Abstract
Background
Corrected QT (QTc) interval has been reported to be associated with type 2 diabetes. This study aimed to explore the relationship between different glucose tolerance and QTc intervals among middle-aged and older Chinese individuals.
Methods
We conducted a cross-sectional analysis that included 9898 subjects (3194 men and 6704 women) in a Chinese population. Glucose tolerance was studied during the oral glucose tolerance test (OGTT). Insulin, blood pressure, hemoglobin A1c (HbA1c), serum lipids, hepatic transaminases and waist-to-hip ratio were assessed. The QTc interval was derived from ECG recordings, and the subjects were stratified based on different glucose tolerance.
Results
QTc interval levels were increased significantly in the subjects with abnormal glucose metabolism compared with the normal glucose regulation group. Multiple regression analyses showed that the QTc interval was significantly associated with fasting plasma glucose, 2-h OGTT plasma glucose and HbA1c. The odds ratio of prolonged QTc was 1.396 for impaired glucose regulation (IFG)/impaired fasting glucose (IGT) (95% CI 0.126–1.730), and 1.342 for type 2 diabetes (95% CI 0.142–1.577) after all potential confounders were adjusted.
Conclusions
Impaired glucose tolerance (IGR) and diabetes are associated with prolonged QTc intervals among middle-aged and older Chinese individuals. Abnormal glucose regulation can be used to monitor the QTc interval in the population.
Similar content being viewed by others
Introduction
The risk of sudden death is increased in patients with diabetes mellitus and impaired glucose tolerance [1, 2]. Abnormal cardiac autonomic nervous system function is one of the most significant complications of diabetes [3]. The QT interval is defined as the total time required for ventricular myocardial depolarization and repolarization [4]. Corrected QT (QTc) is very important for the prediction, risk assessment and treatment of ventricular arrhythmias [2]. The relationship between a prolonged QTc interval and an increased risk of sudden death has been traditionally explored in familial long QT syndrome sudden cot death, and congestive heart failure, as well as in adults with diabetes mellitus [2, 5,6,7,8].
Although several studied have described the relationship between QTc interval prolongation, diabetic complications, and an increased mortality rate in adults [5, 9,10,11], the influence of abnormal glucose metabolism on the QTc interval has not been fully clarified. Previous studies have confirmed that the prevalence of prolonged QTc interval is increased in people with type 1 and type 2 diabetes as compared to non-diabetics, and the abnormalities in cardiac repolarisation may be caused by complications of diabetes rather than diabetes itself [5, 10, 12, 13]. Most recently, Kurnaz E et al. found that QTc prolongation already exists in a significant proportion of children and adolescents with newly diagnosed type 1 diabetes [14].
However, up to date, only a few studies have reported conflicting data on the relationship between the QTc interval and glucose metabolism regulation, especially the status of impaired glucose regulation (IGR) [5, 6, 10, 12]. In this study, we aimed to investigate the influence of different glucose tolerance on QTc intervals in a cross-sectional study of Chinese individuals aged 40–70 years.
Material and methods
Study population
The present study is one part of the risk evaluation of cancers in Chinese diabetic individuals, i.e., the longitudinal study (REACTION) which was a population-based cross-sectional study among middle-aged and elderly Chinese individuals in 25 communities across mainland China. The studied individuals were aged 40 to 70 years old. The details of the study design have been described previously [15, 16]. All participants came from the Chongming District in Shanghai, China and 9930 suitable subjects were recruited. After the exclusion of 1940 individuals with self-reported coronary heart disease, or taking medicine affecting heart rhythm, 7990 subjects (2489 men and 5501 women) were found to be suitable for the current analysis. Written informed consent was obtained from all of the participants. Approval was given by the Institutional Review Board of Xinhua Hospital affiliated with Shanghai Jiaotong University School of Medicine.
Data collection
A standardized questionnaire was used by trained physicians to collect the baseline data. The measurements of weight, height, waist circumference, hip circumference and blood pressure have been described previously. Smoking (yes/no) and alcohol drinking (yes/no) were estimated with an interview preceding the physical examination. Body mass index (BMI) was calculated as weight in kilograms divided by the square of height in metres.
A 12-lead ECG was recorded at a paper speed of 50 mm/s on a six-channel recorder. The QT interval was measured from the beginning of the QRS complex to the end of the T-wave. The QT interval corrected for the previous cardiac R-R cycle length (QTc) was calculated according to the formula proposed previously and known as Bazett’s formula: QTc = QT/(RR) 1/2 [17]. QTc is the mean of QTc from five consecutive cycles in lead V5. QTc > 440 ms was considered abnormally prolonged [18].
After overnight fasting for at least 10 h, fasting and 2-h oral glucose tolerance test (OGTT) blood samples were collected from all the participants in tubes containing EDTA centrifuged at 4 °C and stored at − 80 °C untill analysis. Fasting glucose, 2-h OGTT plasma glucose, triglycerides, total cholesterol (TC), high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, and serum creatinine (Scr) were measured on an automatic analyser (Hitachi 7080; Tokyo, Japan). The homeostasis model assessment of insulin resistance (HOMA-IR) was calculated based on the equation described by Matthews et al. [19].
Definitions of impaired glucose regulation and type 2 diabetes
Impaired glucose regulation was defined as impaired fasting glucose (IFG, fasting plasma glucose level ≥ 6.1 and < 7.0 mmol/l) and/or impaired glucose tolerance (IGT, 2-h OGTT plasma glucose level ≥ 7.8 and < 11.1 mmol/l). Isolated IFG: fasting plasma glucose ≥ 6.1 mmol/l and < 7.0 mmol/l and a 2 h OGTT plasma glucose < 7.8 mmol/l. Isolated IGT: 2-h OGTT plasma glucose ≥ 7.8 mmol/l and < 11.1 mmol/l and a fasting glucose < 6.1 mmol/l. IFG/IGT: fasting plasma glucose between 6.1 and 6.9 mmol/l and a 2-h OGTT plasma glucose 7.8–11.0 mmol/l. Type 2 diabetes was diagnosed by the 1999 World Health Organization criteria (fasting plasma glucose level ≥ 7.0 mmol/l and/or a 2-h OGTT plasma glucose level ≥ 11.1 mmol/l [20]. A fasting glucose level < 6.1 mmol/l and a 2-h OGTT plasma glucose level < 7.8 mmol/l were defined as normal glucose regulation (NGR).
Statistical analysis
Data management and statistical analysis were performed with the SPSS Statistical Package (version 22.0; SPSS Inc., Chicago, IL). Normally distributed data were expressed as means ± SD, whereas variables with a skewed distribution were reported as median (inter-quartile range). Comparisons of means and proportions were performed with the t-test and χ2 tests, respectively. Crude and partial correlation and multivariable stepwise regression analysis were used to investigate the association of QTc interval with cardiovascular and metabolic related parameters. A multiple linear regression analysis was performed to determine the associations of different glucose tolerance with QTc interval. Finally, a multivariate logistic regression model was used to evaluate the ORs and 95% CIs of different glucose tolerance for prolonged QTc. The statistical analyses were adjusted for potential confounders including age, sex, insulin, SBP, DBP, CHOL, TG, smoking, and drinking. P < 0.05 was considered statistically significant.
Results
Clinical characteristics of the participants
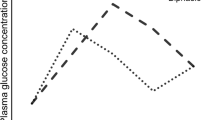
The clinical characteristics of the participants stratified by glucose tolerance are summarized in Table 1. The study involved 3412 participants with NGR, 2475 with IGR and 1833 with type 2 diabetes. Among the participants with IGR, 773 (9.67%) had isolated IFG, 1255 (15.7%) had isolated IGT, and 717 (8.97%) had combined IFG/IGT. The NGR and diabetes groups had the most favorable and unfavorable metabolic profiles, respectively. Across different glucose tolerance levels, the subjects with higher blood glucose levels were more likely to be older, and have a higher waist-to-hip ratio, higher lipid profiles, and higher levels of blood pressure. Some liver and kidney function indicators (such as AST, ALT, GGT, SCr) were significantly different among groups. In addition, the QTc interval was significantly increased in the subjects with isolated IFG, isolated IGT, combined IFG and IGT, and type 2 diabetes compared with the subjects with normal glucose regulation (416.63 ± 24.79, 418.53 ± 24.20,419.2 ± 27.35, and 420.79 ± 25.49, respectively, vs. 415.6 ± 24.30, p < 0.05) (Fig. 1). The QTc interval was positively associated with abnormal glucose metabolism.
Adjusted means (± SD) of QTc interval in subjects with NGR, IGR (isolated IFG, isolated IGT), combined IFG/IGT and diabetes mellitus (DM). Subjects with type 2 diabetes mellitus and IGR (isolated IFG, isolated IGT and IFG/IGT) had higher level of QTc interval than those with NGR (both p < 0.0001). There was no significant difference among the subgroups of IGR (p = 0.35) and between the IGR and type 2 diabetes mellitus groups (p = 0.61)
Association between QTc interval and clinical characteristics
Table 2 presents the results of correlation analyses of the QTc interval with metabolism-related parameters. QTc levels correlated positively with age, sex, WHR, SBP, DBP, PPG, FPG, HbA1c, insulin, HOMA-IR, LDL, CHO, TG, smoking and drinking. After adjusting for age and sex, partial correlation coefficient values indicated that WHR, SBP, DBP, PPG, FPG, HbA1c, insulin, HOMA-IR, CHO and TG were still positively associated with the QTc interval. However, neither LDL (r = 0.021; P = 0.28), smoking (r = − 0.002; P = 0.12) or drinking (r = − 0.007; P = 0.39) correlated with the QTc interval.
Determinants of QTc interval
A multiple regression analysis with a stepwise model was used to assess the independent variables that may affect QTc levels. The variables entered in the model were as follows: age, sex, FPG, PPG, HbA1c, SBP, DBP, CHOL, TG, WHR, insulin, BMI, smoking, and drinking. The main determinants of QTc levels were age (β = 0.125, p < 0.001), sex (β = 0.192, p < 0.001), FPG (β = 0.065, p < 0.001), PPG (β = 0.052, p < 0.001), HbA1c (β = 0.043, p < 0.001), SBP (β = 0.044, p 0.003), DBP (β = 2.498, p 0.012), and TG (β = 0.049, p < 0.001) (Table 3).
Associations between different glucose tolerance and QTc intervals
To further explore the relationship between different glucose tolerance and QTc intervals, multiple regression analyses were performed. The analyses revealed that isolated IGT (β = 0.044, p 0.002), combined IFG/IGT (β = 0.032, p 0.023) and type 2 diabetes (β = 0.076, p < 0.001) were significantly associated with the QTc interval after adjusting for all potential confounders (age, sex, insulin, SBP, DBP, CHOL, TG, smoking, and drinking) (Table 4).
Associations between different glucose tolerance and QTc prolongation
In the present study, we considered QTc > 440 ms as abnormally prolonged. Taking NGR as a reference, isolated IGT, combined IFG and IGT, and type 2 diabetes were all risk factors for prolonged QTc.The OR of IGT for QTc prolongation was 1.131 (95% CI 1.059–1.356), the OR of IFG + IGT for QTc prolongation was 1.396 (95% CI 1.126–1.73), and the OR of T2DM for QTc prolongation was 1.34 (95% CI 1.142–1.577) (P for trend < 0.001) after adjusting for age, sex, insulin, SBP, DBP, CHOL, TG, smoking, and drinking (Table 5).
Discussion
The main finding of this study is the effect of different glucose tolerance on the QTc interval. QTc interval prolongation is more common in patients with isolated IGT, combined IFG and IGT, and type 2 diabetes than in participants with NGR. Moreover, this association is independent of potentially confounding covariates.
Cardiac autonomic dysfunction present in prediabetes may lead to repolarization disturbances and may increase the risk of ventricular arrhythmias and sudden cardiac death [21]. Many ECG parameters, such as QTc prolongation, enhanced QT dispersion, the short-term variability of the QT interval, p-wave dispersion and heart rate variability were verified in patients with diabetes and IGT [13, 21,22,23]. ECG abnormalities were independently associated with an increased risk of developing coronary heart disease in a population of Middle Eastern women [24]. QTc prolongation is reported to be an independent risk factor for coronary heart disease in type 1 and type 2 diabetes and has been described to be a prominent predictor of cardiac death even in newly diagnosed type 2 diabetes patients [25, 26]. Many studies have demonstrated that QTc prolongation has direct detrimental effects on deadly arrhythmias and the risk of sudden death with myocardial ischemia [6, 13]. Additionally, hypoglycemia is associated with a significant prolongation of the QTc interval [27]. The above studies support the role of the QTc interval in predicting the risks of mortality. In this study, we found that the QTc interval was prolonged in prediabetes and diabetes which indicates that people with IGR and diabetes may be at high risk of arrhythmia and cardiovascular disease (CVD) mortality.
Previously published articles have reported that age [13], sex [5], BMI [28], hypertension [29], insulin concentration and hyperglycemia [30] are all risk factors for prolonged QTc interval in patients with diabetes. Consistent with the above studies, our analysis showed that age, sex, SBP, DBP, PPG, FPG, HbA1c, TG were positively correlated with the QTc interval. However, after further adjusting for the related confounding variables, BMI, insulin, HOMA-IR, HDL, LDL and CHO were no longer independently related to the QTc interval. In agreement with our results, the EURODIAB Prospective Complications Study demonstrated that female sex and higher values of HbA1c and systolic blood pressure were associated with a higher incidence of prolonged QTc, whereas BMI and physical activity within the range of 21.5–23.2 kg/m2 were displayed as protective factors [31].
In the present study, we further evaluated the association between different glucose tolerance and prolonged QTc intervals using a logistic regression model. Our results agreed with previous findings that patients with IGR, a prediabetic condition, were correlated with QTc interval [21, 31]. However, surprisingly, impaired fasting glucose was not found to significantly influence the QTc interval, which is not in agreement with other studies [32, 33]. The reason for this is probably because the participants in our study were from only one area and more women were recruited than men.
Hyperglycemia may cause QTc prolongation by several mechanisms including the stimulation of protein kinase C, which can reduce the synthesis and release of endothelial derived nitric oxide [34], consequently resulting in a decrease in Na-K-ATPase activity. The reduced nitric oxide bioavailability during hyperglycemia may also be responsible for the decreased activity of Ca2+ ATPase [35], an enzyme that maintains a low concentration of Ca2+ ions in the cell. Decreased activity of Na-K-ATPase will increase the intracellular calcium concentration and extend the QTc interval. Moreover, hyperglycemia is related to increased sympathetic activity as shown by increased plasma catecholamine concentrations [36]. Sympathetic stimulation unopposed by vagal activity may also induce ventricular electrical instability.
In summary, our study shows that prediabetes and diabetes appear to be independent risk factors for QTc interval prolongation after controlling for potential cofounders.The QTc interval is remarkably associated with glucose metabolism indices (including FPS, PPS, and HbA1c). In view of this, it is reasonable to consider whether the QTc interval alone should become a novel target for clinical intervention. Our study supports the concept that postprandial glucose level elevation may have an important role in the pathogenesis of cardiovascular complications [31]. Consistent with the previous study [37], we found that hyperglycemia, especially postprandial is an important risk factor for QTc prolongation in this study. Impaired glucose regulation appears to be an indication to evaluate and monitor the QTc interval in the population. We believe that our data are relevant in terms of public health. Further research is needed to verify whether glucose control among people with impaired glucose regulation may be useful in reducing QTc interval prolongation and ultimately limiting the risk of ventricular arrhythmia and sudden death.
The advantage of the present study is that we evaluated the relationship between all different glycometabolism conditions and the QTc interval in a large-scale population. Our study also has some limitations. First, given the cross-sectional nature of the study design, a cause-effect relationship between abnormal glucose metabolism and the QTc interval could not be verified. Further prospective studies are needed to analyze the potential pathophysiological mechanism of this correlation. Second, inaddition to the QTc interval, other markers of cardiac repolarization abnormalities, such as QT dispersion, were not evaluated. Third, because our data were obtained from middle-aged and older subjects, and the gender proportions were unbalanced, it has yet to be seen whether our results can be generalized to younger populations or other ethnic groups. Last, the QTc intervals were from one ECG which might not represent the true resting heart rate.
Availability of data and materials
The data that support the findings of this study are available from the corresponding author on reasonable request. Inquiries for data access may be sent to the following e-mail address: suqing@xinhuamed.com.cn.
Abbreviations
- QTc:
-
Corrected QT
- IGR:
-
Impaired glucose regulation
- IFG:
-
Impaired fasting glucose
- IGT:
-
Impaired glucose tolerance
- DM:
-
Diabetes mellitus
- WHR:
-
Waist-to-hip ratio
- BMI:
-
Body mass index
- SBP:
-
Systolic blood pressure
- DBP:
-
Diastolic blood pressure
- OGTT:
-
Oral glucose tolerance test
- FPG:
-
Fasting plasma glucose
- 2 h PPG:
-
2-H postload plasma glucose
- HbA1c:
-
Hemoglobin A1c
- TC:
-
Total cholesterol
- TG:
-
Triglycerides
- LDL:
-
Low-density lipoprotein-cholesterol
- HDL:
-
High-density lipoprotein-cholesterol
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- SCr:
-
Serum creatinine
References
Curb JD, Rodriguez BL, Burchfiel CM, Abbott RD, Chiu D, Yano K. Sudden death, impaired glucose tolerance, and diabetes in Japanese American men. Circulation. 1995;91(10):2591–5.
Svane J, Pedersen-Bjergaard U, Tfelt-Hansen J. Diabetes and the risk of sudden cardiac death. Curr Cardiol Rep. 2020;22(10):112.
Agashe S, Petak S. Cardiac autonomic neuropathy in diabetes mellitus. Methodist DeBakey Cardiovasc J. 2018;14(4):251–6.
Rautaharju PM, Manolio TA, Psaty BM, Borhani NO, Furberg CD. Correlates of QT prolongation in older adults (the Cardiovascular Health Study). Cardiovasc Health Study Collaborative Research Group. Am J Cardiol. 1994;73(13):999–1002.
Subbalakshmi NK, Adhikari PM, Sathyanarayana Rao KN, Jeganathan PS. Influencing factors of QTc among the clinical characteristics in type 2 diabetes mellitus. Diabetes Res Clin Pract. 2010;88(3):265–72.
Cox AJ, Azeem A, Yeboah J, Soliman EZ, Aggarwal SR, Bertoni AG, Carr JJ, Freedman BI, Herrington DM, Bowden DW. Heart rate-corrected QT interval is an independent predictor of all-cause and cardiovascular mortality in individuals with type 2 diabetes: the Diabetes Heart Study. Diabetes Care. 2014;37(5):1454–61.
Van Niekerk C, Van Deventer BS, du Toit-Prinsloo L. Long QT syndrome and sudden unexpected infant death. J Clin Pathol. 2017;70(9):808–13.
Schwartz PJ, Wolf S. QT interval prolongation as predictor of sudden death in patients with myocardial infarction. Circulation. 1978;57(6):1074–7.
Yang XH, Su JB, Zhang XL, Zhao LH, Xu F, Wang XQ, Cheng XB. The relationship between insulin sensitivity and heart rate-corrected QT interval in patients with type 2 diabetes. Diabetol Metab Syndr. 2017;9:69.
Arildsen H, May O, Christiansen EH, Damsgaard EM. Increased QT dispersion in patients with insulin-dependent diabetes mellitus. Int J Cardiol. 1999;71(3):235–42.
Veglio M, Sivieri R, Chinaglia A, Scaglione L, Cavallo-Perin P. QT interval prolongation and mortality in type 1 diabetic patients: a 5-year cohort prospective study. Neuropathy Study Group of the Italian Society of the Study of Diabetes, Piemonte Affiliate. Diabetes Care. 2000;23(9):1381–3.
Su JB, Yang XH, Zhang XL, Cai HL, Huang HY, Zhao LH, Xu F, Chen T, Cheng XB, Wang XQ, et al. The association of long-term glycaemic variability versus sustained chronic hyperglycaemia with heart rate-corrected QT interval in patients with type 2 diabetes. PLoS ONE. 2017;12(8):e0183055.
Veglio M, Giunti S, Stevens LK, Fuller JH, Perin PC, Group EICS. Prevalence of Q-T interval dispersion in type 1 diabetes and its relation with cardiac ischemia: the EURODIAB IDDM Complications Study Group. Diabetes Care. 2002;25(4):702–7.
Kurnaz E, Erdeve SS, Ozgur S, Keskin M, Ozbudak P, Cetinkaya S, Aycan Z. Congenital long-QT syndrome in type 1 diabetes: a unique association. Turk J Pediatr. 2019;61(5):791–3.
Ning G, Reaction Study Group. Risk Evaluation of cAncers in Chinese diabeTic Individuals: a lONgitudinal (REACTION) study. J Diabetes. 2012;4(2):172–3.
Qin L, Yang Z, Gu H, Lu S, Shi Q, Xing Y, Li X, Li R, Ning G, Su Q. Association between serum uric acid levels and cardiovascular disease in middle-aged and elderly Chinese individuals. BMC Cardiovasc Disord. 2014;14:26.
Luo S, Michler K, Johnston P, Macfarlane PW. A comparison of commonly used QT correction formulae: the effect of heart rate on the QTc of normal ECGs. J Electrocardiol. 2004;37(Suppl):81–90.
Fagher K, Londahl M. The impact of metabolic control and QTc prolongation on all-cause mortality in patients with type 2 diabetes and foot ulcers. Diabetologia. 2013;56(5):1140–7.
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–9.
Gabir MM, Hanson RL, Dabelea D, Imperatore G, Roumain J, Bennett PH, Knowler WC. The 1997 American Diabetes Association and 1999 World Health Organization criteria for hyperglycemia in the diagnosis and prediction of diabetes. Diabetes Care. 2000;23(8):1108–12.
Orosz A, Baczko I, Nyiraty S, Korei AE, Putz Z, Takacs R, Nemes A, Varkonyi TT, Balogh L, Abraham G, et al. Increased short-term beat-to-beat QT interval variability in patients with impaired glucose tolerance. Front Endocrinol. 2017;8:129.
Tarvainen MP, Laitinen TP, Lipponen JA, Cornforth DJ, Jelinek HF. Cardiac autonomic dysfunction in type 2 diabetes-effect of hyperglycemia and disease duration. Front Endocrinol. 2014;5:130.
Mone P, Pansini A, Calabro F, De Gennaro S, Esposito M, Rinaldi P, Colin A, Minicucci F, Coppola A, Frullone S, et al. Global cognitive function correlates with P-wave dispersion in frail hypertensive older adults. J Clini Hypertens. 2022;24(5):638–43.
Hadaegh F, Mohebi R, Bozorgmanesh M, Saadat N, Sheikholeslami F, Azizi F. Electrocardiographic abnormalities improve classification of coronary heart disease risk in women: Tehran Lipid and Glucose Study. Atherosclerosis. 2012;222(1):110–5.
Rana BS, Lim PO, Naas AA, Ogston SA, Newton RW, Jung RT, Morris AD, Struthers AD. QT interval abnormalities are often present at diagnosis in diabetes and are better predictors of cardiac death than ankle brachial pressure index and autonomic function tests. Heart. 2005;91(1):44–50.
Kimura S, Nakao S, Kitaura A, Iwamoto T, Houri K, Matsushima M, Hamasaki S. Sevoflurane causes greater QTc interval prolongation in chronically hyperglycemic patients than in normoglycemic patients. PLoS ONE. 2017;12(12):e0188555.
Mylona M, Liatis S, Anastasiadis G, Kapelios C, Kokkinos A. Severe iatrogenic hypoglycaemia requiring medical assistance is associated with concurrent prolongation of the QTc interval. Diabetes Res Clin Pract. 2020;161:108038.
Festa A, D’Agostino R Jr, Rautaharju P, Mykkanen L, Haffner SM. Relation of systemic blood pressure, left ventricular mass, insulin sensitivity, and coronary artery disease to QT interval duration in nondiabetic and type 2 diabetic subjects. Am J Cardiol. 2000;86(10):1117–22.
Sohaib SM, Papacosta O, Morris RW, Macfarlane PW, Whincup PH. Length of the QT interval: determinants and prognostic implications in a population-based prospective study of older men. J Electrocardiol. 2008;41(6):704–10.
Li X, Ren H, Xu ZR, Liu YJ, Yang XP, Liu JQ. Prevalence and risk factors of prolonged QTc interval among Chinese patients with type 2 diabetes. Exp Diabetes Res. 2012;2012:234084.
Solini A, Passaro A, D’Elia K, Calzoni F, Alberti L, Fellin R. The relationship of plasma glucose and electrocardiographic parameters in elderly women with different degrees of glucose tolerance. Aging. 2000;12(4):249–55.
Brown DW, Giles WH, Greenlund KJ, Valdez R, Croft JB. Impaired fasting glucose, diabetes mellitus, and cardiovascular disease risk factors are associated with prolonged QTc duration. Results from the Third National Health and Nutrition Examination Survey. J Cardiovasc Risk. 2001;8(4):227–33.
van Noord C, Sturkenboom MC, Straus SM, Hofman A, Kors JA, Witteman JC, Stricker BH. Serum glucose and insulin are associated with QTc and RR intervals in nondiabetic elderly. Eur J Endocrinol. 2010;162(2):241–8.
Tesfamariam B, Brown ML, Cohen RA. Elevated glucose impairs endothelium-dependent relaxation by activating protein kinase C. J Clin Investig. 1991;87(5):1643–8.
Davis FB, Davis PJ, Nat G, Blas SD, MacGillivray M, Gutman S, Feldman MJ. The effect of in vivo glucose administration on human erythrocyte Ca2+-ATPase activity and on enzyme responsiveness in vitro to thyroid hormone and calmodulin. Diabetes. 1985;34(7):639–46.
Fiorentini A, Perciaccante A, Valente R, Paris A, Serra P, Tubani L. The correlation among QTc interval, hyperglycaemia and the impaired autonomic activity. Auton Neurosci Basic Clin. 2010;154(1–2):94–8.
Decode Study Group EDEG. Is the current definition for diabetes relevant to mortality risk from all causes and cardiovascular and noncardiovascular diseases? Diabetes Care. 2003;26(3):688–96.
Acknowledgements
The authors gratefully acknowledge the support of the study participants, study staff, and partner organizations participating in the baseline survey and follow-up investigation.
Funding
This work was supported by the National Natural Science Foundation of China (81970669). Hospital Funded Clinical Research, Xin Hua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine (19XHCR07B).
Author information
Authors and Affiliations
Contributions
NL, and HZ drafted the manuscript and performed the experiments. Su Q and Qin L conceived of and designed the study. HG, SL and LQ recruited the subjects, processed the samples, and contributed to the acquisition of the data. XL, and YN analyzed the data. ZY revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All procedures performed in this study involving human participants were approved by the Ethics Committee of the Xinhua Hospital affiliated to Shanghai Jiaotong University School of Medicine and performed in accordance with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Written informed consent was obtained from all the respondents.
Consent for publication
Not applicable.
Competing interests
No potential competing interest relevant to this article were reported.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Lin, N., Zhang, H., Li, X. et al. The influence of different glucose tolerance on QTc interval: a population-based study. BMC Cardiovasc Disord 23, 47 (2023). https://doi.org/10.1186/s12872-023-03081-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12872-023-03081-6