Abstract
Introduction
Coronary artery disease (CAD) is considered an independent risk factor for COVID-19. However, no study has specifically examined the clinical manifestations and outcomes of COVID-19 in patients with ischemic heart disease (IHD).
Methods
In a retrospective case-control study between 20 March 2020 to 20 May 2020, the medical record of 1611 patients with laboratory-confirmed SARS-CoV-2 infection was reviewed. IHD was defined as a history of an abnormal coronary angiography, coronary angioplasty, coronary artery bypass graft (CABG), or chronic stable angina. Demographic data, past medical history, drug history, symptoms, vital signs, laboratory findings, outcome, and death were investigated from medical records.
Results
1518 Patients (882 men (58.1%)) with a mean age of 59.3 ± 15.5 years were included in the study. Patients with IHD (n = 300) were significantly less likely to have fever (OR: 0.170, 95% CI: 0.34–0.81, P < 0.001), and chills (OR: 0.74, 95% CI: 0.45–0.91, P < 0.001). Patients with IHD were 1.57 times more likely to have hypoxia (83.3% vs. 76%, OR: 1.57, 95% CI: 1.13–2.19, P = 0.007). There was no significant difference in terms of WBC, platelets, lymphocytes, LDH, AST, ALT, and CRP between the two groups (P > 0.05). After adjusting for demographic characteristics, comorbidities and vital signs, the risk factors for mortality of these patients were older age (OR: 1.04 and 1.07) and cancer (OR: 1.03, and 1.11) in both groups. In addition, in the patients without IHD, diabetes mellitus (OR: 1.50), CKD (OR: 1.21) and chronic respiratory diseases (OR: 1.48) have increased the odds of mortality. In addition, the use of anticoagulants (OR: 2.77) and calcium channel blockers (OR: 2.00) has increased the odds of mortality in two groups.
Conclusion
In comparison with non-IHD, the symptoms of SARS-CoV-2 infection such as fever, chills and diarrhea were less common among patients with a history of IHD. Also, older age, and comorbidities (including cancer, diabetes mellitus, CKD and chronic obstructive respiratory diseases) have been associated with a higher risk of mortality in patients with IHD. In addition, the use of anticoagulants and calcium channel blockers has increased the chance of death in two groups without and with IHD.
Similar content being viewed by others
Introduction
Coronavirus belongs to a family of viruses that are abundant in animals throughout the world. However, few cause disease in humans with symptoms such as pneumonia, fever, breathing difficulty and lung infection [1]. One of these viruses with the current reference name of Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), which has recently caused pathogenesis in humans and quickly caused a pandemic, has been named coronavirus disease 19 [2] by the World Health Organization (WHO) [3, 4]. To date (30 July 2022), about 572 M people have been infected with COVID-19, of which 6.39 M have died. This number is increasing day by day [5].
The clinical manifestation of COVID-19 is non-specific and ranges from asymptomatic to severe conditions and death. [6, 7]. If it is symptomatic, the most frequent symptoms are fever, fatigue, cough, myalgia and complicated dyspnea, whereas less frequent symptoms include hemoptysis, diarrhea, headache and runny nose [1, 8,9,10]. Comorbidities, i.e., diabetes, hypertension, cirrhosis, cancer, and coronary artery disease (CAD), and aging cause more severe disease and even death [11]. As a common disease, coronary artery disease (CAD) accounts for one-third of the world’s deaths. The most common, life-threatening type of CAD, ischemic heart disease (IHD), is expected to increase from one in 11 cases in 2019 to one in six in 2050 [12]. Therefore, IHD is recognized as a major threat to sustainable development in the 21st century [13,14,15].
Due to the prevalence of IHD and being a risk factor for severe COVID-19, early diagnosis and proper management of COVID-19 are very important. However, the early detection of these patients requires accurate knowledge of clinical presentations. Despite the high prevalence of IHD and its potential risks associated with COVID-19, no study evaluated the clinical manifestations of SARS-CoV-2 in patients with IHD. Thus, the present study aimed to evaluate the clinical manifestation of COVID-19 in patients with a history of IHD.
Methods and material
Study design
This retrospective case-control study was conducted in Khorshid Hospital, the main and first center of COVID-19 in Isfahan province, Iran, from 20 March 2020 to 20 May 2020.
Patient enrollment
All patients with moderate to severe COVID-19 whose diagnosis was confirmed by real-time reverse transcription polymerase chain reaction (rRT-PCR) test of nasal and pharyngeal swab specimens [10] and outcome of discharge or death prior to 20 May were enrolled in the study. The diagnostic criteria for COVID-19 were made based on the WHO Interim Guideline [16] and severity was defined according to Coronavirus Disease 2019 Treatment Guidelines by the National Institute of Health [17]. Moderate illness was defined as evidence of lower respiratory disease during clinical assessment or imaging, with SpO2 ≥ 94% on room air at sea level. Severe illness was defined as having SpO2 < 94% on room air at sea level, PaO2/FiO2 < 300 mm Hg, a respiratory rate > 30 breaths/min, or lung infiltrates > 50%.
The study consisted of two groups; [1] Case group (IHD) included patients with ischemic heart disease, which was defined as a history of abnormal epicardial coronary artery disease, coronary angioplasty, coronary artery bypass surgery (CABG) and chronic stable angina, and [2] Control group (non-IHD) included patients without any documented coronary artery diseases. Besides, patients with microvascular, whose exercise-related angina or evidence of ischemia in non-invasive tests are associated with no stenoses or mild-to-moderate stenoses (40–60%), revealed by ICA or CTA, that are deemed functionally non-relevant, were included. Abnormal epicardial coronary artery disease was defined as minimal if the narrowing is visually less than 50%, moderate between 50% and 70%, and severe with a diameter reduction of 70% or more [18]. Also, chronic stable angina was defined according to the 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes [19]. Initial evaluations identified 1,611 patients with a definitive diagnosis of COVID-19. Medical records of identified patients were evaluated for coronary artery disease documentation and congestive heart failure (CHF). Patients with CHF or acute coronary syndrome were excluded from the study.
Data collection
The clinical and laboratory data of patients were collected from medical records, including demographics data, underlying disease, drug history, vital signs at admission, clinical symptoms, laboratory findings, comorbid conditions and clinical outcomes.
Comorbidities were recorded based on medical records. The comorbidities are diabetes (DM), chronic kidney disease (CKD), chronic respiratory diseases, hypertension (HTN), end-stage renal disease (ESRD) and malignancies.
Admission vital signs included systolic blood pressure (SBP), diastolic blood pressure (DBP), heart rate (HR), respiratory rate (RR), body temperature [19] and oxygen saturation (SpO2) by pulse oximetry. The abnormal SBP is defined as < 90mmHg (hypotension) or ≥ 140mmHg (hypertension). Also, abnormal DBP, tachycardia, tachypnea, fever (oral temperature) and hypoxemia were defined as ≥ 90mmHg, ≥ 100 beat/min, ≥ 20 beat/min, ≥ 38 °C of and < 93%. Blood pressures were measured by mercury sphygmomanometer (Manufacturer: MDF, 2018, Model: SKU: BP-03,) as Ogedegbe et al. recommendation [20]. Oxygen saturation was assessed by calibrated fingertip pulse oximeter (Manufacturer: Choicemmed, 2019).
In this study, inexpensive and available laboratory tests were considered for the initial evaluation. Complete blood count (CBC) with differential, C-reactive protein (CRP), lactate dehydrogenase (LDH), aspartate aminotransferase [19] and alanine aminotransferase (ALT) were initial laboratory evaluation. In differential CBC, platelets less than 150,000 [21], white blood cells greater than 11,000 or less than 4,000 [22, 23], and lymphocytes less than 1,100 were considered abnormal [24]. Besides, CRP ≥ 10 mg/dl [25], AST > 40 U/L, ALT > 50 U/L and LDH > 250 U/L were considered abnormal [8].
Primary clinical outcomes were defined as hospitalization in the ICU and intubation. Moreover, secondary outcomes were investigated, including length of hospital stay and death.
Data analysis
Initially, the normal distribution of each variable was investigated by the Kolmogorov-Smirnov test. The chi-square and independent T-test were used to analyze qualitative (categorical) and quantitative data, respectively. The Mann-Whitney test was also used for quantitative data without normal distribution. Categorical and quantitative data were reported in number (percentage) and mean ± standard deviation respectively. The odds ratio and 95% confidence interval were estimated by logistic regression to evaluate the association between symptoms and IHD. In order to estimate the relationship between risk factors related to mortality, multivariable regression was used by adjusting demographic characteristics, comorbidities, used drugs and vital signs. A P-value lower than 0.05 was considered statistically significant. STATA (V.12.0) software performed all the statistical analyses.
Results
Evaluations of 1611 medical records revealed 328 patients had a history of IHD and 1,283 patients had no history of IHD. Because of missing records, 28 patients from the IHD group and 65 patients from the non-IHD group were excluded from the study. Finally, data were collected from 300 patients in the IHD group and 1218 patients in the non-IHD group (Fig. 1). Of them, 882 (58.1%) patients were men. The mean age was 59.3 ± 15.5 years. Also, there is no significant difference between the two groups regarding gender and smoking (P > 0.05). However, analysis exhibited a significant difference in age between IHD and non-IHD groups, 67.99 ± 11.17 vs. 57.14 ± 15.70 years (P < 0.001). Of the IHD group, 52 and 33 patients had a history of Percutaneous Coronary Intervention and CABG, respectively.
Comorbidities
Among comorbidities, there was no significant difference between groups regarding the history of chronic respiratory diseases and cancer (P > 0.05). However, the history of HTN, DM, CKD, and ESRD was significantly higher in patients with IHD (P < 0.05) (Table 1).
Drug history
Angiotensin-converting enzyme (ACE) inhibitors, beta-adrenergic blockers, calcium channel blockers (CCBs), diuretics, anti-hyperglycemic agents, angiotensin II receptor blockers [19], antilipemic agents, anticoagulant and anticonvulsant were significantly higher in IHD group compared to non-IHD (P < 0.05) (Table 2).
Clinical presentations
The five most common symptoms in the IHD group were cough (73.7%), fever (65%), shortness of breath (62.3%), fatigue (59.3%), and chills (50.7%), respectively. On the other hand, the five most common symptoms in the non-IHD group were fever (75.7%), cough (71.8%), shortness of breath (61.6%), chills (60.1%), and weakness and fatigue (59.4%), respectively. Analyses showed patients with IHD were significantly less likely to present symptoms, including fever (OR: 0.170, 95% CI: 0.34–0.81, P < 0.001) and chills (OR: 0.74, 95% CI: 0.45–0.91, P < 0.001). The other symptoms had no significant difference between the two groups (P > 0.05) (Table 3).
Vital signs
At the time of admission, body temperature (T) (37.42 ± 2.21 vs. 37.52 ± 0.99 °C, P = 0.500), DBP (79.1 ± 13.97 vs. 80.11 ± 35.15 mmHg, P = 0.555), SBP (131.58 ± 57.46 vs. 132 ± 22.03 mmHg, P = 0.800) and tachycardia (27.3% vs. 33%, P = 0.059) were not statistically significantly different between IHD and non-IHD groups. However, the mean HR (90.02 ± 17.33 vs. 93 ± 15.72 beat/min, P = 0.003) and SaO2 (85.1 ± 10.34 vs. 87.85 ± 27.46%, P = 0.026) were significantly lower in IHD group.
Laboratory findings.
A quantitative comparison of laboratory data showed that white blood cell counts, platelets, lymphocytes, lactate dehydrogenase (LDH), aspartate transaminase [19], alanine transaminase (ALT) and C-reactive protein (CRP) were not significantly different between the two groups (Table 3).
Clinical outcomes
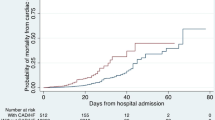
Length of hospitalization was significantly higher in the IHD group (8.4 ± 5.57 days vs.7.5 ± 5.5 days, P = 0.042). However, the ICU admission, ICU length of stay, the need to mechanical ventilation, duration of mechanical ventilation, death and time from admission to death were not significantly different between the IHD and non-IHD groups (P > 0.05) (Table 4).
Finally, the results of the multivariate regression analysis of mortality risk factors in each of the two groups with and without IHD showed that in patients without IHD, age, length of hospitalization, use of anti-coagulants, comorbidities (including chronic respiratory diseases, diabetes, cancer and CKD) significantly increase the odds of mortality (P < 0.05). However, increasing DBP and SaO2 decreased the odds of mortality (P < 0.05). In the group of patients with IHD, older age, use of calcium channel blockers, increased HR, and RR and having cancer can increase the odds of mortality (P < 0.05) (Table 5).
Discussion
The present study on “comparing the clinical and laboratory characteristics and outcomes of patients with IHD and patients without IHD” reported that the five most common symptoms in patients with IHD were cough, fever, shortness of breath, fatigue and chills. On the other hand, the five most common symptoms in patients without IHD were fever, cough, shortness of breath, chills and fatigue. Only three symptoms, fever, chills and diarrhea, were significantly less frequent in IHD patients. The mean heart rate and systolic blood pressure were significantly lower in IHD patients. Also, hypoxemia was significantly higher in patients with IHD. Among clinical outcomes, only the hospital stay of patients with IHD was significantly longer. Also, it was shown that older age, use of calcium channel blockers, increased HR, and RR and positive history of cancer were associated with increased mortality in patients with IHD.
In the previous studies, the early stages of the SARS-CoV-2 infection are characterized by mild constitutional symptoms and upper respiratory tract infection. The most common symptoms in the early stages of the disease, the viral phase, include fever, cough, sore throat, shortness of breath, malaise, fatigue and headache [26,27,28]. Subsequently, nasal congestion, rhinorrhea and sneezing may occur [1, 8, 26, 29, 30]. The inflammatory phase, which usually begins in the second week of the disease, manifests with symptoms such as dyspnea, tachypnea, hypoxemia, diarrhea, and abdominal pain [10, 26, 27, 31]. These symptoms are due to RNAemia and cytokine release [2, 6, 21, 26, 29]. The presence of viral phase and inflammatory phase symptoms in our patients may be justified by not classifying patients at different times from the onset of symptoms to hospitalization.
A case-control study on 859 patients, 113 patients with heart disease (HD) and 746 without heart disease, by Gonzalo Cabezón Villalba et al. reported that the mean age of patients with heart disease compared to the control group was significantly higher, 75.6 vs. 67 (P < 0.001) [32]. Besides, the history of DM, CKD and HTN in the HD group was significantly more than the non-HD group (38.1 vs. 16.5%, 14.2 vs. 5.8%, 76.1 vs. 45.4%, P < 0.001). Our findings in patients with IHD also supported their findings regarding the mean age and comorbidities.
The study by Gonzalo Cabezón Villalba showed that cough is the only symptom that occurs significantly lesser in patients with heart disease. Regarding fever and diarrhea, they did not find any significant difference [32]. however, our study revealed that patients with a history of IHD reported less fever, chills and diarrhea than the control group. It seems that these symptoms could not help diagnose because patients with IHD usually receive drugs such as acetylsalicylic acid (ASA) and calcium channel blockers (CCBs) like amlodipine verapamil and diltiazem as secondary prevention. ASA has both anti-inflammatory (high doses) and antithrombotic effects (low doses) [33]. However, ASA is prescribed in low doses, 80 mg/day, in case of secondary prevention in patients with IHD. Thus, it could be suggested that ASA is not responsible for reducing these symptoms in these patients. In addition, a side effect of calcium channel blockers is constipation resulting from colonic motor activity inhibition [34, 35]. Therefore, patients consuming calcium channel blockers may report diarrhea lesser than healthy people.
The study on patients with heart disease reported no significant difference in the complaint of dyspnea between groups [32]. Our study is in line with their study. The onset of dyspnea and tachypnea were not statistically significantly different between patients with and without IHD. Although patients with IHD had less oxygen saturation than the control group, the complaint of dyspnea had no significant difference. Nevertheless, we do not have a proper explanation for this finding. The accuracy of this finding needs further study in the future.
The prevalence of CAD in patients with COVID-19 was variable between 2.5 and 40% in previous studies [8, 21, 28, 36]. In the present study, 20% of the patients had a history of IHD. Some studies have cited the association between cardiovascular disease (CVD) and severe COVID-19 as a secondary outcome of their study. The death rates of patients with CAD were significantly higher compared with other patients in the study of Zhou et al. (24% vs. 1%, p < 0.0001) and Wu et al. (10.5% vs. 2.3%, P < 0.001) [37, 38]. Mehra et al. cited coronary artery disease (10.2%, vs. 5.2%, OR: 2.70, 95% CI: 2.08–3.51), heart failure (15.3%, vs. 5.6%, OR: 2.48, 95% CI: 1.62–3.79), and cardiac arrhythmia (11.5%, vs. 5.6%, OR: 1.95; 95% CI: 1.33–2.86) as independent factors associated with an increased risk of hospital death in patients with COVID-19 [39]. Gonzalo Cabezón Villalba et al. reported that the in-hospital mortality in patients with heart disease was higher than the control group (35.4% vs. 18.2%, p < 0.001). However, in the present study, IHD did not increase adverse outcomes and mortality rates in patients with COVID-19. This discrepancy is probably due to our definition of IHD patients. Though previous studies considered all types of heart disease as CAD, the definition of IHD in the present study was only considered as a positive history of abnormal coronary angiography, coronary angioplasty, CABG and chronic stable angina. In addition, by adjustment of other risk factors, e.g., comorbidities and age, only the net effect of IHD was assessed.
In a comparison of our results with studies on a healthy population, a systematic review and meta-analysis of 42 studies and 423,117 patients showed that acute kidney injury, COPD, diabetes, hypertension, CVD, cancer, increased D-dimer, male gender, older age, current smoker, and obesity are associated with a higher mortality rate [40]. In our study, some of these factors, including cancer, COPD, older age, diabetes and hypertension, have been identified as risk factors in the non-IHD group.
We had some limitations. First, the unavailability of echocardiography results and the nature of the study (retrospective) made us diagnose CHF only based on prior medical records and/or symptoms and signs. Also, we excluded patients with CHF because the cause of heart failure, i.e., ischemic or structural, was not known. Second, since Our hospital was the center for COVID-19 patients in the region only during the mentioned time (20 March 2020 to 20 May 2020), it could not be possible to include further subvariants of SARS-CoV-2 in the study and this study just assessed the very first peak of COVID-19.
Conclusion
According to the results of the current study, age, comorbidities (including diabetes, CKD, HTN, ESRD, and dialysis), fever, and length of hospitalization in the IHD group were significantly more than in the group without IHD. While chill symptoms and high HR in the group without IHD were significantly more than the IHD group. In the evaluation of the risk factors related to the mortality of these patients, it was found that patients with older age and cancer in both groups were associated with a higher risk of mortality. In addition, in the group of patients without IHD, diabetes, CKD and chronic respiratory diseases have increased the odds of mortality. In addition, the use of anticoagulants and calcium channel blockers has increased the odds of mortality in two groups without and with IHD, respectively.
Data availability
• The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Abbreviations
- IHD:
-
Ischemic Heart Disease
- CAD:
-
Coronary Artery Disease
- CABG:
-
Coronary Artery Bypass Graft
- DM:
-
Diabetes Mellitus
- CKD:
-
Chronic Kidney Disease
- HTN:
-
Hypertension
- ESRD:
-
End Stage Renal Disease
References
Adhikari SP, Meng S, Wu Y-J, Mao Y-P, Ye R-X, Wang Q-Z, et al. Epidemiology, causes, clinical manifestation and diagnosis, prevention and control of coronavirus disease (COVID-19) during the early outbreak period: a scoping review. Infect Dis poverty. 2020;9(1):1–12.
Ram-Mohan N, Kim D, Zudock EJ, Hashemi MM, Tjandra KC, Rogers AJ, et al. SARS-CoV-2 RNAemia predicts clinical deterioration and Extrapulmonary Complications from COVID-19. Clin Infect Dis. 2021;74(2):218–26.
Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y et al. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. N Engl J Med. 2020.
Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. New England Journal of Medicine; 2020.
WHO. 2020 [Available from: https://covid19.who.int/.
Lakhani HV, Pillai SS, Zehra M, Sharma I, Sodhi K. Systematic review of clinical insights into novel coronavirus (COVID-19) pandemic: persisting challenges in US rural population. Int J Environ Res Public Health. 2020;17(12):4279.
Xu G, Yang Y, Du Y, Peng F, Hu P, Wang R et al. Clinical Pathway for Early Diagnosis of COVID-19: Updates from Experience to Evidence-Based Practice. Clin Rev Allergy Immunol. 2020:1–12.
Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. The Lancet. 2020;395(10223):507–13.
Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–9.
Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The lancet. 2020;395(10223):497–506.
Li T, Wei C, Li W, Hongwei F, Shi J. Beijing Union Medical College Hospital on” pneumonia of novel coronavirus infection” diagnosis and treatment proposal (V2. 0). Med J Peking Union Med Coll Hosp. 2020.
Khan MA, Hashim MJ, Mustafa H, Baniyas MY, Al Suwaidi SKBM, AlKatheeri R et al. Global Epidemiology of Ischemic Heart Disease: Results from the Global Burden of Disease Study. Cureus. 2020;12(7).
Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Executive summary: heart disease and stroke statistics—2016 update: a report from the American Heart Association. Circulation. 2016;133(4):447–54.
Roth GA, Johnson C, Abajobir A, Abd-Allah F, Abera SF, Abyu G, et al. Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J Am Coll Cardiol. 2017;70(1):1–25.
Prabhakaran D, Jeemon P, Sharma M, Roth GA, Johnson C, Harikrishnan S, et al. The changing patterns of cardiovascular diseases and their risk factors in the states of India: the global burden of Disease Study 1990–2016. The Lancet Global Health. 2018;6(12):e1339–e51.
Organization WH. Clinical management of severe acute respiratory infection when novel coronavirus (2019-nCoV) infection is suspected: interim guidance. Clinical management of severe acute respiratory infection when novel coronavirus (2019-nCoV) infection is suspected: Interim guidance 2020. p. 21-.
NIAID-RML C. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. Nih gov Published. 2020.
Libby P, Bonow RO, Mann DL, Tomaselli GF, Bhatt DL, Solomon SD, et al. Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine. Elsevier; 2021.
Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41(3):407–77.
Ogedegbe G, Pickering T. Principles and techniques of blood pressure measurement. Cardiol Clin. 2010;28(4):571–86.
Guan W-j, Ni Z-y, Hu Y, Liang W-h, Ou C-q, He J-x, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–20.
Mank V, Brown K. Leukocytosis. StatPearls [Internet]. 2020.
Annamalai A. Leukopenia. Medical Management of Psychotropic Side Effects. Springer; 2017. pp. 177–84.
Huang I, Pranata R. Lymphopenia in severe coronavirus disease-2019 (COVID-19): systematic review and meta-analysis. J Intensive Care. 2020;8(1):1–10.
Bartoletti M, Giannella M, Scudeller L, Tedeschi S, Rinaldi M, Bussini L, et al. Predictors of severe respiratory failure in hospitalized patients with SARS-CoV-2 infection. Development and Validation of a Prediction Model (PREDI-CO Study; 2020.
Cascella M, Rajnik M, Cuomo A, Dulebohn SC, Di Napoli R. Features, evaluation and treatment coronavirus (COVID-19). Statpearls [internet]: StatPearls Publishing; 2020.
Guo Y-R, Cao Q-D, Hong Z-S, Tan Y-Y, Chen S-D, Jin H-J, et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak–an update on the status. Military Med Res. 2020;7(1):1–10.
Lovato A, de Filippis C. Clinical presentation of COVID-19: a systematic review focusing on Upper Airway symptoms. Ear Nose & Throat Journal. 2020;99(9):569–76.
Singhal T. A review of coronavirus disease-2019 (COVID-19). Indian J Pediatr. 2020:1–6.
Meo S, Alhowikan A, Al-Khlaiwi T, Meo I, Halepoto D, Iqbal M, et al. Novel coronavirus 2019-nCoV: prevalence, biological and clinical characteristics comparison with SARS-CoV and MERS-CoV. Eur Rev Med Pharmacol Sci. 2020;24(4):2012–9.
Wang Y, Wang Y, Chen Y, Qin Q. Unique epidemiological and clinical features of the emerging 2019 novel coronavirus pneumonia (COVID-19) implicate special control measures. J Med Virol. 2020;92(6):568–76.
Cabezón Villalba G, Amat-Santos IJ, Dueñas C, Lopez Otero D, Catala P, Aparisi A, et al. Impact of the presence of heart disease, cardiovascular medications and cardiac events on outcome in COVID-19. Cardiol J. 2021;28(3):360–8.
Bianconi V, Violi F, Fallarino F, Pignatelli P, Sahebkar A, Pirro M. Is acetylsalicylic acid a safe and potentially useful choice for adult patients with COVID-19? Drugs. 2020:1–14.
Bassotti G, Calcara C, Annese V, Fiorella S, Roselli P, Morelli A. Nifedipine and verapamil inhibit the sigmoid colon myoelectric response to eating in healthy volunteers. Dis colon rectum. 1998;41(3):377–80.
Devasahayam J, Pillai U, Uppaluri C. Acute severe intestinal obstruction secondary to amlodipine toxicity. QJM: An International Journal of Medicine. 2012;105(5):467–9.
Gan JM, Kho J, Akhunbay-Fudge M, Choo HM, Wright M, Batt F, et al. Atypical presentation of COVID-19 in hospitalised older adults. Ir J Med Sci (1971 -). 2021;190(2):469–74.
Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. The lancet. 2020.
Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239–42.
Mehra MR, Desai SS, Kuy S, Henry TD, Patel AN. Cardiovascular disease, drug therapy, and mortality in COVID-19. N Engl J Med. 2020.
Dessie ZG, Zewotir T. Mortality-related risk factors of COVID-19: a systematic review and meta-analysis of 42 studies and 423,117 patients. BMC Infect Dis. 2021;21(1):855.
Acknowledgements
Not applicable.
Funding
No funding.
Author information
Authors and Affiliations
Contributions
MT, RS, FS and MM were contributed to design the study. NK, NH and MD were contributed to gathering the data. MD and FS were contributed to analyzing and interpreting the results. MM, NK and NH were contributed to writing the manuscript. MT, RS, MD and Fs were contributed to revising the initial manuscript. All authors approved the final version of manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests
Ethics approval and consent to participate
This study was approved by the ethics committee of Isfahan University of Medical Sciences with the ethical code number: IR.MUI.MED.REC.1399.291. Written informed consent was obtained from patients after explaining the study to them. This study was conducted in accordance with the Declaration of Helsinki and national ethical guidelines.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Tajmirriahi, M., Sami, R., Mansourian, M. et al. The clinical manifestation and outcome of COVID-19 in patients with a history of ischemic heart disease; a retrospective case-control study. BMC Cardiovasc Disord 23, 241 (2023). https://doi.org/10.1186/s12872-023-03256-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12872-023-03256-1