Abstract
Background
Inflammation has been implicated in the progressive exacerbation of valvular atrial fibrillation (VAF) and thrombogenesis. This study aimed to analyze the association of systemic inflammation as measured by six indices with left atrial thrombus (LAT) in patients with VAF.
Methods
This comparative cross-sectional analytical study included 434 patients with VAF. Logistic regression analysis was used to assess the predictive value of LAT using six inflammation indices: neutrophil-to-lymphocyte ratio, monocyte-to-lymphocyte ratio (MLR), white blood cell-to-mean platelet volume ratio, neutrophil-to-mean platelet volume ratio, systemic immune inflammation index, and systemic inflammation response index. Receiver operating characteristic curves were plotted, and the area under these curves (AUC) were calculated to evaluate the discriminative ability of the indices.
Results
Transesophageal echocardiography revealed LAT in 143 (32.9%) patients. All six indices reflected a positive correlation with C-reactive protein levels. Multivariate logistic analysis revealed that these indices were independent predictors of LAT, and MLR appeared to perform best (odds ratio 12.006 [95% confidence interval (CI) 3.404–42.347]; P < 0.001; AUC 0.639 [95% CI 0.583–0.694]; P < 0.001).
Conclusions
Selected inflammatory indices were significantly and independently associated with LAT among patients with VAF.
Similar content being viewed by others
Background
Atrial fibrillation (AF) is the most common type of cardiac arrhythmia and its health-related burden continues to be significant worldwide. The estimated prevalence of AF among adults is 2–4%, and it is expected to increase owing to extended longevity [1]. AF has been proved to an important contributor to stroke, resulting in substantial morbidity and mortality. Approximately, 30% of AF patients, who experience stroke, die within 1 year, and 15–30% of stroke survivors remain permanently disabled [2]. AF can be classified into “valvular AF (VAF)” and “nonvalvular AF (NVAF)” [1]. Compared with the latter, the former heralds in greater embolic risk. Patients with VAF who have experienced embolic events have recurrences at a rate of 15–40 events per 100 patient-months, which is the highest rate of thromboembolism ever reported in AF [3]. Notably, VAF related stroke occurs in a much younger population with consequent loss of human power and resultant economic burdens [4].
Inflammatory immune response is a determinant of initiation and progressive exacerbation of VAF. Degenerative remodeling, extensive fibrosis and evidence of ongoing inflammation has been found in the left atria of VAF patients by histopathological studies [5, 6]. It has been well recognized that the left atrial thrombus (LAT) is the primary cause of stroke in AF patients [7]. Chronic systemic inflammation and oxidative injury play a vital role in LAT formation in patients with AF, which may lead to endothelial dysfunction and a hypercoagulable state. Inflammatory biomarkers, such as C-reactive protein (CRP), were proved to be associated with the presence of LAT in patients with AF [8].
Several hematological indices, including neutrophil-to-lymphocyte ratio (NLR), monocyte-to-lymphocyte ratio (MLR), white blood cell-to-mean platelet volume ratio (WMR), neutrophil-to-mean platelet volume ratio (NMR), systemic immune inflammation index (SII), and system inflammation response index (SIRI), are also believed to reflect inflammation [9]. These leukocyte-derived indices integrate information from the innate and adaptive immunity to avoid relying on the absolute value of a single leukocyte subtype caused by infection or dehydration. Evidence from observational studies has demonstrated a significant association between these inflammation indices and the incidence and severity of many cardiovascular diseases, such as heart failure, myocardial infarction, and hypertension [10]. However, to date, no studies have investigated the predictive value of inflammation indices for LAT in VAF patients. The objective of this study was to determine the association of NLR, MLR, WMR, NMR, SII and SIRI with the risk of LAT formation in patients with VAF undergoing transesophageal echocardiography (TEE).
Methods
Study population
A total of 448 patients with documented VAF who underwent TEE at Henan Provincial People’s Hospital between January 2015 and September 2022 were retrospectively collected. VAF refers to AF patients with moderate/severe mitral stenosis and those with mechanical prosthetic heart valve(s) [1]. First-diagnosed AF was defined as AF not diagnosed previously, irrespective of its duration or the presence/severity of AF-related symptoms [1]. Patients with hematological malignancies, acute and/or chronic infection, or missing complete blood count data were excluded (n = 14). Ultimately, 434 participants were enrolled in the current study (Fig. 1). The study protocol was approved by the Ethics Committee of the Henan Provincial People’s Hospital. The need for informed consent was waived by the Ethics Committee of the Henan Provincial People’s Hospital, because of the retrospective nature of the study.
Data collection
Patients’ demographic characteristics, including age, gender, comorbidities (coronary heart disease, hypertension, heart failure, type of AF, diabetes, and stroke), echocardiographic parameters, laboratory variables, and medications (oral anticoagulants, beta-blockers, diuretics and renin-angiotensin system inhibitors) were obtained from patients’ medical records before TEE.
Biochemical analyses
Venous blood samples were collected from each patient after 12 h fasting on the day before TEE. All these tests were performed at the core laboratory of Henan Provincial People’s Hospital using standard techniques. Biochemical parameters were determined by Hitachi 7180 biochemistry autoanalyzer. The Cockcroft-Gault equation was used to estimate the glomerular filtration rate.
Calculation of inflammation indices
The inflammation indices were determined from the first blood test results after admission. The ratios were calculated using the following equations:
Echocardiographic data
TEE was performed by two qualified physicians to detect thrombus formation in the left atrium. LAT was defined as a well-circumscribed, highly reflective mass with a different texture from the atrial wall and uniform consistency [11]. The severity of mitral stenosis was evaluated using the peak and mean gradients obtained at the mitral inflow velocities using continuous wave Doppler ultrasound scanning from the apical view under transthoracic echocardiography before TEE. Mitral valve area was calculated using the pressure half-time method and planimetry of the mitral valve orifice in early diastole from the short-axis view. Patients with a valve area < 1.5 cm2 or a mean gradient > 5 mmHg were considered to have moderate/severe mitral stenosis. Left atrial diameter (LAD) and left ventricular ejection fraction (LVEF) were measured from M-mode or 2D view in the parasternal long-axis projection. Discrepancies were resolved through discussions or by an expert physician when needed.
Statistical analysis
Continuous variables were presented as the means ± standard deviations or medians (inter-quartile ranges). The Student’s t tests and Mann–Whitney U tests were used to compare normally and non-normally distributed variables, respectively. Categorical variables in each group, expressed as percentages, were compared using the χ2 test. Spearman test was used to evaluate the correlation between inflammation indices and CRP levels. Restricted cubic splines were used to assess the dose–response association between inflammation indices and the risk for LAT. Four knots were placed at the 5th, 35th, 65th, and 95th percentiles of inflammation indices. A univariate logistic regression model was used to assess the association between the inflammation indices and LAT, followed by multivariate adjustments. Model 1 was also adjusted for age and gender. Model 2 was additionally adjusted for thrombotic factors (coronary heart diseases, heart failure, hypertension, diabetes, stroke). Model 3 was adjusted for the factors in model 2 in addition to oral anticoagulants, LAD and LVEF. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated to express the risk. Receiver operating characteristic (ROC) curves were plotted to identify the cut-off values of inflammation indices that could be used to predict LAT. The area under the ROC curves (AUC) were calculated and compared pairwise. A value of P < 0.05 was considered significant in all conditions. Statistical analyses were performed using SPSS Statistics 26.0 (SPSS, Chicago, IL, USA) and R 4.1.2 (R Core Team, Vienna, Austria).
Results
Baseline characteristics
The mean age of the 434 patients was 56.94 ± 9.12 years, and only 36.2% were men. 32.7% of included patients received oral anticoagulant at admission. LAT was observed in 143 (32.9%) patients. Patients with LAT were more likely to be male, and had heart failure and diabetes, with larger LAD and higher CRP levels, than those without LAT. All the six inflammation indices were significantly higher in patients with LAT (Table 1).
Relationship between inflammation indices and CRP
In correlation analysis, the six inflammation indices were all positively correlated with CRP levels (P < 0.001, Fig. 2). NLR had the highest correlation coefficient (r = 0.447), whereas WMR had the lowest (r = 0.277).
Correlations between inflammation indices and CRP. NLR neutrophil/lymphocyte ratio, MLR monocyte/lymphocyte ratio, WMR white blood/mean platelet volume ratio, NMR neutrophil/mean platelet volume ratio, SII systemic immune inflammation index, SIRI system inflammation response index, CRP C-reactive protein
Dose–response association between inflammation indices and risk for LAT
To continuously assess the association of inflammation indices with the risk of LAT, dose–response curves were constructed (Fig. 3). Log-transformed NLR (P for non-linearity < 0.001), MLR (P for non-linearity < 0.001), NMR (P for non-linearity = 0.003) and SIRI (P for non-linearity < 0.001) all had a non-linear and positive correlation with the risk of LAT. The risk increased when the log-transformed NLR, MLR, NMR and SIRI were greater than 0.338, 0.619, 0.477 and 0.064, respectively. Linear and positive associations were found between log-transformed WMR (P for non-linearity = 0.129), SII (P for non-linearity = 0.126), and the risk of LAT. The risk increased when the log-transformed WMR and SII were above 0.255 and 2.610, respectively.
Dose–response curve of inflammation indices and risk of left atrial thrombus. NLR neutrophil/lymphocyte ratio, MLR monocyte/lymphocyte ratio, WMR white blood/mean platelet volume ratio, NMR neutrophil/mean platelet volume ratio, SII systemic immune inflammation index, SIRI system inflammation response index, OR odds ratio, CI confidence interval
Logistic regression analysis of the association between inflammation indices and LAT
Considering the possible covariance between the inflammation indices, separate logistic regression analyses were performed. As shown in Table 2, unadjusted logistic regression analyses revealed that all the six inflammation indices were associated with an increased rate of LAT. After adjusting for age and gender (model 1), inflammation indices maintained a significant association with LAT. The strength of this association was not attenuated after additional adjustment for thrombotic factors (coronary heart diseases, heart failure, hypertension, diabetes, and stroke; model 2). Finally, the significant association of all the six inflammation indices with the risk of LAT were still consistent after further adjustment for oral anticoagulants, LAD and LVEF (model 3).
Discriminative ability of inflammation indices
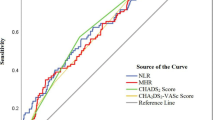
The comparisons among various inflammation indices for predicting LAT were summarized in Table 3. MLR had the highest AUC (0.639 [95% CI 0.583–0.694]; P < 0.001). The ROC curves for inflammation indices were presented in Fig. 4. According to pair-wise comparison of the AUCs, MLR appeared to perform better than the other five indices (Table 4).
Receiver operating characteristic curves for inflammation indices as a predictor of left atrial thrombus. NLR neutrophil/lymphocyte ratio, MLR monocyte/lymphocyte ratio, WMR white blood/mean platelet volume ratio, NMR neutrophil/mean platelet volume ratio, SII systemic immune inflammation index, SIRI system inflammation response index
Discussion
To our knowledge, the present study was the first to examine the predictive value of inflammation indices for LAT in patients with VAF. Our principal finding was that elevated inflammation indices were robustly associated with an increased risk for LAT. These indices could reveal the level of systemic inflammation due to their positive correlation with conventional inflammation biomarkers such as CRP. Systemic inflammation was one of the common determinants of VAF and thrombogenesis. Among the six inflammation indices, MLR yielded the highest AUC (0.639), confirming its predictive value. Additionally, the inflammation indices are extensively and readily obtainable at low cost in the laboratory and clinical fields, and VAF is largely limited to low- and middle- income countries, so these indices may be proposed as predictors for the incidence of LAT.
To date, there has been a paucity of information regarding the association between inflammation indices and LAT or stroke in patients with AF. These studies were limited to NVAF patients and the index of NLR. A study including 207 NVAF patients demonstrated that NLR was significantly and positively correlated with the CHA2DS2-VASc score and CRP, and a higher NLR was associated with a 3.872-fold risk for LAT [12]. Yalcin et al. [13] found a similar significant association in a larger sample, and reported that a higher NLR was an independent risk factor for LAT in 309 patients with NVAF (OR 1.59). Fukuda et al. [14] demonstrated that an elevated NLR was an independent risk factor for spontaneous echo contrast in TEE (OR 1.86) and had greater left atrial volume index, left atrial appendage area and left atrial appendage wall motion velocity during atrial contraction. This study revealed an association between NLR and the left atrial appendage function in relation to thrombogenesis. In a cohort study involving 981 patients with AF, NLR was also significantly associated with the first episode of stroke independent of the CHA2DS2-VASc score and this association had a dose–response pattern [15]. Similar to these previous studies, our study verified the predictive value of NLR for LAT formation in VAF patients. Moreover, we found that other inflammation indices were also independent risk factors for LAT and some were not worse than the NLR. Inflammation indices can be mutually complementary. NLR had a poor sensitivity for LAT (46.2%), while NMR had the best sensitivity (81.8%). A combination of inflammation indices may improve the accuracy of predicting LAT.
The presence of systemic inflammation in VAF patients was found by either histopathological examination of the left atrium or by measuring serum inflammatory biomarkers. In local cardiac tissue, VAF patients exhibited higher infiltration of neutrophils and resting stage dendritic cells, while those with sinus rhythm exhibited higher infiltration of follicular helper T cells [16]. Neutrophil-mediated inflammatory responses were mainly associated with neutrophil extracellular traps that recruited other inflammatory cells such as macrophages to amplify the inflammatory response and promoted collagen synthesis leading to fibrosis. Sharma et al. [17] found a progressive increase in the level of inflammatory biomarkers (CRP, interleukin‐6 and sCD‐40L) in rheumatic mitral stenosis patients with sinus rhythm, subclinical transient AF and chronic AF. CRP, interleukin‐6 and sCD‐40L had also been recognized as predictors of thromboembolic complications in AF patients in several studies [8, 18, 19]. Inflammatory cytokines had prothrombotic effects such as upregulation of tissue factors from monocyte-macrophages, increased fibrinogen expression, reduced expression of protein C and related proteins, and increased platelet reactivity. Inflammation also caused and accelerated the electrical and structural remodeling of the atria, resulting in ineffective irregular contraction and blood stasis. Blood stasis in the left atrium played a vital role in thrombogenic tendency among patients with AF. During inflammatory reactions, blood cell subtype counts and the balance between them changed. A combination of these cell counts might better reflect alterations in systemic inflammation. Inflammation indices in our study were all positively correlated with CRP; therefore, their levels also reflected the extent of systemic inflammation. Furthermore, it was reported that the NLR could be reduced by anti-inflammatory therapy using canakinumab [20]. Another promising pharmacological agent, sodium-glucose cotransporter 2 inhibitor, could also decrease the level of NLR by directly targeting inflammatory pathways such as nucleotide-binding domain-like receptor protein-3 inflammasome [21, 22]. Recent evidence suggested that inflammation indices could identify high-risk patients with thromboembolic diseases such as acute pulmonary embolism or peripheral arterial disease [23, 24]. Therefore, based on our study and findings from previous investigations, systemic inflammation might underlie the association between the indices examined in our study and LAT in VAF patients.
Our study had several limitations, the first of which was its retrospective design, which was not specifically constructed to assess the endpoints reported in this article. As such, prospective studies with larger sample sizes are required to confirm our results. In addition, many factors, such as smoking, alcohol, and mental stress, can induce chronic systemic inflammation; however, these data were not collected in our study. Finally, inflammatory indices were measured once at admission and subsequent temporal changes were not observed. Dynamic monitoring of these indices may provide additional information.
Conclusion
Elevated inflammatory indices were associated with an increased risk for LAT in patients with VAF. Because these indices are extensively used and readily available in the clinical field, we propose that they could be used as cost-effective predictors for thromboembolic risk, which would benefit a large subset of patients with VAF in developing countries.
Availability of data and materials
The datasets generated and analysed during the current study are not publicly available due to the Henan Provincial People’s Hospital regulations, but are available from the corresponding author on reasonable request.
Abbreviations
- AF:
-
Atrial fibrillation
- NVAF:
-
Non-valvular atrial fibrillation
- VAF:
-
Valvular atrial fibrillation
- LAT:
-
Left atrial thrombus
- CRP:
-
C-reactive protein
- NLR:
-
Neutrophil-to-lymphocyte ratio
- MLR:
-
Monocytes-to-lymphocyte ratio
- WMR:
-
White blood cell-to-mean platelet volume ratio
- NMR:
-
Neutrophil-to-mean platelet volume ratio
- SII:
-
Systemic immune inflammation index
- SIRI:
-
System inflammation response index
- TEE:
-
Transesophageal echocardiography
- LAD:
-
Left atrium diameter
- LVEF:
-
Left ventricular ejection fraction
- ROC:
-
Receiver operating characteristic curve
- AUC:
-
Area under the curve
- OR:
-
Odds ratio
- CI:
-
Confidence interval
References
Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomstrom-Lundqvist C, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the Diagnosis and Management of Atrial Fibrillation of the European Society of Cardiology (ESC) Developed with the Special Contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42:373–498.
Menke J, Lüthje L, Kastrup A, Larsen J. Thromboembolism in atrial fibrillation. Am J Cardiol. 2010;105:502–10.
De Caterina R, Camm AJ. What is “valvular” atrial fibrillation? A reappraisal. Eur Heart J. 2014;35(47):3328–35.
John B, Lau C-P. Atrial fibrillation in valvular heart disease. Card Electrophysiol Clin. 2021;13(1):113–22.
Sharma S, Sharma G, Hote M, Devagourou V, Kesari V, Arava S, et al. Light and electron microscopic features of surgically excised left atrial appendage in rheumatic heart disease patients with atrial fibrillation and sinus rhythm. Cardiovasc Pathol. 2014;23:319–26.
Yongjun Q, Huanzhang S, Wenxia Z, Hong T, Xijun X. Histopathological characteristics and oxidative injury secondary to atrial fibrillation in the left atrial appendages of patients with different forms of mitral valve disease. Cardiovasc Pathol. 2013;22:211–8.
Manning WJ, Silverman DI, Waksmonski CA, Oettgen P, Douglas PS. Prevalence of residual left atrial thrombi among patients with acute thromboembolism and newly recognized atrial fibrillation. Arch Intern Med. 1995;155:2193–8.
Cianfrocca C, Loricchio ML, Pelliccia F, Pasceri V, Auriti A, Bianconi L, et al. C-reactive protein and left atrial appendage velocity are independent determinants of the risk of thrombogenesis in patients with atrial fibrillation. Int J Cardiol. 2010;142:22–8.
Li Q, Ma X, Shao Q, Yang Z, Wang Y, Gao F, et al. Prognostic impact of multiple lymphocyte-based inflammatory indices in acute coronary syndrome patients. Front Cardiovasc Med. 2022;9: 811790.
Seo IH, Lee YJ. Usefulness of complete blood count (CBC) to assess cardiovascular and metabolic diseases in clinical settings: a comprehensive literature review. Biomedicines. 2022;10:2697.
Hahn RT, Abraham T, Adams MS, Bruce CJ, Glas KE, Lang RM, et al. Guidelines for performing a comprehensive transesophageal echocardiographic examination: recommendations from the American Society of Echocardiography and the Society of Cardiovascular Anesthesiologists. J Am Soc Echocardiogr. 2013;26:921–64.
Tang L, Xia Y, Fang L. Correlation between left atrial thrombosis and neutrophil-to-lymphocyte ratio upon non-valvular atrial fibrillation. Clin Lab. 2022;68:326–33.
Yalcin M, Aparci M, Uz O, Isilak Z, Balta S, Dogan M, et al. Neutrophil-lymphocyte ratio may predict left atrial thrombus in patients with nonvalvular atrial fibrillation. Clin Appl Thromb Hemost. 2015;21:166–71.
Fukuda Y, Okamoto M, Tomomori S, Matsumura H, Tokuyama T, Nakano Y, et al. In paroxysmal atrial fibrillation patients, the neutrophil-to-lymphocyte ratio is related to thrombogenesis and more closely associated with left atrial appendage contraction than with the left atrial body function. Intern Med. 2018;57:633–40.
Saliba W, Barnett-Griness O, Elias M, Rennert G. Neutrophil to lymphocyte ratio and risk of a first episode of stroke in patients with atrial fibrillation: a cohort study. J Thromb Haemost. 2015;13:1971–9.
Jiang F, Zhang W, Lu H, Tan M, Zeng Z, Song Y, et al. Prediction of herbal medicines based on immune cell infiltration and immune- and ferroptosis-related gene expression levels to treat valvular atrial fibrillation. Front Genet. 2022;13: 886860.
Sharma G, Ghati N, Sharique M, Sharma S, Shetkar S, Karmakar S, et al. Role of inflammation in initiation and maintenance of atrial fibrillation in rheumatic mitral stenosis—an analytical cross-sectional study. J Arrhythm. 2020;36:1007–15.
Duygu H, Barisik V, Kurt H, Turk U, Ercan E, Kose S. Prognostic value of plasma soluble CD40 ligand in patients with chronic non-valvular atrial fibrillation. Europace. 2008;10:210–4.
Conway DS, Buggins P, Hughes E, Lip GY. Relationship of interleukin-6 and C-reactive protein to the prothrombotic state in chronic atrial fibrillation. J Am Coll Cardiol. 2004;43:2075–82.
Adamstein NH, MacFadyen JG, Rose LM, Glynn RJ, Dey AK, Libby P, et al. The neutrophil-lymphocyte ratio and incident atherosclerotic events: analyses from five contemporary randomized trials. Eur Heart J. 2021;42:896–903.
Paolisso P, Bergamaschi L, Santulli G, Gallinoro E, Cesaro A, Gragnano F, et al. Infarct size, inflammatory burden, and admission hyperglycemia in diabetic patients with acute myocardial infarction treated with SGLT2-inhibitors: a multicenter international registry. Cardiovasc Diabetol. 2022;21(1):77.
Cesaro A, Gragnano F, Paolisso P, Bergamaschi L, Gallinoro E, Sardu C, et al. In-hospital arrhythmic burden reduction in diabetic patients with acute myocardial infarction treated with SGLT2-inhibitors: insights from the SGLT2-I AMI PROTECT study. Front Cardiovasc Med. 2022;9:1012220.
Ozbeyaz NB, Gokalp G, Gezer AE, Algul E, Sahan HF, Aydinyilmaz F, et al. Novel marker for predicting the severity and prognosis of acute pulmonary embolism: platelet-to-hemoglobin ratio. Biomark Med. 2022;16(12):915–24.
Ozbeyaz NB, Gokalp G, Algul E, Sahan HF, Aydinyilmaz F, Guliyev I, et al. Platelet-hemoglobin ratio predicts amputation in patients with below-knee peripheral arterial disease. BMC Cardiovasc Disord. 2022;22(1):337.
Acknowledgements
None.
Funding
This study was supported by Joint Construction Project of Henan Medical Science and Technology Research Plan (No. LHGJ20210093).
Author information
Authors and Affiliations
Contributions
YZ and HXF contributed to the conception or design of the work. XWS, YZ, JFM and XQW contributed to the acquisition, analysis, or interpretation of data for the work. YZ and XWS drafted the manuscript. HXF critically revised the manuscript. All gave final approval and agree to be accountable for all aspects of work ensuring integrity and accuracy.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of the Henan Provincial People’s Hospital. The need for informed consent was waived by the Ethics Committee of the Henan Provincial People’s Hospital, because of the retrospective nature of the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhou, Y., Song, X., Ma, J. et al. Association of inflammation indices with left atrial thrombus in patients with valvular atrial fibrillation. BMC Cardiovasc Disord 23, 9 (2023). https://doi.org/10.1186/s12872-023-03036-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12872-023-03036-x