury in diabetic rat heart. Free Radic Res. 2011;45(10):1173–83.
Guaiquil VH, Golde DW, Beckles DL, Mascareno EJ, Siddiqui MA. Vitamin C inhibits hypoxia-induced damage and apoptotic signaling pathways in cardiomyocytes and ischemic hearts. Free Radic Biol Med. 2004;37(9):1419–29.
Mickle DA, Li RK, Weisel RD, Birnbaum PL, Wu TW, Jackowski G, et al. Myocardial salvage with trolox and ascorbic acid for an acute evolving infarction. Ann Thorac Surg. 1989;47(4):553–7.
May JM, Harrison FE. Role of vitamin C in the function of the vascular endothelium. Antioxid Redox Signal. 2013;19(17):2068–83.
Ashor AW, Lara J, Mathers JC, Siervo M. Effect of vitamin C on endothelial function in health and disease: a systematic review and meta-analysis of randomised controlled trials. Atherosclerosis. 2014;235(1):9–20.
Basili S, Tanzilli G, Mangieri E, Raparelli V, Di Santo S, Pignatelli P, et al. Intravenous ascorbic acid infusion improves myocardial perfusion grade during elective percutaneous coronary intervention: relationship with oxidative stress markers. JACC Cardiovasc Interv. 2010;3(2):221–9.
Gao Z, Spilk S, Momen A, Muller MD, Leuenberger UA, Sinoway LI. Vitamin C prevents hyperoxia-mediated coronary vasoconstriction and impairment of myocardial function in healthy subjects. Eur J Appl Physiol. 2012;112(2):483–92.
McNulty PH, Robertson BJ, Tulli MA, Hess J, Harach LA, Scott S, et al. Effect of hyperoxia and vitamin C on coronary blood flow in patients with ischemic heart disease. J Appl Physiol. 2007;102(5):2040–5.
Hemilä H, Chalker E, de Man AME. Vitamin C may improve left ventricular ejection fraction: a Meta-analysis. Front Cardiovasc Med. 2022;9:789729.
Hemilä H, Suonsyrjä T. Vitamin C for preventing atrial fibrillation in high risk patients: a systematic review and meta-analysis. BMC Cardiovasc Disord. 2017;17(1):49.
Cowled P, Fitridge R. Pathophysiology of Reperfusion Injury. In: Fitridge R, Thompson M, editors. Mechanisms of vascular disease: a reference book for vascular specialists [Internet]: Adelaide (AU). University of Adelaide Press; 2011.
Becker LB. New concepts in reactive oxygen species and cardiovascular reperfusion physiology. Cardiovasc Res. 2004;61(3):461–70.
Oudemans-van Straaten HM, Spoelstra-de Man AM, de Waard MC. Vitamin C revisited. Crit Care. 2014;18(4):460.
Bagatini MD, Martins CC, Battisti V, Gasparetto D, da Rosa CS, Spanevello RM, et al. Oxidative stress versus antioxidant defenses in patients with acute myocardial infarction. Heart Vessels. 2011;26(1):55–63.
Lassnigg A, Punz A, Barker R, Keznickl P, Manhart N, Roth E, et al. Influence of intravenous vitamin E supplementation in cardiac surgery on oxidative stress: a double-blinded, randomized, controlled study. Br J Anaesth. 2003;90(2):148–54.
Rodemeister S, Duquesne M, Adolph M, Nohr D, Biesalski HK, Unertl K. Massive and long-lasting decrease in vitamin C plasma levels as a consequence of extracorporeal circulation. Nutrition. 2014;30(6):673–8.
Spoelstra-de Man AME, Elbers PWG, Oudemans-van Straaten HM. Making sense of early high-dose intravenous vitamin C in ischemia/reperfusion injury. Crit Care. 2018;22(1):70.
Delafontaine P, Anwar A. Vitamin C and percutaneous coronary intervention. JACC Cardiovasc Interventions. 2010;3(2):230–2.
Higgins JPT, Savović J, Page MJ, Elbers RG, Sterne JAC. Chapter 8: assessing risk of bias in a randomized trial 2021. Available from: www.training.cochrane.org/handbook.
Friedrich JO, Adhikari NKJ, Beyene J. Ratio of means for analyzing continuous outcomes in meta-analysis performed as well as mean difference methods. J Clin Epidemiol. 2011;64(5):556–64.
Wang ZJ, Hu WK, Liu YY, Shi DM, Cheng WJ, Guo YH, et al. The effect of intravenous vitamin C infusion on periprocedural myocardial injury for patients undergoing elective percutaneous coronary intervention. Can J Cardiol. 2014;30(1):96–101.
Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14(1):135.
Oktar GL, Sinci V, Kalaycioglu S, Soncul H, Gökgöz L, Halit V, et al. Biochemical and hemodynamic effects of ascorbic acid and alpha-tocopherol in coronary artery surgery. Scand J Clin Lab Invest. 2001;61(8):621–9.
Dingchao H, Zhiduan Q, Liye H, Xiaodong F. The protective effects of high-dose ascorbic acid on myocardium against reperfusion injury during and after cardiopulmonary bypass. Thorac Cardiovasc Surg. 1994;42(5):276–8.
Schwarzer G, Carpenter JR, Rucker G. Meta-analysis with R. London: Springer; 2015.
R Project for Statistical Computing. Available from: https://www.r-project.org.
Gültekin Y, Güzel A, Karakaya A, Beşoğul Y. Combined effects of the implementation of magnesium and ascorbic acid on myocardial ischemia-reperfusion in open heart surgery. Anatol Curr Med J. 2021;3(4):319–26.
Hemilä H. Gültekin (2021) paper has a substantial number of identical data with the 20-year earlier Demirag (2001) paper. Pubpeer 2022 Feb. Available from: https://pubpeer.com/publications/8150B66EC0289FA4BF7C9ADB1C48A9#1.
Rostami AR, Sharifi M, Kamali AR, Kalantari M. Evaluation of the effect of combination of N-Acetylcysteine and vitamin C on improving outcomes following CABG. J Arak Univ Med Sci. 2016;18(10):29–39.
Shafaei-Bajestani N, Talasaz AH, Salarifar M, Pourhosseini H, Sadri F, Jalali A. Potential role of vitamin C Intracoronary Administration in preventing Cardiac Injury after primary percutaneous coronary intervention in patients with ST-Elevation myocardial infarction. J Res Pharm Pract. 2019;8(2):75–82.
Moludi J, Alizadeh M, Chehri G, Jafari-Vayghyan H, Foroumandi E, Maleki V, et al. The effect of vitamin C supplementation on cardiac enzymes after coronary artery bypass graft: a double-blind Randomized Control Trial. Curr Nutr Food Sci. 2020;16(5):833–8.
Hemilä H. Statistical problems in the vitamin C trial with PCI patients by Shafaei-Bajestani. (2019). Pubpeer 2022 Feb. Available from: https://pubpeer.com/publications/1B59AD863839D55215841C1124C2D3#1.
Hemilä H. Statistical problems in the vitamin C trial with CABG patients by Moludi. (2020). Pubpeer 2022 Feb. Available from: https://pubpeer.com/publications/2E9E504061A7DC2AF6B2692D8632F1#1.
Antonic M, Lipovec R, Gregorcic F, Juric P, Kosir G. Perioperative ascorbic acid supplementation does not reduce theincidence of postoperative atrial fibrillation in on-pump coronary artery bypass graft patients. J Cardiol. 2017;69(1):98–102.
Demirag K, Askar FZ, Uyar M, Cevik A, Ozmen D, Mutaf I, et al. The protective effects of high dose ascorbic acid and diltiazem on myocardial ischaemia-reperfusion injury. Middle East J Anaesthesiol. 2001;16(1):67–79.
Wood L, Egger M, Gluud LL, Schulz KF, Jüni P, Altman DG, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta-epidemiological study. BMJ. 2008;336(7644):601.
Rodrigo R, Prieto JC, Aguayo R, Ramos C, Puentes Á, Gajardo A, et al. Joint cardioprotective effect of vitamin C and other antioxidants against Reperfusion Injury in patients with Acute Myocardial Infarction undergoing percutaneous coronary intervention. Molecules. 2021;26(18):5702.
Ellis GR, Anderson RA, Chirkov YY, Morris-Thurgood J, Jackson SK, Lewis MJ, et al. Acute effects of vitamin C on platelet responsiveness to nitric oxide donors and endothelial function in patients with chronic heart failure. J Cardiovasc Pharmacol. 2001;37(5):564–70.
Erbs S, Gielen S, Linke A, Möbius-Winkler S, Adams V, Baither Y, et al. Improvement of peripheral endothelial dysfunction by acute vitamin C application: different effects in patients with coronary artery disease, ischemic, and dilated cardiomyopathy. Am Heart J. 2003;146(2):280–5.
Hornig B, Arakawa N, Kohler C, Drexler H. Vitamin C improves endothelial function of conduit arteries in patients with chronic heart failure. Circulation. 1998;97(4):363–8.
Vitamin -C. Requirement of human adults experimental study of vitamin-C deprivation in man: a preliminary report by the vitamin C subcommittee of the accessory food factors committee, medical research council. The Lancet. 1948;251(6510):853–8.
Sament S. Cardiac disorders in scurvy. N Engl J Med. 1970;282(5):282–3.
Shafar J. Rapid reversion of electrocardiographic abnormalities after treatment in two cases of scurvy. The Lancet. 1967;290(7508):176–8.
Eddy L, Hurvitz R, Hochstein P. A protective role for ascorbate in induced ischemic arrest associated with cardiopulmonary bypass. J Appl Cardiol. 1990;5:409–14.
Devanandan P, Puvvada RC, Muthukumar VA. Effects of vitamin C supplementation on the glycemic control and cardiovascular risk in type II diabetes Mellitus. J Res Pharm. 2020;24(2):182–7.
Lee JY, Kim CJ, Chung MY. Effect of high-dose vitamin C on oxygen free radical production and myocardial enzyme after tourniquet ischaemia-reperfusion injury during bilateral total knee replacement. J Int Med Res. 2010;38(4):1519–29.
Holse C, Aasvang EK, Vester-Andersen M, Rasmussen LS, Wetterslev J, Christensen R, et al. Hyperoxia and Antioxidants for Myocardial Injury in Noncardiac Surgery: A 2 × 2 Factorial, Blinded, Randomized Clinical Trial. Anesthesiology. 2022;136(3):408–19.
Sesso HD, Buring JE, Christen WG, Kurth T, Belanger C, MacFadyen J, et al. Vitamins E and C in the prevention of cardiovascular disease in men: the Physicians’ Health Study II randomized controlled trial. JAMA. 2008;300(18):2123–33.
Ellis SG, Chew D, Chan A, Whitlow PL, Schneider JP, Topol EJ. Death following creatine kinase-MB elevation after coronary intervention: identification of an early risk period: importance of creatine kinase-MB level, completeness of revascularization, ventricular function, and probable benefit of statin therapy. Circulation. 2002;106(10):1205–10.
Emadi N, Nemati MH, Ghorbani M, Allahyari E. The effect of high-dose vitamin C on biochemical markers of myocardial Injury in Coronary artery bypass surgery. Braz J Cardiovasc Surg. 2019;34(5):517–24.
Levine M, Padayatty SJ, Espey MG. Vitamin C: a concentration-function approach yields pharmacology and therapeutic discoveries. Adv Nutr. 2011;2(2):78–88.
Padayatty SJ, Sun H, Wang Y, Riordan HD, Hewitt SM, Katz A, et al. Vitamin C pharmacokinetics: implications for oral and intravenous use. Ann Intern Med. 2004;140(7):533–7.
Padayatty SJ, Levine M, Vitamin C. The known and the unknown and Goldilocks. Oral Dis. 2016;22(6):463–93.
Rozemeijer S, van der Horst FAL, de Man AME. Measuring vitamin C in critically ill patients: clinical importance and practical difficulties-Is it time for a surrogate marker? Crit Care. 2021;25(1):310.
Rozemeijer S, Smit B, Elbers PWG, Girbes ARJ, Oudemans-van Straaten HM, de Man AME. Rapid screening of critically ill patients for low plasma vitamin C concentrations using a point-of-care oxidation–reduction potential measurement. Intensive Care Medicine Experimental. 2021;9(1):40.
Dietary Reference Intakes for, Vitamin C, Vitamin E, Selenium. and Carotenoids.: Washington (DC): National Academies Press (US); 2000 [5:Vitamin C]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK225480/.
Padayatty SJ, Sun AY, Chen Q, Espey MG, Drisko J, Levine M, Vitamin C. Intravenous use by complementary and alternative medicine practitioners and adverse Effects. PLoS ONE. 2010;5(7):e11414.
Lamontagne F, Masse M-H, Menard J, Sprague S, Pinto R, Heyland DK, et al. Intravenous vitamin C in adults with Sepsis in the Intensive Care Unit. N Engl J Med. 2022;386(25):2387–98.
Hemilä H, Chalker E. Abrupt termination of vitamin C from ICU patients may increase mortality: secondary analysis of the LOVIT trial. Eur J Clin Nutr. 2023;77(4):490–4.
Acknowledgements
Not applicable.
Funding
There were no sources of financial support for the review, nor were there funders or sponsors involved.
Author information
Authors and Affiliations
Contributions
S.R. and A.M.E.d.M. initiated the study. S.R., M.v.B. and H.H. performed the searches and screened the records. S.R. and H.H. performed the statistical analyses. S.R. prepared Figs. 1 and 2, and H.H. prepared Figs. 3 and 4. All authors wrote and reviewed the main manuscript text.
Corresponding author
Ethics declarations
Competing interests
Grant of the Netherlands Organisation for Health Research and Development for the VITaCCA trial (https://clinicaltrials.gov/ct2/show/NCT03509662) for A.M.E. de Man and S. Rozemeijer. The other authors do not have any competing interests.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Rozemeijer, S., Hemilä, H., van Baaren, M. et al. Vitamin C may reduce troponin and CKMB levels after PCI and CABG: a meta-analysis. BMC Cardiovasc Disord 23, 475 (2023). https://doi.org/10.1186/s12872-023-03459-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12872-023-03459-6