Abstract
Background
A link between uric acid (UA) levels and cardiovascular diseases has been previously reported. However, its importance as a risk factor is still controversial. This study sought to determine whether elevated serum uric acid levels are associated with cardiovascular disease (CVD) in middle-aged and elderly Chinese individuals.
Methods
We conducted a population-based cross-sectional study in Shanghai, with a total of 8510 participants aged ≥40 years. The CVD included diagnosed coronary heart disease (CHD) and stroke. MetS was defined according to the updated National Cholesterol Education Program Adult Treatment Panel III criteria for Asian Americans.
Results
Uric acid levels were positively associated with BMI, waist circumference, triglycerides, systolic blood pressure, diastolic blood pressure, glycohemoglobin, fasting plasma glucose, postprandial 2-hour plasma glucose (all P < 0.05), and negatively associated with HDL-cholesterol (P < 0.001). The prevalence of CVD significantly increased with increasing quartiles of UA in those without MetS group (p trend < 0.001), but not necessarily increased in those with MetS. After adjustment for metabolic syndrome and other cardiovascular risk factors, multivariate logistic regression analysis showed that odds ratios (OR) for CHD, stroke, and CVD in those in the fourth quartiles were 2.34 (95% confidence interval [CI] 1.73 to 3.45), 2.18 (95% CI 1.86 to 3.28), and 2.16 (95% CI 1.80 to 3.29), respectively, compared with those in the first quartile of UA.
Conclusions
Elevated serum uric acid level was associated with CVD, independent of conventional cardiovascular disease risk factors and metabolic syndrome.
Similar content being viewed by others
Background
During the past two decades, China has experienced rapid economic growth and the ageing of its population. Resulting changes in lifestyle and longer life expectancy have led to an increased burden of cardiovascular and other chronic diseases [1–3]. The metabolic syndrome (MetS) is characterized by a clustering of cardiovascular risk factors. Previous studies have demonstrated an association of metabolic syndrome with the development of cardiovascular disease (CVD) [3–5] and increased risk of mortality from CVD [6, 7].
Uric acid is the metabolic end product of purine metabolism in humans, excess accumulation can lead to various diseases [8]. Recently, a series of controversial and conflicting findings from epidemiological studies were reported [9–21]. Previous studies have demonstrated a strong relationship between serum uric acid levels and coronary heart disease (CHD) and some studies suggested that uric acid may be an independent risk factor for cardiovascular disease [9–17]. Moreover, recently a meta-analysis showed that hyperuricemia may increase the risk of CHD events, independently of traditional CHD risk factors [18]. However, the nature of the relationship between uric acid and cardiovascular disease remains a subject of debate [19–21].
Furthermore, although previous studies have analyzed the relationship between uric acid and CVD, thus far, evidence from large sample populations about the relationship between uric acid and CVD in Chinese people is scarce. In the present study, we undertook in large-scale Chinese populations. We first investigated the association between uric acid levels and confounding factors including metabolic syndrome. In addition, we also assessed whether there is an independent association of uric acid with cardiovascular disease in individuals subdivided according to metabolic syndrome status.
Methods
Study population and design
From May 2011 and November 2011, a population-based cross-sectional survey (Chongming Health Investigation) was conducted in Chongming District, Shanghai, China. A two-stage stratified sampling method was used. First, 12 residential communities or streets were randomly selected from the Chongming District. Of these, 8 urban communities and 4 rural communities were chosen to represent people with high to low socioeconomic status. Secondly, Within each community/street, all eligible individuals were sampled, with the exception that in households with more than one eligible individual, one individual was randomly selected. During the recruiting phase, inhabitants aged ≥40 years in these 12 communities were invited by telephone or door-to-door visit to participate in this study. A total of 9,930 subjects completed the survey, yielding a response rate of 92.4%. Each participant signed an informed consent form before completing the questionnaire. The protocol was approved by the Institutional Review Board of Xinhua Hospital affiliated with Shanghai Jiao-Tong University School of Medicine.After excluding subjects with missing data regarding serum uric acid (n = 1194) or coronary heart disease (n = 129) or stroke (n = 97), 8510 participants were included in the final analysis (Figure 1).
Data collection
A standardized questionnaire was used by trained physicians to collect information such as age, sex, medications, education level (6, 7–9, or ≥10 years in school). The history of chronic diseases and current use of medications were recorded. The smoking or alcohol consumption habit was defined as never, current (smoking or consuming alcohol regularly in the past 6 months), or ever (cessation of smoking or alcohol consumption for more than 6 months). The type, amount, and frequency of alcohol consumption were collected. Based on the alcohol content of the beverages reported, mean daily alcohol consumption was calculated and expressed in grams per day. Physical activity at leisure time was estimated using the short form of the International Physical Activity Questionnaire by adding questions on frequency and duration of moderate and vigorous activities and walking [22].
Anthropometric measurements were performed by the trained personnel using a standardized protocol. Height was measured to the nearest 0.1 cm, and weight was recorded to the nearest 0.1 kg while participants were wearing lightweight clothing and no shoes. Body mass index (BMI) was defined as body weight in kilograms divided by height squared in meters. Waist circumference (WC) was measured to the nearest 0.1 cm at the umbilical level with the participant in a standing position. Hip circumference was measured over the widest part of the gluteal region, and the waist-to-hip ratio was calculated as a measure of central obesity. Three blood pressure recordings were obtained from the right arm of patients in a sitting position after 30 min of rest; measurements were taken in 5-min intervals, and mean values were calculated.
Biochemical measurements
Peripheral venous blood samples were collected after an overnight fast. The fasting glucose, glucose 2 h after oral glucose tolerance test were measured with the use of the glucose oxidase method on an autoanalyzer (Modular P800; Roche, Basel, Switzerland). Triglycerides (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-c), low-density lipoprotein cholesterol (LDL-c), and serum uric acid were measured using chemiluminescence methods on the autoanalyzer (Modular E170; Roche). Glycated hemoglobin(HbA1c) was measured with the use of the Chromatography method on an autoanalyzer (D10; Bio-Rad, USA).
Diagnosis of CVD
Hypertension was defined as diastolic blood pressure ≥90 mm Hg, systolic blood pressure of ≥140 mm Hg, or current medication for hypertension (as defined by WHO 1999) [16]. Coronary heart disease (CHD) and stroke were defined using the WHO MONICA criteria [23]. Myocardial infarction was diagnosed by a representative set of electrocardiogram, cardiac enzyme values, and typical symptoms. Angina was defined as use of nitroglycerine, experience of typical chest pain, and electrocardiogram changes compatible with ischemic heart disease. Strokes were defined as events requiring hospitalization; this information was verified from local hospital records, and 82% of the cases were confirmed using computed tomography and magnetic resonance imaging. Cardiovascular disease in the present study was defined by the presence of one or more of these two outcomes: CHD and stroke. Subjects with a fasting plasma glucose level of 7.0 mmol/l and/or a 2-h plasma glucose level of 11.1 mmol/l during an oral glucose tolerance test and/or who were receiving antidiabetic medications were diagnosed with diabetes mellitus. The diagnosis of cardiovascular events was based on self-reports, confirmed by hospital medical records and further clinical examinations carried out at the time of the survey (including electrocardiogram and ankle-arm systolic blood pressure index).
Definition of MetS
The MetS was defined based upon the updated NCEP-ATPIII for Asian Americans [24] as presenting 3 or more of the following components: 1) waist cirumference ≥90 cm for men or ≥80 cm for women; 2) triglycerides ≥1.7 mmol/l; 3) HDL cholesterol <1.03 mmol/l for men or <1.30 mmol/l for women; 4) blood pressure ≥130/85 mm Hg or current use of anti-hypertensive medications; and 5) fasting glucose ≥5.6 mmol/l or previously diagnosed type 2 diabetes or on oral antidiabetic agents or insulin.
Statistical analysis
Normally distributed data were expressed as means ± SD, whereas variables with a skewed distribution were reported as median (interquartile range) and log transformed to approximate normality before analysis. Categorical variables were represented by frequency and percentage. The association of demographic, medical, metabolic and clinical characteristics with serum uric acid quartiles was assessed using multivariate linear or logistic regression analyses, adjusting for age and gender, for continuous and dichotomous variables, respectively. The correlations among serum uric acid, anthropometric indices and metabolic features adjusting for age and gender were obtained using Spearman partial correlation analysis. Sampling weights was used when estimating prevalence of CVD. The trend for age- and sex-adjusted prevalence of CVD according to quartile of uric acid and the presence of metabolic syndrome were analysed using linear regression model. Multivariate logistic regression models were used to estimate the odds ratios (ORs) for CHD, stroke and CVD. Potential confounding variables including age, gender, smoking, alcohol drinking, physical activity, educational level, education, BMI, and metabolic syndrome were controlled in the regression models. All statistical analysis were performed with the SPSS Statistical Package (version 15.0; SPSS Inc., Chicago, IL). P < 0.05 was considered statistically significant.
Results
Characteristics of participants according to serum uric acid quartiles
Included were 8510 individuals who had both uric acid and CVD assessments (Figure 1). The mean age of this cohort was 55.90 ± 7.89 years, and approximately 32% were men. Baseline demographic and medical characteristics for both genders combined and divided by uric acid quartiles are presented in Table 1. Higher uric acid levels were associated with higher proportion of metabolic syndrome, proportion of men, age, smoking, alcohol consumption, systolic blood pressure, diastolic blood pressure, BMI, waist circumference, hip circumference, waist to hip ratio, fasting plasma glucose, postprandial 2-hour plasma glucose, glycohemoglobin, triglycerides, total cholesterol, LDL-cholesterol, presence of stroke, presence of CHD, hypertension, diabetes (all P < 0.05). In contrast, the patients with higher uric acid levels displayed lower levels of HDL cholesterol (P < 0.001) (Table 1).
Association between serum uric acid and CHD, stroke and CVD
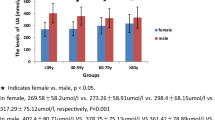
Partial Spearman correlation analysis demonstrated the strongest correlation between uric acid and triglycerides among various metabolic features (Table 2). Moreover there was strong partial correlation between uric acid and BMI, TC, Waist circumference (WC), systolic blood pressure (SBP) and diastolic blood pressure (DBP), adjusting for age and gender, (all P < 0.001) (Table 2). As presented in Table 3, the ORs for CHD, stroke and CVD were higher with increasing uric acid quartiles (all p < 0.001 for linear trend). In the highest uric acid quartile, the adjusted ORs of CHD, stroke and CVD were 2.34 (95% confidence interval [CI] 1.73 to 3.45), 2.18 (95% CI 1.86 to 3.27), and 2.16 (95% CI 1.80 to 3.29), respectively, compared with those in the first quartile of UA (Table 3).We further analyzed the prevalence of CVD according to quartile of uric acid and the presence of metabolic syndrome. After adjustments for age, gender, alcohol drinking, smoking, education, physical activity, TC, LDL, the prevalence of CVD in subjects with metabolic syndrome increased with uric acid quartile (p < 0.001 for linear trend) (Figure 2). No trend was observed in subject without metabolic syndrome (p = 0.217 for linear trend).
Prevalence of CVD according to quartiles of uric acid and the presence of metabolic syndrome (adjusted for age, gender, alcohol drinking, smoking, education, physical activity, TC, LDL). Data are expressed as percentages ± SE. P < 0.001 for trend in MS(−) group, and p = 0.217 for trend in MS(+) group.
Discussion
In the present study, we found that serum uric acid levels showed association with the risk of CVD from the cross-sectional data in middle-aged and elderly Chinese individuals, independent of conventional cardiovascular risk factors including components of metabolic syndrome.
Several previous studies in literature have documented the relationship between serum uric acid levels and cardiovascular diseases [9–17, 25]. Increased serum uric acid was found to be associated with important risk factors for atherosclerosis like hypertension [26–29], abdominal obesity [28], diabetes mellitus [29, 30], the metabolic syndrome [17], hypertriglyceridemia [31], endothelial dysfunction [32] and renal failure [33]. However, whether uric acid is an independent risk factor for cardiovascular mortality is still a controversy. Difficulties in determining whether uric acid should be considered a cardiovascular risk factor may be explained by its frequent association with other cardiovascular risk factors [34] for which uric acid is considered as a risk marker or epiphenomenon or even an adaptive change to protect from atherosclerosis due to its antioxidant properties [35] and the controversial and conflicting findings from epidemiological studies [36].
The metabolic syndrome is characterized by a clustering of cardiovascular risk factors, including abdominal obesity, high blood pressure, increased glucose concentration, and dyslipidemia, is a common basis for the development of atherosclerosis, especially CVD [37–39]. Moreover, previous studies claimed that hyperuricemia was a new component of metabolic syndrome. Furthermore, in our study, the proportion of metabolic syndrome is in parallel with increasing serum uric acid quartiles. Thus, in order to explore whether elevated serum uric acid levels are associated with CVD independent of metabolic syndrome, we further analyzed the prevalence of CVD according to quartile of uric acid and the presence of metabolic syndrome. We found that prevalence of CVD is significantly increased with increasing quartiles of uric acid in without MetS group (p trend < 0.001), but not necessarily increased in those with metabolic syndrome. Additional factors besides metabolic syndrome may also play a key role in the development of CVD in hyperuricemia subjects, which requires further research.
The present study may have some implications. In particular, this study may strengthen the need for interventional studies with uric acid-lowering therapies to maintain UA levels in the lower safe range. Moreover, allopurinol – a xanthine oxidase inhibitor – has been shown recently to significantly improve endothelial function and abolish vascular oxidative stress [40], has a clinically relevant antiischaemic effect and has been well tolerated in patients with angina [41]. In analogy with homocysteine-lowering therapy interventional studies with UA-lowering agents, apart from exploring clinical benefits of these agents, may provide valuable additional information regarding causality in the uric acid–cardiovascular disease relationship.
The major strength of our present study was that the large sample size and representative sample with a relatively high response rate. Several limitations of our study have also to be addressed. First, due to the cross-sectional nature of the present study, no causal relationships can be established. Large prospective studies are in urgent need to confirm the relationship between serum uric acid levels and CVD. Second, although most potential confounders were carefully controlled, since some of the study subjects may had several chronic disease, we could not eliminate the possible effect of underlying diseases and medications used for these diseases on the present findings.
Conclusion
In conclusion, the present study showed that elevated serum uric acid level was associated with CVD, independent of conventional CVD risk factors and the presence of metabolic syndrome. The study added more evidence to the notion that the risk of CVD increased in subjects with hyperuricemia.
References
Wu Z, Yao C, Zhao D, Wu G, Wang W, Liu J, Zeng Z, Wu Y: Sino-MONICA project: a collaborative study on trends and determinants in cardiovascular diseases in China, part I: morbidity and mortality monitoring. Circulation. 2001, 103: 462-468. 10.1161/01.CIR.103.3.462.
Yang Z, Wang X, Wen J, Ye Z, Li Q, He M, Lu B, Ling C, Wu S, Hu R: Prevalence of non-alcoholic fatty liver disease and its relation to hypoadiponectinaemia in the middle-aged and elderly Chinese population. Arch Med Sci. 2011, 665-672.
He Y, Jiang B, Wang J, Feng K, Chang Q, Fan L, Li X, Hu FB: Prevalence of the metabolic syndrome and its relation to cardiovascular disease in an elderly Chinese population. J Am Coll Cardiol. 2006, 47: 1588-1594. 10.1016/j.jacc.2005.11.074.
Haffner SM, Valdez RA, Hazuda HP, Mitchell BD, Morales PA, Stern MP: Prospective analysis of the insulin-resistance syndrome (Syndrome X). Diabetes. 1992, 41: 715-722. 10.2337/diab.41.6.715.
Isomaa B, Almgren P, Tuomi T, Forsén B, Lahti K, Nissén M, Taskinen MR, Groop L: Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care. 2001, 24: 683-689. 10.2337/diacare.24.4.683.
Trevisan M, Liu J, Bahsas FB, Menotti A: Syndrome X and mortality:a population-based study. Risk Factor and Life Expectancy Research Group. Am J Epidemiol. 1998, 148: 958-966. 10.1093/oxfordjournals.aje.a009572.
Lakka HM, Laaksonen DE, Lakka TA, Niskanen LK, Kumpusalo E, Tuomilehto J, Salonen JT: The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA. 2002, 288: 2709-2716. 10.1001/jama.288.21.2709.
So A, Thorens B: Uric acid transport and disease. J Clin Invest. 2010, 120: 1791-1799. 10.1172/JCI42344.
Neogi T, Ellison RC, Hunt S, Terkeltaub R, Felson DT, Zhang Y: Serum uric acid is associated with carotid plaques: the National Heart, Lung, and Blood Institute Family Heart Study. J Rheumatol. 2009, 36: 378-384.
Bos MJ, Koudstaal PJ, Hofman A, Witteman JC, Breteler MM: Uric acid is a risk factor for myocardial infarction and stroke: the Rotterdam study. Stroke. 2006, 37: 1503-1507. 10.1161/01.STR.0000221716.55088.d4.
Franse LV, Pahor M, Di Bari M, Shorr RI, Wan JY, Somes GW, Applegate WB: Serum uric acid, diuretic treatment and risk of cardiovascular events in the Systolic Hypertension in the Elderly Program (SHEP). J Hypertens. 2000, 18: 1149-1154. 10.1097/00004872-200018080-00021.
Juraschek SP, Tunstall-Pedoe H, Woodward M: Serum uric acid and the risk of mortality during 23 years follow-up in the Scottish Heart Health Extended Cohort Study. Atherosclerosis. 2014, 233: 623-629. 10.1016/j.atherosclerosis.2014.01.026.
Ito H, Abe M, Mifune M, Oshikiri K, Antoku S, Takeuchi Y, Togane M: Hyperuricemia is independently associated with coronary heart disease and renal dysfunction in patients with type 2 diabetes mellitus. PLoS One. 2011, 6: e27817-10.1371/journal.pone.0027817.
Freedman DS, Williamson DF, Gunter EW, Byers T: Relation of serum uric acid to mortality and ischemic heart disease. The NHANES I Epidemiologic Follow-up Study. Am J Epidemiol. 1995, 141: 637-644.
Krishnan E, Baker JF, Furst DE, Schumacher HR: Gout and the risk of acute myocardial infarction. Arthritis Rheum. 2006, 54: 2688-2696. 10.1002/art.22014.
Verdecchia P, Schillaci G, Reboldi G, Santeusanio F, Porcellati C, Brunetti P: Relation between serum uric acid and risk of cardiovascular disease in essential hypertension. The PIUMA study. Hypertension. 2000, 36: 1072-1078. 10.1161/01.HYP.36.6.1072.
Li Q, Yang Z, Lu B, Wen J, Ye Z, Chen L, He M, Tao X, Zhang W, Huang Y, Zhang Z, Qu S, Hu R: Serum uric acid level and its association with metabolic syndrome and carotid atherosclerosis in patients with type 2 diabetes. Cardiovasc Diabetol. 2011, 10: 72-10.1186/1475-2840-10-72.
Kim SY, Guevara JP, Kim KM, Choi HK, Heitjan DF, Albert DA: Hyperuricemia and coronary heart disease: a systematic review and meta-analysis. Arthritis Care Res (Hoboken). 2010, 62: 170-180.
Culleton BF, Larson MG, Kannel WB, Levy D: Serum uric acid and risk for cardiovascular disease and death: the Framingham Heart Study. Ann Intern Med. 1999, 131: 7-13. 10.7326/0003-4819-131-1-199907060-00003.
Brand FN, McGee DL, Kannel WB, Stokes J, Castelli WP: Hyperuricemia as a risk factor of coronary heart disease: The Framingham Study. Am J Epidemiol. 1985, 121: 11-18.
Moriarity JT, Folsom AR, Iribarren C, Nieto FJ, Rosamond WD: Serum uric acid and risk of coronary heart disease: Atherosclerosis Risk in Communities (ARIC) Study. Ann Epidemiol. 2000, 10: 136-143.
Guidelines for data processing and analysis of the International Physical Activity Questionnaire (IPAQ). 2006, Available at: http://www.ipaq.ki.se/
WHO/MONICA-Project: Multinational Monitoring of Trends andDeterminants in Cardiovascular Diseases (MONICA Project) and Manual of Operation. 1983, Geneva, Switzerland: World Health Organization, Cardiovascular Disease Unit
Liu J, Grundy SM, Wang W, Smith SC, Vega GL, Wu Z, Zeng Z, Wang W, Zhao D: Ethnic-Specific Criteria for the Metabolic Syndrome. Diabetes Care. 2006, 29: 1414-1416. 10.2337/dc06-0481.
Takayama S, Kawamoto R, Kusunoki T, Abe M, Onji M: Uric acid is an independent risk factor for carotid atherosclerosis in a Japanese elderly population without metabolic syndrome. Cardiovasc Diabetol. 2012, 11: 2-10.1186/1475-2840-11-2.
Perlstein TS, Gumieniak O, Williams GH, Sparrow D, Vokonas PS, Gaziano M, Weiss ST, Litonjua AA: Uric acid and the development of hypertension: the normative aging study. Hypertension. 2006, 48: 1031-1036. 10.1161/01.HYP.0000248752.08807.4c.
Shankar A, Klein R, Klein BE, Nieto FJ: The association between serum uric acid level and long-term incidence of hypertension: Population-based cohort study. J Hum Hypertens. 2006, 20: 937-945. 10.1038/sj.jhh.1002095.
Masuo K, Kawaguchi H, Mikami H, Ogihara T, Tuck ML: Serum uric acid and plasma norepinephrine concentrations predict subsequent weight gain and blood pressure elevation. Hypertension. 2003, 42: 474-480. 10.1161/01.HYP.0000091371.53502.D3.
Nakanishi N, Okamoto M, Yoshida H, Matsuo Y, Suzuki K, Tatara K: Serum uric acid and risk for development of hypertension and impaired fasting glucose or Type II diabetes in Japanese male office workers. Eur J Epidemiol. 2003, 18: 523-530.
Dehghan A, Van Hoek M, Sijbrands EJ, Hofman A, Witteman JC: High serum uric acid as a novel risk factor for type 2 diabetes. Diabetes Care. 2008, 31: 361-362.
Rathmann W, Funkhouser E, Dyer AR, Roseman JM: Relations of hyperuricemia with the various components of the insulin resistance syndrome in young black and white adults: the CARDIA study. Coronary Artery Risk Development in Young Adults. Ann Epidemiol. 1998, 8: 250-261.
Khosla UM, Zharikov S, Finch JL, Nakagawa T, Roncal C, Mu W, Krotova K, Block ER, Prabhakar S, Johnson RJ: Hyperuricemia induces endothelial dysfunction. Kidney Int. 2005, 67: 1739-1742. 10.1111/j.1523-1755.2005.00273.x.
Iseki K, Ikemiya Y, Inoue T, Iseki C, Kinjo K, Takishita S: Significance of hyperuricemia as a risk factor for developing ESRD in a screened cohort. Am J Kidney Dis. 2004, 44: 642-650. 10.1053/j.ajkd.2004.06.006.
Davies KJ, Sevanian A, Muakkassah-Kelly SF, Hochstein P: Uric acid-iron ion complexes. A new aspect of the antioxidant functions of uric acid. Biochem J. 1986, 235: 747-754.
Fang J, Alderman MH: Serum uric acid and cardiovascular mortality the NHANES I epidemiologic follow-up study, 1971–1992. National Health and Nutrition Examination Survey. JAMA. 2000, 283: 2404-2410. 10.1001/jama.283.18.2404.
Niskanen LK, Laaksonen DE, Nyyssonen K, Alfthan G, Lakka HM, Lakka TA: Uric acid level as a risk factor for cardiovascular and all-cause mortality in middle-aged men: a prospective cohort study. Arch Intern Med. 2004, 164: 1546-1551. 10.1001/archinte.164.14.1546.
Chen L, Yang Z, Lu B, Li Q, Ye Z, He M, Huang Y, Wang X, Zhang Z, Wen J, Liu C, Qu S: Hu R:Serum CXC ligand 5 is a new marker of subclinical atherosclerosis in type 2 diabetes. Clin Endocrinol (Oxf). 2011, 75: 766-770. 10.1111/j.1365-2265.2011.04119.x.
Yang Z, Zhang Z, Wen J, Wang X, Lu B, Yang Z, Zhang W, Wang M, Feng X, Ling C, Wu S, Hu R: Elevated serum chemokine CXC ligand 5 levels are associated with hypercholesterolemia but not a worsening of insulin resistance in Chinese people. J Clin Endocrinol Metab. 2010, 95: 3926-3932. 10.1210/jc.2009-2194.
Huang Y, Yang Z, Ye Z, Li Q, Wen J, Tao X, Chen L, He M, Wang X, Lu B, Zhang Z, Zhang W, Qu S, Hu R: Lipocalin-2, glucose metabolism and chronic low-grade systemic inflammation in Chinese people. Cardiovasc Diabetol. 2012, 11: 11-10.1186/1475-2840-11-11.
Rajendra NS, Ireland S, George J, Belch JJ, Lang CC, Struthers AD: Mechanistic insights into the therapeutic use of high-dose allopurinol in angina pectoris. J Am Coll Cardiol. 2011, 58: 820-828. 10.1016/j.jacc.2010.12.052.
Noman A, Ang DS, Ogston S, Lang CC, Struthers AD: Effect of high-dose allopurinol on exercise in patients with chronic stable angina: a randomised, placebo controlled crossover trial. Lancet. 2010, 375: 2161-2167. 10.1016/S0140-6736(10)60391-1.
Pre-publication history
The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-2261/14/26/prepub
Acknowledgements
This work was supported by the Shanghai Science and Technology Commission (10411956600), National Natural Science Foundation of China (81000332, 81370953, 81370935), Shanghai Health System Outstanding Young Talents Training Program (XYQ2013098), Chinesse Society of Endocrinology (13040580443). We thank the field workers for their contribution and the participants for their cooperation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
Conceived and designed the experiments: QS. Analyzed the data: LQ, ZY. Contributed reagents/materials/analysis tools: LQ, ZY, HG, SL, QS, YX, XL, RL, GN. Wrote the paper: LQ, ZY. All authors read and approved the final manuscript.
Li Qin, Zhen Yang contributed equally to this work.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Qin, L., Yang, Z., Gu, H. et al. Association between serum uric acid levels and cardiovascular disease in middle-aged and elderly Chinese individuals. BMC Cardiovasc Disord 14, 26 (2014). https://doi.org/10.1186/1471-2261-14-26
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2261-14-26