Abstract
Purpose of Review
Patients with severe tricuspid regurgitation (TR) are at risk for significant morbidity and mortality. Transcatheter tricuspid valve interventions (TTVI) may offer patients less invasive treatment alternatives to surgery. This review evaluates the most common class of device currently used worldwide to treat TR, tricuspid transcatheter edge-to-edge repair (T-TEER) and orthotopic transcatheter tricuspid valve replacement (TTVR), both of which are now approved in the USA and Europe.
Recent Findings
The first pivotal randomized clinical trial, TRILUMINATE, demonstrated that T-TEER can safely reduce TR and is associated with improved health status outcomes. However, results of this trial have raised questions about whether this device can provide sufficient TR reduction to impact clinical outcomes. Orthotopic TTVR has recently gained attention with initial data suggesting near-complete TR elimination.
Summary
The current review examines the technical features and anatomic limitations of the most commonly used devices for T-TEER and orthotopic TTVR, discusses the current clinical data for these devices, and offers a theoretical construct for device selection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There has been growing awareness regarding the prevalence and impact of tricuspid regurgitation (TR) on outcomes [1•]. Clinically significant TR is highly prevalent, afflicting nearly 5 million individuals in the United States and Europe with increasing prevalence in patients of advanced age and women and is associated with substantial morbidity and mortality [1,2,3,4,5]. There are however no Class I medical therapy recommendations to treat symptomatic severe TR in the current guidelines given the paucity of evidence in this understudied population [6, 7]. To add to the management challenges, the only Class I indication for surgical therapy in the American guidelines is in the setting of correction of concomitant left-sided valve surgery [6] with isolated tricuspid valve (TV) surgery associated with high morbidity and mortality [8, 9]. The poor outcomes associated with isolated TV surgery is in large part due to the late presentation of these patients, related to a number of factors: (1) lack of guideline recommendations and limited validation for risk assessment scores [10], (2) underappreciation of the independent association of TR with outcomes particularly in the setting of secondary disease [11], (3) non-specific symptoms preventing early clinical diagnosis [10], and (4) underutilization of quantitative imaging modalities for both TR and right ventricular (RV) assessment [12, 13].
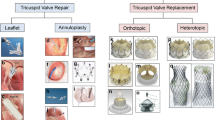
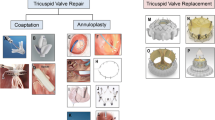
These challenges form the justification for the rapid growth of transcatheter tricuspid valve interventions (TTVI) currently under investigation [14]. These fall into 4 broad categories based on their primary mechanism of action: leaflet approximation, annular reduction, orthotopic valve replacement, and heterotopic valve replacement. Both a tricuspid transcatheter edge-to-edge repair (T-TEER) and a transcatheter tricuspid valve replacement (TTVR) systems have been recently approved in the USA, and devices belonging to all four categories have now been approved for use in Europe, although annular reduction and heterotopic valve replacement devices have not seen broad adoption. The majority TTVI implants have been leaflet approximation and orthotopic valve replacement devices. The current review will examine the technical features and anatomic limitations of the most commonly used devices for T-TEER and orthotopic TTVR, discuss the current clinical data related to patient selection and outcomes, and offer a theoretical construct for device choice.
Current Device Technology
Tricuspid Transcatheter Edge-to-Edge Repair
Tricuspid-specific TEER is currently the most used TTVI treatment strategy across the world. The T-TEER devices aim to reproduce the Alfieri surgical technique by facilitating improved leaflet approximation to reduce valvular regurgitation [15]. These devices grasp and bring together opposing leaflets, thereby reducing coaptation gaps and severity of TR. Of the T-TEER devices, TriClipTM (Abbott Structural Heart, Santa Clara, CA) and PASCAL (Edwards Lifesciences, Irvine, CA) are the most extensively studied. TEER in the tricuspid position is typically performed under general anesthesia with two-dimensional (2D) and three-dimensional (3D) transesophageal echocardiogram (TEE) guidance which has challenges given the far-field imaging of a large valve with thin leaflets [12, 16]. The recent commercial availability of 3D intracardiac echocardiography has provided an adjunctive imaging tool for this procedure [17]. While T-TEER has the potential to substantially reduce TR, < 60% of patients will achieve ≤ mild TR [18,19,20].
The TriClipTM system uses a right heart-specific guide and delivery system and the 4th generation implants: NT and XT clip sizes (4 mm width; 9 [NT] and 12 [XT] mm arm length), a wider implant size of 6 mm is available with both arm lengths (6 mm width; 9 [NTW] and 12 [XTW] mm arm length). The two rigid arms of cobalt-chromium alloy have flexible nitinol-based “grippers” with longitudinally arranged frictional elements, four for the NT and NTW and six for the XT and XTW. There is independent and controlled gripper action and an active locking mechanism. There are two working catheters for positioning the device, the clip delivery system (CDS), and the guide catheter; for optimal steerability, the two markers of the CDS must “straddle” the tip of the guide.
The PASCAL system for either mitral or tricuspid TEER has three working catheters with the device attached to the distal end of the inner implant catheter. With this design, there is high range of motion and maneuverability without dictating the relative positions of the catheters. The device itself is nitinol throughout with two spring-loaded, curved paddles (10 mm wide for P10 and 6 mm wide for ACE) with horizontally arranged retention elements along the distal end of the paddles and a central spacer of varying diameters (smaller for the ACE). The nitinol clasps can be controlled individually, enabling either simultaneous or independent leaflet capture and with passive closing mechanism (based on nitinol shape memory).
Transcatheter Tricuspid Valve Replacement
Orthotopic TTVR has the potential to completely eliminate TR, with anatomic feasibility dependent on the anchoring mechanism of the particular device. Potential mechanisms for maintenance of device stability include the use of radial force against the annular anatomy, tricuspid leaflet/annular engagement, and non-TV anchoring mechanisms such as the septum or vena cava [21].
The EVOQUE tricuspid valve replacement system (Edwards Lifesciences, Irvine, CA) is the most extensively studied of these devices [22,23,24,25,26]. The device is comprised of a 27-mm trileaflet bovine pericardial tissue valve implant in a nitinol frame with diameters of 44 mm, 48 mm, and 52 mm. Nine anchors are attached to the outer frame for implantation stability, with a sealing skirt to minimize paravalvular leak. The delivery system is inserted over an echocardiographically positioned guidewire and advanced across the TV. After position and trajectory are confirmed, the nine anchors are exposed by retracting the capsule to ensure that anchors remain below the leaflet tips and above the papillary muscle heads. During further expansion, anchor tips are positioned below the annulus ensuring leaflets are captured. At each stage of the procedure, all nine anchors must be individually imaged for correct positioning [27]. After optimal anchor positioning and confirmed leaflet capture, the EVOQUE valve is fully deployed and released from the delivery system. TEE-guided positioning this TTVR utilizes advanced 3D imaging capabilities and is frequently less challenging than the T-TEER devices.
Anatomic Suitability
The Tricuspid Valve Academic Research Consortium (TVARC) [28] defines that the adequate performance of a transcatheter device whose purpose is a reduction in TR should include the absence of tricuspid stenosis (TV area ≥ 1.5 cm2 or TV area index ≥ 0.9 cm2 /m2 [≥ 0.75 if BMI > 30], Doppler index < 2.2, mean gradient < 5 mmHg) and reduction of total TR to optimal (≤ mild [1+]) or acceptable (≤ moderate [2+]). Multiple studies have shown that worse outcomes are associated with greater severity of residual TR [18, 29,30,31]. Thus, anatomic parameters which may predict procedural success may be used to identify suitable candidates for these procedures and are shown in Fig. 1.
T-TEER
In most T-TEER studies, procedural success is defined as a TR reduction to ≤ moderate or 2+. In this setting, predictors of success have been identified in various small studies and include the following: location of jet, size of the coaptation gap, leaflet morphology (i.e., number of leaflets [32]), leaflet thickness or calcification, leaflet mobility, complexity of subvalvular apparatus, severity of TR, leaflet-to-annulus ratio, location and extent of CIED-related TR, tethering height, and right atrial volume (Fig. 1) [30, 33,34,35,36,37,38,39,40]. Very large coaptation gaps, torrential TR, markedly thickened or immobile leaflets, and CIED-related TR where the device is adherent to leaflets or subvalvular apparatus precluding adequate TR reduction may be relative exclusion for this technology. In addition, visualization of the leaflets during the procedure is required and the use of TEE with or without adjunctive intracardiac echocardiographic imaging, heavily relying on 3D functions, is also an “anatomic” requirement.
TTVR
The anatomic requirements for TTVR are primarily related to the ability to position the device within the annular plane and the anchoring mechanism. Thus, different devices will have different anatomic restrictions. A balloon-expandable valve implantation within a surgical valve prosthesis or ring was the first type of TTVR performed [41,42,43]. However TTVR for native TV disease has progressed rapidly, moving from a transatrial approach [44,45,46] to transfemoral [23, 24, 47] or transjugular venous approaches [48]. Unlike the T-TEER devices, large coaptation gaps, torrential TR, complex leaflet morphologies, markedly thickened or immobile leaflets, and CIED-related TR are not typically exclusions to TTVR. For these devices, significant determinants of feasibility are the size of the current devices as well as the ability to steer the device to obtain a coaxial implantation trajectory, in large part determined by the size of the implant device and available right heart space. Although also described for T-TEER [49], acute afterload mismatch and RV failure following obliteration of severe TR may theoretically be a greater concern for TTVR since ≤ mild TR may be achieved in > 90% of patients [26, 50••].
Review of Device Outcomes
Outcomes for the T-TEER and TTVR are limited to early feasibility studies, and post-market registries with only one randomized controlled trial (RCT) currently reported. To understand difference in efficacy and outcomes of these trials, it is important to know the baseline characteristics of the trials (Table 1) and the reported procedural outcomes (Table 2).
T-TEER
The TRILUMINATE early feasibility study demonstrated sustained TR reduction after TriClipTM in an 85-patient study with 2-year follow-up [18, 51, 52]. Echocardiographic markers of right heart size and hemodynamics and quality of life parameters all similarly demonstrated persistent favorable trends at 2 years. Moreover, when compared to baseline data prior to intervention, significant reductions in all-cause hospitalizations were present after T-TEER therapy in individuals with 2-year follow-up (0.66 events per patient year vs. 1.30 events per patient year, p < 0.0001).
TriClipTM initially received CE Mark approval in Europe, and the FDA recently approved this device in the USA after reviewing the TRILUMINATE Pivotal RCT results [19••]. This study randomized 350 patients of intermediate or greater surgical risk patients with severe TR to T-TEER with medical therapy versus medical therapy alone in 1:1 fashion. The primary composite endpoint was a hierarchical composite of all-cause death or tricuspid-valve surgery, heart failure hospitalization, and improvement in quality of life measured by KCCQ at 1 year. Baseline patient characteristics of the control and intervention arms of this RCT are presented in Table 1. Mean age of enrolled patients was approximately 78 ± 7 years with approximately 70% presenting with massive or torrential TR. While the primary endpoint was met and favored the T-TEER group (win ratio, 1.48; 95% confidence interval [CI], 1.06–2.13; p = 0.02), this was primarily driven by improvements in KCCQ score at 1 year (mean difference, 11.7; 95%CI, 6.8–16.6; p < 0.001). Changes in KCCQ scores were significantly associated with degree of residual TR and magnitude of TR reduction at 1 year, and similar improvements in KCCQ were observed across several patient subgroups. No benefits with regards to rates of all-cause death/ TV surgery or heart failure hospitalizations were observed with T-TEER therapy. Reductions in TR severity were noted with TriClipTM, as a significantly greater proportion of patients had moderate or less TR at 30 days compared with the medical therapy group (87.0% vs. 4.8%, p < 0.001) and similar findings were noted at 1-year follow up (88.9% vs. 5.7%). The majority of patients (98.3%) who underwent T-TEER did not experience major adverse events within 30 days. While procedural success rates were high (87%), major bleeding (5.2%), SLDA (7%), tricuspid mean gradient ≥ 5 mmHg (5%), device embolization (0%), and device thrombosis (0%) were not frequently observed events (Table 2).
A dedicated analysis of health status outcomes delved into further describing the benefits with regards to quality-of-life parameters in the TRILUMINATE trial [53]. Results of this analysis confirmed that health status benefits of T-TEER persisted from 1 month after randomization through 1-year follow-up (mean KCCQ between-group difference 10.4 points, 95% CI 6.3–14.6). Patients who received T-TEER were more likely to be alive and well at 1 year when compared to patients in the medical therapy group (number needed to treat, 3.5). While results were largely consistent across subgroups, patients with preserved cardiac index (≥ 2 L/min/m2) appeared more likely to benefit compared to individuals with reduced cardiac index.
More recently, the bRIGHT post-approval study presented further safety and performance data of T-TEER with TriClipTM (Tables 1 and 2) [30]. This prospective, single-arm, open-label, multicenter, post-market registry performed at 26 sites in Europe evaluated outcomes after T-TEER in 511 patients with largely massive or torrential TR (88%) and significant concurrent heart failure symptomatology (80% with New York Heart Association [NYHA] Class III or IV). Successful device implantation was observed in 504 (99%) of patients, and procedural success (implantation success with at least one grade TR reduction noted on discharge or 30 days when appropriate) was achieved in 451 (91%) of patients. After TriClipTM therapy, 80% of patients were noted to have moderate or less TR at on discharge, and these findings were fairly consistent at 30 days. Significant improvements in NYHA functional class, KCCQ scores, and RV echocardiographic parameters were noted at 30 days. The overall adverse event rate at 30 days was 2.7% with a cardiovascular mortality rate noted to be low (0.8%).
The TRILUMINATE Pivotal trial proved that T-TEER with TriClipTM can be a safe and effective treatment for sustained reduction in severe TR (Tables 1 and 2). That the degree of reduction was associated with improvements in KCCQ scores suggests a mechanistic relationship between TR reduction quality-of-life metrics. However, given open label trial and lack of a sham control, it remains unclear if the perceived benefits in KCCQ improvement may have been, at least in part, due to unblinded nature of the study. Moreover, lack of benefits with regards to all-cause death or need for TV surgery, heart failure hospitalizations, or 6-min walk test were not encouraging. Questions remain regarding whether outcomes of TRILUMINATE may have been impacted by patient selection or duration of follow-up after intervention. Specifically with regards to patient selection, key differences between the TRILUMINATE RCT and bRIGHT have been noted. In addition to observed differences in baseline characteristics, patients in bRIGHT more frequently had massive and torrential TR as well as trends towards higher proportion of New York Heart Association (NYHA) III/IV symptoms, lower KCCQ scores, and more frequently were admitted with heart failure hospitalizations in the year prior to enrollment [19, 30]. Thus, it remains to be determined whether specifically patient subsets (e.g., potentially higher risk and more symptomatic patients) may derive more benefit from T-TEER therapy.
After description of the initial PASCAL compassionate use experience in high surgical risk or inoperable patients [54], results of the CLASP TR Early Feasibility Study up to 1 year after treatment have been made available [55, 56]. The recently presented 1-year report summarizes outcomes after T-TEER with PASCAL in a cohort of 65 patients [56]. Significant reductions in TR severity and improvements in NYHA functional class, KCCQ score, and 6-min walk test were observed at 30 days, and the initial 30-day benefits with regards to these parameters remained consistent at 1-year follow-up. Rates of major adverse events were 9.2% at 30 days and 16.9% at 1 year, driven mainly by cardiovascular mortality and severe bleeding events. Only three (4.6%) SLDA events were observed in this study (Table 2).
Given narrower profile and longer clasps, some have suggested that the PASCAL Ace device design may prove to be beneficial for use in the tricuspid space in the presence of anatomical characteristics such as dense chordae, annular shape and size, and wide coaptation gaps [57]. The recent report from the PASTE multicenter registry (PASCAL for Tricuspid Regurgitation—A European Registry) studied 235 high-risk patients, most with ≥ severe, functional TR, with after commercial approval in Europe provided more insights [31]. Overall procedural success was 78%, and sustained reduction in TR or ≤ moderate TR in 78% of patients at furthest follow-up available (~ 6 months). This analysis suggested that treatment with both the PASCAL and PASCAL Ace device may result in similar results, resulting in comparable reduction in TR to moderate or less by echocardiographic core laboratory analysis.
The currently underway, pivotal, CLASP II TR RCT (NCT04097145) which aims to randomize 870 patients in 2:1 fashion between treatment with PASCAL T-TEER and guideline-directed medical therapy will certainly provide more insights regarding TTVI treatment of severe, symptomatic TR. The primary endpoint of this study is all-cause mortality, RV assist-device implantation or heart transplant, TV intervention, heart failure hospitalizations, and quality of life improvement at 24 months of follow up. Data from the roll-in cohort of 73 patients appears to be promising, with significant improvements in TR severity, NYHA class, KCCQ score, and RV remodeling and function noted in this non-randomized patient cohort [58].
TTVR
Several TTVR devices are under development including the following: LuX-Valve (Jenscare biotechnology Co. Ningbo, China), Trisol (Trisol Medical, Yokneam, Israel), CardioValve (CardioValve Ltd., Yehuda, Israel), VDyne (VDyne Inc. Maple Grove, Minnesota), and Topaz (Tricares, Aschheim, Germany). Currently, the largest number of TTVR implants have been the EVOQUE tricuspid valve replacement system.
First-in-human experience with the EVOQUE system in 27 patients presented with follow-up data available up 1 year after TTVR [23, 24]. At baseline, all patients had ≥ severe TR with 89% experiencing NYHA class III or IV symptoms. Significant reductions in TR severity and improvements in NYHA functional class were noted at 30 days. By 1 year, 96% of patients had ≤ moderate TR, and sustained improvements in NYHA functional class were noted. The overall mortality rate at 1 year was 7%.
Subsequently, the prospective, single-arm, multicenter TRISCEND (Edwards EVOQUE Tricuspid Valve Replacement: Investigation of Safety and Clinical Efficacy after Replacement of Tricuspid Valve with Transcatheter Device) studied outcomes in 56 patients after TTVR (Tables 1 and 2) [25]. At baseline, 91% of patients had ≥ severe TR. Thirty-day outcomes demonstrated reduction in TR to mild or less in 98% of patients. Composite major adverse event rate at 30 days was 26.8%, due to 1 cardiovascular death after a failed intervention, 2 reinterventions for device embolization, 1 major access site or vascular complication, and 15 non-fatal bleeding events. Significant improvements in NYHA functional class, KCCQ scores, and 6-min walk tests were also noted at 30 days. A larger analysis of 176 patients with 1-year follow-up demonstrated high rates of device success (94.4%) with 97.6% of patients having no to mild TR at 1-year follow-up [26]. Low rates of cardiovascular mortality (9.4%) were noted at 1 year, and the Kaplan–Meier estimate for heart failure hospitalization was 11.6 ± 2.6%. High rates of severe bleeding (25.5%) and pacemaker requirement were observed (13.3%) at 1 year.
The pivotal TRISCEND II RCT (NCT04482062) randomized 400 patients to the EVOQUE TTVR system versus optimal medical therapy for severe TR. Results of the first 150 patients who were randomized and treated were presented at the 2023 Transcatheter Cardiovascular Therapeutics conference [50••]. Baseline clinical characteristics of these patients are presented in Table 3. At enrollment, > 50% of patients had greater than severe or massive TR. Initial findings demonstrated a 77.1% reduction in TR at 6-month follow-up in patients randomized to the EVOQUE TTVR system (N = 96) when compared with medical therapy (N = 37) (p < 0.001). A substantial difference in KCCQ scores (Δ = 17.8) was also noted between the TTVR and medical therapy groups at 6 months. Trends in NYHA functional class and 6-min walk test also seemed to favor TTVR. Notably, this study also lacked a sham control in the medical therapy group. These results led to recent FDA approval of the EVOQUE TTVR system. Complete 1-year results of the total 400 patient cohort, including further clinical and echocardiographic outcomes, are forthcoming.
Algorithm for Device Choice
Following medical optimization and imaging assessment with transthoracic echocardiogram and TEE for severe symptomatic TR, the structural heart valve team must consider a number of anatomic and clinical factors when determining optimal device selection for an individual patient (Tables 3, Fig. 2).
Etiology of TR
The new etiologic classification divides TR into primary diseases of the leaflets, secondary diseases (with normal leaflets), and CIED-related TR. Secondary disease is further divided into atrial secondary disease with annular and atrial dilatation being the main driver of leaflet malcoaptation and ventricular secondary disease with ventricular dilatation and leaflet tethering resulting in malcoaptation. These etiologies may determine the appropriateness of each class of TTVI.
T-TEER may address primary TR related to degenerative disease but is not appropriate for diseases resulting in leaflet thickening and restriction such as rheumatic disease or carcinoid valvulopathy. TTVR has been used to treat all types of primary disease [23]. Late-stage secondary disease resulting in extreme tethering or low leaflet-to-annulus ratios may be more difficult treat with T-TEER (see discussion of coaptation gaps below); for these patients, TTVR may be effective in reducing TR to ideal levels. Orthotopic TTVR devices are primarily limited by the large annular dimensions which exceed available device sizes. Efforts are underway to develop larger TTVR device sizes for such anatomic circumstances.
CIED-related TR has been recognized as a predictor of TR progression [59, 60]. The diagnosis can typically be made using transthoracic echocardiography with the use of advanced 3D imaging [61]. In the T-TEER trials, up to 23% of patients have a prior pacemaker; however, in the TTVR trials, up to 43% of patients have a prior pacemaker (Table 1). This difference is likely related to the feasibility and efficacy of T-TEER in the setting of CIED-related TR. T-TEER may be feasible even in the setting of CIED-related disease, if there is TR seen distant to the interaction. TTVR can be implanted whether there is CIED-related or CIED-incidental TR. However, the risks for lead interaction or dysfunction with TTVR must also be carefully considered. In the setting of a pre-existing CIED lead across the TV annulus, TTVR will result in “jailing” of the lead which may cause CIED dysfunction [43] and result in difficulty with lead extraction. Lead extraction is a management option not only to reduce the TR in CIED-related disease, but also to allow for easier TTVI implantation. Although transvenous lead extraction is relatively safe, finding an alternative pacing strategy must first be determined particularly in pacer-dependent patients [62]. In circumstances where there are no alternative pacing options if CIED dysfunction occurs or a new pacemaker is required after TTVI, or the patient is at high risk for future pacer infection making jailing the lead undesirable, T-TEER may be preferred over TTVR.
Large Coaptation Gap
Large coaptation gaps have been demonstrated to be a key anatomic predictor of procedural success with T-TEER [34]. Larger coaptation gaps (i.e., > 7 mm) may be associated with greater residual TR and can technically limit optimal placement of T-TEER devices despite availability of longer device arms and independent leaflet capture in both contemporary T-TEER devices as well as the presence of a central spacer with PASCAL. Conversely, large coaptation gaps represent favorable anatomy for orthotopic TTVR, as such a strategy is not dependent on approximating native leaflets.
Leaflet Tethering
While T-TEER devices can be feasible in the setting of leaflet tethering, significant tethering (> 10 mm) is observed in the setting of advanced RV remodeling and can contribute towards presence of larger coaptation gaps. Therefore, the use of T-TEER in this circumstance is associated with residual TR and poor procedural success. Leaflet tethering typically does not limit consideration to proceed with orthotopic TTVR, assuming annular dimensions are within range for a given device.
Leaflet Number and Morphology
Non-trileaflet TV anatomy may pose additional challenges to T-TEER therapy, especially in the presence of dense chords, regurgitant jets which extend into commissures, nonuniform leaflet sizes, and limited grasping area which may result in inadequate leaflet grasp. Such anatomic variants may be feasible for T-TEER depending on the additional complexities to the valve apparatus in addition to the non-trileaflet morphology [32]. Abnormal leaflet morphologies including thickened, shortened, or immobile leaflets (e.g., in the setting of carcinoid, endocarditis, or rheumatic heart disease) or leaflet perforation are not favorable anatomies for T-TEER. However, T-TEER may be feasible in primary TR with flail or prolapsed leaflets. Non-trileaflet valves or the abovementioned morphologies generally should not limit the ability to proceed with orthotopic TTVR with the caveat that careful assessment and consideration may be required in the setting of orthotopic TTVR devices which require anchoring on the ventricular aspects of TV leaflets.
Right Heart Anatomy
The implantation of the devices depends on the ability to achieve coaxiality with landing zone; thus, both approach angle (typically from the vena cava) and the size of the right atrium and RV may affect procedural success. Smaller RV dimensions can limit the ability to maneuver large-bore delivery systems for orthotopic TTVR devices, increasing potential risk for chordal entanglement or RV injury or perforation, and thus depending on RV imaging assessment may range from feasible to unfavorable anatomy for orthotopic TTVR. Given the small profile of the T-TEER device, right heart size is not typically a restriction.
Though afterload mismatch may occur with any TTVI strategy, given that the increase in RV afterload relates directly to the degree of TR reduction, there is greater potential for severe acute increases in afterload with orthotopic TTVR in the setting of near-complete elimination of TR with this strategy.
Antithrombotic Considerations
Patients with severe TR may be at risk for bleeding due a number of reasons including hepatic or renal dysfunction and coagulopathy. As current practice is to initiate oral anticoagulation after orthotopic TTVR given potential for valve thrombosis in the setting of slower flow and lower pressure in the right heart, patients with a contraindication for anticoagulation may not be optimal candidates for this strategy. It remains to be seen whether alternate antithrombotic strategies, such as short-term anticoagulation followed by antiplatelet therapy, may be an acceptable alternative to long-term anticoagulation after TTVR. Conversely, T-TEER generally does not require anticoagulation in the post-procedural setting and may thus be a more attractive option in patients with risk factors for bleeding.
TEE Imaging
TEE imaging is required for both T-TEER and TTVR; however, severe challenges with intraprocedural imaging of valve leaflets and subvalvular anatomy are more likely to render T-TEER feasible or potentially even unfavorable depending on the severity of TEE limitations compared when compared with TTVR. The use of advanced 3D imaging (both TEE and intracardiac echocardiography) have reduced imaging limitations of all TTVI procedures.
Conclusions
Significant advances have been made with regards to transcatheter treatment for valvular heart disease since the inception of the field. There are a number of clinical and anatomic considerations specific to the TR that make catheter-based treatment strategies challenging. T-TEER and TTVR are the most extensively evaluated transcatheter treatments for TR to date. As the field continues to progress, treatment algorithms specific to certain anatomic and physiologic subsets will likely continue to emerge [10, 27, 63, 64]. The recent TRILUMINATE and TRISCEND II pivotal RCTs results prove that T-TEER and TTVR can reduce TR and associated symptoms and highlight some of the strengths and limitations of these two therapies. As these therapies are now FDA approved, it is likely an increasing number of patients will be considered for and treated with these devices. Whether such treatments can improve clinical endpoints such as mortality and heart failure hospitalizations is yet to be determined. Much will be learned from the results of the ongoing and upcoming clinical studies evaluating such therapies (Table 4).
Abbreviations
- 2D:
-
Two-dimensional
- 3D:
-
Three-dimensional
- CIED:
-
Cardiovascular implantable electronic device
- KCCQ:
-
Kansas City Cardiomyopathy Questionnaire
- NYHA:
-
New York Heart Association
- RCT:
-
Randomized clinical trial
- RV:
-
Right ventricle
- TEE:
-
Transesophageal echocardiography
- TR:
-
Tricuspid regurgitation
- T-TEER:
-
Tricuspid transcatheter edge-to-edge repair
- TTVI:
-
Transcatheter tricuspid valve intervention
- TTVR:
-
Transcatheter tricuspid valve replacement
- TV:
-
Tricuspid valve
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
• Hahn RT. Tricuspid regurgitation. N Engl J Med. 2023;388:1876–91. This comprehensive review summarizes the anatomy, pathophysiology, epidemiology, and available clinical data for tricuspid valve disease.
Nath J, Foster E, Heidenreich PA. Impact of tricuspid regurgitation on long-term survival. J Am Coll Cardiol. 2004;43:405–9.
Offen S, Playford D, Strange G, Stewart S, Celermajer DS. Adverse prognostic impact of even mild or moderate tricuspid regurgitation: insights from the National Echocardiography Database of Australia. J Am Soc Echocardiogr. 2022;35:810–7.
Taramasso M, Benfari G, van der Bijl P, Alessandrini H, Attinger-Toller A, Biasco L, et al. Transcatheter versus medical treatment of patients with symptomatic severe tricuspid regurgitation. J Am Coll Cardiol. 2019;74:2998–3008.
Henning RJ. Tricuspid valve regurgitation: current diagnosis and treatment. Am J Cardiovasc Dis. 2022;12:1–18.
Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP 3rd, Gentile F, et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. J Am Coll Cardiol. 2021;77:450–500.
Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, et al. 2021 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. 2022;43:561–632.
Chen Q, Bowdish ME, Malas J, Roach A, Gill G, Rowe G, et al. Isolated tricuspid operations: the society of thoracic surgeons adult cardiac surgery database analysis. Ann Thorac Surg. 2023;115:1162–70.
Scotti A, Sturla M, Granada JF, Kodali SK, Coisne A, Mangieri A, et al. Outcomes of isolated tricuspid valve replacement: a systematic review and meta-analysis of 5,316 patients from 35 studies. EuroIntervention. 2022;18:840–51.
Hahn RT, Brener MI, Cox ZL, Pinney S, Lindenfeld J. Tricuspid regurgitation management for heart failure. JACC Heart Fail. 2023;11:1084–102.
Wang N, Fulcher J, Abeysuriya N, McGrady M, Wilcox I, Celermajer D, et al. Tricuspid regurgitation is associated with increased mortality independent of pulmonary pressures and right heart failure: a systematic review and meta-analysis. Eur Heart J. 2019;40:476–84.
Hahn RT, Badano LP, Bartko PE, Muraru D, Maisano F, Zamorano JL, et al. Tricuspid regurgitation: recent advances in understanding pathophysiology, severity grading and outcome. Eur Heart J Cardiovas Imaging. 2022.
Hahn RT, Lerakis S, Delgado V, Addetia K, Burkhoff D, Muraru D, et al. Multimodality imaging of right heart function: JACC scientific statement. J Am Coll Cardiol. 2023;81:1954–73.
Agarwal V, Hahn R. Tricuspid regurgitation and right heart failure: the role of imaging in defining pathophysiology, presentation, and novel management strategies. Heart Fail Clin. 2023;19:505–23.
Alfieri O, Denti P. Alfieri stitch and its impact on mitral clip. Eur J Cardiothorac Surg. 2011;39:807–8.
Hahn RT, Saric M, Faletra FF, Garg R, Gillam LD, Horton K, et al. Recommended standards for the performance of transesophageal echocardiographic screening for structural heart intervention: from the American Society of Echocardiography. J Am Soc Echocardiogr. 2022;35:1–76.
Eleid MF, Alkhouli M, Thaden JJ, Zahr F, Chadderdon S, Guerrero M, et al. Utility of intracardiac echocardiography in the early experience of transcatheter edge to edge tricuspid valve repair. Circ Cardiovasc Interv. 2021;14:e011118.
von Bardeleben RS, Lurz P, Sorajja P, Ruf T, Hausleiter J, Sitges M, et al. Two-year outcomes for tricuspid repair with a transcatheter edge-to-edge valve repair from the transatlantic TRILUMINATE trial. Circ Cardiovasc Interv. 2023;16:e012888.
•• Sorajja P, Whisenant B, Hamid N, Naik H, Makkar R, Tadros P, et al. Transcatheter repair for patients with tricuspid regurgitation. N Engl J Med. 2023;388:1833–42. This study marks the first pivotal randomized clinical trial evaluating the use of triscuspid transcatheter edge-to-edge repair in patients with tricuspid regurgitation.
Maznyczka A, Pilgrim T. Antithrombotic treatment after transcatheter valve interventions: current status and future directions. Clin Ther. 2023.
Greenbaum AB, Babaliaros VC, Eng MH. Orthotopic transcatheter tricuspid valve replacement. Interv Cardiol Clin. 2022;11:87–94.
Webb J, Hensey M, Fam N, Rodés-Cabau J, Daniels D, Smith R, et al. Transcatheter mitral valve replacement with the transseptal EVOQUE system. JACC Cardiovasc Interv. 2020;13:2418–26.
Fam NP, von Bardeleben RS, Hensey M, Kodali SK, Smith RL, Hausleiter J, et al. Transfemoral transcatheter tricuspid valve replacement with the EVOQUE system: a multicenter, observational, first-in-human experience. JACC Cardiovasc Interv. 2021;14:501–11.
Webb JG, Chuang AM, Meier D, von Bardeleben RS, Kodali SK, Smith RL, et al. Transcatheter tricuspid valve replacement with the EVOQUE system: 1-year outcomes of a multicenter, first-in-human experience. JACC Cardiovasc Interv. 2022;15:481–91.
Kodali S, Hahn RT, George I, Davidson CJ, Narang A, Zahr F, et al. Transfemoral tricuspid valve replacement in patients with tricuspid regurgitation: TRISCEND study 30-day results. JACC Cardiovasc Interv. 2022;15:471–80.
Kodali S, Hahn RT, Makkar R, Makar M, Davidson CJ, Puthumana JJ, et al. Transfemoral tricuspid valve replacement and one-year outcomes: the TRISCEND study. Eur Heart J. 2023;44:4862–73.
Tomlinson S, Rivas CG, Agarwal V, Lebehn M, Hahn RT. Multimodality imaging for transcatheter tricuspid valve repair and replacement. Front Cardiovasc Med. 2023;10:1171968.
Hahn RT, Lawlor MK, Davidson CJ, Badhwar V, Sannino A, Spitzer E, et al. Tricuspid valve academic research consortium definitions for tricuspid regurgitation and trial endpoints. J Am Coll Cardiol. 2023;82:1711–35.
Dannenberg V, Koschutnik M, Donà C, Nitsche C, Mascherbauer K, Heitzinger G, et al. Invasive hemodynamic assessment and procedural success of transcatheter tricuspid valve repair-important factors for right ventricular remodeling and outcome. Front Cardiovasc Med. 2022;9:891468.
Lurz P, Besler C, Schmitz T, Bekeredjian R, Nickenig G, Mollmann H, et al. Short-term outcomes of tricuspid edge-to-edge repair in clinical practice. J Am Coll Cardiol. 2023;82:281–91.
Wild MG, Löw K, Rosch S, Gerçek M, Higuchi S, Massberg S, et al. Multicenter experience with the transcatheter leaflet repair system for symptomatic tricuspid regurgitation. JACC Cardiovasc Interv. 2022;15:1352–63.
Hahn RT, Weckbach LT, Noack T, Hamid N, Kitamura M, Bae R, et al. Proposal for a standard echocardiographic tricuspid valve nomenclature. JACC Cardiovasc Imaging. 2021.
Praz F, Muraru D, Kreidel F, Lurz P, Hahn RT, Delgado V, et al. Transcatheter treatment for tricuspid valve disease. EuroIntervention. 2021;17:791–808.
Besler C, Orban M, Rommel KP, Braun D, Patel M, Hagl C, et al. Predictors of procedural and clinical outcomes in patients with symptomatic tricuspid regurgitation undergoing transcatheter edge-to-edge repair. JACC Cardiovasc Interv. 2018;11:1119–28.
Ruf TF, Hahn RT, Kreidel F, Beiras-Fernandez A, Hell M, Gerdes P, et al. Short-term clinical outcomes of transcatheter tricuspid valve repair with the third-generation MitraClip XTR system. JACC Cardiovasc Interv. 2021;14:1231–40.
Tanaka T, Sugiura A, Kavsur R, Vogelhuber J, Öztürk C, Becher MU, et al. Leaflet-to-annulus index and residual tricuspid regurgitation following tricuspid transcatheter edge-to-edge repair. EuroIntervention. 2022.
Taramasso M, Gavazzoni M, Pozzoli A, Alessandrini H, Latib A, Attinger-Toller A, et al. Outcomes of TTVI in patients with pacemaker or defibrillator leads: data from the TriValve registry. JACC Cardiovasc Interv. 2020;13:554–64.
Lurz J, Rommel KP, Unterhuber M, Besler C, Noack T, Borger M, et al. Safety and efficacy of transcatheter edge-to-edge repair of the tricuspid valve in patients with cardiac implantable electronic device leads. JACC Cardiovasc Interv. 2019;12:2114–6.
Kitamura M, Kresoja KP, Besler C, Leontyev S, Kiefer P, Rommel KP, et al. Impact of tricuspid valve morphology on clinical outcomes after transcatheter edge-to-edge repair. JACC Cardiovasc Interv. 2021;14:1616–8.
Sugiura A, Tanaka T, Kavsur R, Öztürk C, Vogelhuber J, Wilde N. Leaflet configuration and residual tricuspid regurgitation after transcatheter edge-to-edge tricuspid repair. JACC Cardiovasc Interv. 2021;14:2260–70.
Webb JG, Wood DA, Ye J, Gurvitch R, Masson JB, Rodés-Cabau J, et al. Transcatheter valve-in-valve implantation for failed bioprosthetic heart valves. Circulation. 2010;121:1848–57.
Godart F, Baruteau AE, Petit J, Riou JY, Sassolas F, Lusson JR, et al. Transcatheter tricuspid valve implantation: a multicentre French study. Arch Cardiovasc Dis. 2014;107:583–91.
McElhinney DB, Aboulhosn JA, Dvir D, Whisenant B, Zhang Y, Eicken A, et al. Mid-term valve-related outcomes after transcatheter tricuspid valve-in-valve or valve-in-ring replacement. J Am Coll Cardiol. 2019;73:148–57.
Navia JL, Kapadia S, Elgharably H, Harb SC, Krishnaswamy A, Unai S, et al. First-in-human implantations of the navigate bioprosthesis in a severely dilated tricuspid annulus and in a failed tricuspid annuloplasty ring. Circ Cardiovasc Interv. 2017;10.
Hahn RT, George I, Kodali SK, Nazif T, Khalique OK, Akkoc D, et al. Early single-site experience with transcatheter tricuspid valve replacement. JACC Cardiovasc Imaging. 2019;12:416–29.
Lu FL, Ma Y, An Z, Cai CL, Li BL, Song ZG, et al. First-in-man experience of transcatheter tricuspid valve replacement with lux-valve in high-risk tricuspid regurgitation patients. JACC Cardiovasc Interv. 2020;13:1614–6.
Barreiro-Perez M, Estevez-Loureiro R, Baz JA, Piñón MA, Maisano F, Puga L, et al. Cardiovalve transfemoral tricuspid valve replacement assisted with CT-fluoroscopy fusion imaging. JACC Cardiovasc Interv. 2022;15:e197–9.
Zhang Y, Lu F, Li W, Chen S, Li M, Zhang X, et al. A first-in-human study of transjugular transcatheter tricuspid valve replacement with the LuX-Valve Plus system. EuroIntervention. 2023;18:e1088–9.
Hagemeyer D, Merdad A, Ong G, Fam NP. Acute afterload mismatch after transcatheter tricuspid valve repair. JACC Case reports. 2022;4:519–22.
•• Kodali S, on behalf of the TRISCEND II investigators. TRISCEND II trial: a randomized trial of transcatheter tricuspid valve replacement in patients with severe tricuspid regurgitation. Presented at: TCT 2023. October 26, 2023. San Francisco, CA. This presentation includes initial data from the first pivotal randomized clinical trial evaluating orthotopic transcatheter tricuspid valve replacement.
Lurz P, Stephan von Bardeleben R, Weber M, Sitges M, Sorajja P, Hausleiter J, et al. Transcatheter edge-to-edge repair for treatment of tricuspid regurgitation. J Am Coll Cardiol. 2021;77:229–39.
Nickenig G, Weber M, Lurz P, von Bardeleben RS, Sitges M, Sorajja P, et al. Transcatheter edge-to-edge repair for reduction of tricuspid regurgitation: 6-month outcomes of the TRILUMINATE single-arm study. Lancet. 2019;394:2002–11.
Arnold SV, Goates S, Sorajja P, Adams DH, Stephan von Bardeleben R, Kapadia SR, et al. Health status after transcatheter tricuspid-valve repair in patients with severe tricuspid regurgitation: results from the TRILUMINATE pivotal trial. J Am Coll Cardiol. 2023.
Fam NP, Braun D, von Bardeleben RS, Nabauer M, Ruf T, Connelly KA, et al. Compassionate use of the PASCAL transcatheter valve repair system for severe tricuspid regurgitation: a multicenter, observational, first-in-human experience. JACC Cardiovasc Interv. 2019;12:2488–95.
Kodali S, Hahn RT, Eleid MF, Kipperman R, Smith R, Lim DS, et al. Feasibility study of the transcatheter valve repair system for severe tricuspid regurgitation. J Am Coll Cardiol. 2021;77:345–56.
Kodali SK, Hahn RT, Davidson CJ, Narang A, Greenbaum A, Gleason P, et al. 1-year outcomes of transcatheter tricuspid valve repair. J Am Coll Cardiol. 2023;81:1766–76.
Aurich M, Volz MJ, Mereles D, Geis NA, Frey N, Konstandin MH, et al. Initial experience with the PASCAL ace implant system for treatment of severe tricuspid regurgitation. Circ Cardiovasc Interv. 2021;14:e010770.
Wang DD. PASCAL (CLASP IITR update). Phoenix, Arizona: TVT 2023; 2023.
Prihadi EA, Delgado V, Leon MB, Enriquez-Sarano M, Topilsky Y, Bax JJ. Morphologic types of tricuspid regurgitation: characteristics and prognostic implications. JACC Cardiovasc Imag. 2019;12:491–9.
Zhang XX, Wei M, Xiang R, Lu YM, Zhang L, Li YD, et al. Incidence, risk factors, and prognosis of tricuspid regurgitation after cardiac implantable electronic device implantation: a systematic review and meta-analysis. J Cardiothorac Vasc Anesth. 2022;36:1741–55.
Stankovic I, Voigt J-U, Burri H, Muraru D, Sade LE, Haugaa KH, et al. Imaging in patients with cardiovascular implantable electronic devices: part 2—imaging after device implantation. A clinical consensus statement of the European Association of Cardiovascular Imaging (EACVI) and the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J Cardiovasc Imag. 2023;25:e33–54.
Sorajja P, Sato H, Abdelhadi R, Zakaib J, Enriquez-Sarano M, Bapat V, et al. The impact and outcomes of RV lead extraction in CIED-related tricuspid regurgitation. JACC Cardiovasc Interv. 2023.
Blusztein DI, Hahn RT. New therapeutic approach for tricuspid regurgitation: transcatheter tricuspid valve replacement or repair. Front Cardiovasc Med. 2023;10.
Sala A, Hahn RT, Kodali SK, Mack MJ, Maisano F. Tricuspid valve regurgitation: current understanding and novel treatment options. J Soc Cardiovas Angiogr Interv. 2023;101041.
Stolz L, Weckbach LT, Hahn RT, Chatfield AG, Fam NP, von Bardeleben RS, et al. 2-year outcomes following transcatheter tricuspid valve replacement using the EVOQUE system. J Am Coll Cardiol. 2023;81:2374–6.
Danenberg HD, Topilsky Y, Planer D, Maor E, Guetta V, Sievert H, et al. Tricuspid valve repair by chordal grasping: mistral first-in-human trial results at 6 months. JACC Cardiovasc Interv. 2023;16:244–6.
Piayda K, Bertog S, Steffan J, Ilioska-Damkohler P, Beeri R, Sievert K, et al. One-year outcomes of transcatheter tricuspid valve repair with the Mistral device. EuroIntervention. 2023;19:e363–5.
Perlman G, Praz F, Puri R, Ofek H, Ye J, Philippon F, et al. Transcatheter tricuspid valve repair with a new transcatheter coaptation system for the treatment of severe tricuspid regurgitation: 1-year clinical and echocardiographic results. JACC Cardiovasc Interv. 2017;10:1994–2003.
Asmarats L, Perlman G, Praz F, Hensey M, Chrissoheris MP, Philippon F, et al. Long-term outcomes of the FORMA transcatheter tricuspid valve repair system for the treatment of severe tricuspid regurgitation: insights from the first-in-human experience. JACC Cardiovasc Interv. 2019;12:1438–47.
Meduri C, Hahn R, Davidson C, Lim S, Nazif T, Ricciardi M, et al. TCT-74 SCOUT study: Trialign results at 30 days from combined US and EU cohort for the treatment of functional TR. J Am Coll Cardiol. 2018;72:B32–3.
Hahn RT, Meduri CU, Davidson CJ, Lim S, Nazif TM, Ricciardi MJ, et al. Early feasibility study of a transcatheter tricuspid valve annuloplasty: SCOUT trial 30-day results. J Am Coll Cardiol. 2017;69:1795–806.
Zhang X, Jin Q, Pan W, Li W, Guo Y, Ma G, et al. First-in-human study of the K-Clip transcatheter annular repair system for severe functional tricuspid regurgitation. Int J Cardiol. 2023;390:131174.
Nickenig G, Weber M, Schüler R, Hausleiter J, Nabauer M, von Bardeleben RS, et al. Tricuspid valve repair with the Cardioband system: two-year outcomes of the multicentre, prospective TRI-REPAIR study. EuroIntervention. 2021;16:e1264–71.
Gray WA, Abramson SV, Lim S, Fowler D, Smith RL, Grayburn PA, et al. Cardioband TREFSI. 1-year outcomes of Cardioband tricuspid valve reconstruction system early feasibility study. JACC Cardiovasc Interv. 2021;15:1921–32.
Dreger H, Mattig I, Hewing B, Knebel F, Lauten A, Lembcke A, et al. Treatment of severe TRIcuspid regurgitation in patients with advanced heart failure with CAval vein implantation of the Edwards Sapien XT VALve (TRICAVAL): a randomised controlled trial. EuroIntervention. 2020;15:1506–13.
Estevez-Loureiro R, Sanchez-Recalde A, Amat-Santos IJ, Cruz-Gonzalez I, Baz JA, Pascual I, et al. 6-month outcomes of the TricValve system in patients with tricuspid regurgitation: the TRICUS EURO study. JACC Cardiovasc Interv. 2022;15:1366–77.
Wild MG, Lubos E, Cruz-Gonzalez I, Amat-Santos I, Ancona M, Andreas M, et al. Early clinical experience with the TRICENTO bicaval valved stent for treatment of symptomatic severe tricuspid regurgitation: a multicenter registry. Circ Cardiovasc Interv. 2022;15:e011302.
Author information
Authors and Affiliations
Contributions
MM and RTH wrote the main manuscript MM generated the Tables RTH generated the Figures All authors reviewed the manuscript
Corresponding author
Ethics declarations
Conflict of Interest
Dr. Madhavan reports institutional educational grant to Columbia University from Boston Scientific Corporation. Dr. Agarwal reports speaker fees from Abbott Structural, and consulting for Moray Medical, HVR Cardio, and ReNiva Inc. Dr. Hahn reports speaker fees from Abbott Structural, Baylis Medical, Boston Scientific Corporation, Edwards Lifesciences, Philips Healthcare, and Siemens Healthineers and is Chief Scientific Officer for the Echocardiography Core Laboratory at the Cardiovascular Research Foundation for multiple industry-sponsored trials, for which she receives no direct industry compensation.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Madhavan, M.V., Agarwal, V. & Hahn, R.T. Transcatheter Therapy for the Tricuspid Valve: A Focused Review of Edge-to-Edge Repair and Orthotopic Valve Replacement. Curr Cardiol Rep 26, 459–474 (2024). https://doi.org/10.1007/s11886-024-02051-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11886-024-02051-4