Abstract
Background
Familial hypercholesterolemia (FH) due to a founder variant in Apolipoprotein B (ApoBR3500Q) is reported in 12% of the Pennsylvania Amish community. By studying a cohort of ApoBR3500Q heterozygotes and homozygotes, we aimed to characterize the biochemical and cardiac imaging features in children and young adults with a common genetic background and similar lifestyle.
Methods
We employed advanced lipid profile testing, carotid intima media thickness (CIMT), pulse wave velocity (PWV), and peripheral artery tonometry (PAT) to assess atherosclerosis in a cohort of Amish ApoBR3500Q heterozygotes (n = 13), homozygotes (n = 3), and their unaffected, age-matched siblings (n = 9). ApoBR3500Q homozygotes were not included in statistical comparisons.
Results
LDL cholesterol (LDL-C) was significantly elevated among ApoBR3500Q heterozygotes compared to sibling controls, though several ApoBR3500Q heterozygotes had LDL-C levels in the normal range. LDL particles (LDL-P), small, dense LDL particles, and ApoB were also significantly elevated among subjects with ApoBR3500Q. Despite these differences in serum lipids and particles, CIMT and PWV were not significantly different between ApoBR3500Q heterozygotes and controls in age-adjusted analysis.
Conclusions
We provide a detailed description of the serum lipids, atherosclerotic plaque burden, vascular stiffness, and endothelial function among children and young adults with FH due to heterozygous ApoBR3500Q. Fasting LDL-C was lower than what is seen with other forms of FH, and even normal in several ApoBR3500Q heterozygotes, emphasizing the importance of cascade genetic testing among related individuals for diagnosis. We found increased number of LDL particles among ApoBR3500Q heterozygotes but an absence of detectable atherosclerosis.
Similar content being viewed by others
Background
Familial hypercholesterolemia (FH) is a genetic disorder with autosomal dominant inheritance leading to elevated low density lipoprotein cholesterol (LDL-C) levels and predisposition to premature atherosclerosis and cardiovascular disease (CVD) [1]. Symptoms of CVD typically emerge in adults, but atherosclerosis begins during childhood [2,3,4].
A founder variant in Apolipoprotein B-100 (NC_000002.12; NM_000384.3; APOB c.10580G > A; p.Arg3527Gln; previously described as p.Arg3500Gln; referenced here as ApoBR3500Q) has a 12% carrier frequency among the Old Order Amish of Lancaster County, Pennsylvania [5]. Adults who are heterozygous for ApoBR3500Q have 58 mg/dL higher LDL-C and are about 4.5 times more likely to have detectable coronary artery calcification [5]. Early identification of the ApoBR3500Q variant in the Amish community may offer an opportunity to prevent or delay development of atherosclerosis by providing treatments to maintain low LDL-C levels [6, 7].
Individuals with FH due to variants in the LDL receptor (LDLR) have increased carotid intima media thickness (CIMT) by age 8–10 years, and there may be presence of aortic lesions on magnetic resonance imaging [1]. Coronary artery calcification can be seen in 25% of adolescents with LDLR FH [8]. In addition to LDL-C levels, other lipid components, such as Apolipoprotein-B (ApoB), Apolipoprotein A-1 (ApoA-1), LDL and HDL particles (LDL-P and HDL-P), small, dense LDL cholesterol (sdLDL-C), and Lipoprotein(a) [Lp(a)] may also be elevated and associated with atherosclerosis. Less is known regarding atherosclerotic risk in children and young adults with FH caused by variants in APOB.
In the current study, we employed both noninvasive cardiac imaging modalities and advanced lipid profile testing to assess premature atherosclerosis risk in a cohort of Amish subjects with ApoBR3500Q and their unaffected, age-matched siblings. By studying a genetically homogenous cohort of subjects with FH and sibling controls, we aimed to identify and characterize children and young adults with premature atherosclerosis at risk for future CVD.
Methods
Subjects
The Institutional Review Board of Penn Medicine-Lancaster General Hospital (Lancaster, PA) approved the research study. We screened 570 subjects from our internal DNA biobankfor the APOB c.10580G > A variant. We re-contacted individuals/families found to harbor the variant to invite them to participate in the study. Subjects from five families consented to research for themselves or on behalf of their affected children. All subjects were naive to lipid-lowering and antihypertensive medications.
Genetic testing and serum analysis
To genotype individuals for the APOB c.10580G > A variant, we developed a high-resolution melt analysis using an unlabeled probe on a LightScanner 32 System (BioFire Diagnostics, Salt Lake City, UT). We designed the primers (F-TATGCGTTGGAGTGTGGCTTCTCC, R-TGTCAAGGGTTCGGTTCTTTCTCG) and probe (P-CACTGAAGACCGTGTGCTCTTGGAATT) using Primer3 (http://bioinfo.ut.ee/primer3-0.4.0/). We validated the assay in patients, their parents, and siblings of known genotype to demonstrate accurate allele discrimination and genotype calls. Whole exome sequencing was performed by Regeneron Research Center (Tarrytown, NY) as previously described [9]. Lipid analysis and other serum measurements were analyzed from fasting blood by a CLIA-certified laboratory (Health Diagnostic Laboratory, Inc, Richmond, VA).
Echocardiogram
Baseline echocardiograms were performed using the GE Vivid i imaging system and included all standard views to obtain baseline information and rule out structural defects and aortic stenosis.
Carotid intima media thickness
All examinations were performed at the start of the day, prior to activity and meals. Height, weight, and resting blood pressure were obtained. A vascular examination was performed utilizing a linear transducer on the GE Vivid i. ECG-gated longitudinal images of the common carotid artery were obtained for both the left and right carotid with focus on capturing the best images of the intima lining proximal to the carotid bulb. CIMT measurements were obtained offline on GE EchoPAC semi-automated software. Triplicate measurements were made on the posterior wall of the vessel 10 mm away from the carotid bulb and averaged. A Meyer’s arc device was used to record the angle of acquisition for reproducibility. All imaging was conducted by a single echo technician and interpreted by one pediatric cardiologist. Both were blinded to the patient’s genotype at the time of data acquisition.
Pulse wave velocity
Pulse wave velocity was calculated as the traversed distance divided by transit time. Three tracings were obtained per subject and averaged. ECG-gated Doppler image was acquired from the right carotid artery and right femoral artery using the linear probe of the GE Vivid i. After the Doppler images were obtained, the distance (cm) from the site of acquisition to the suprasternal notch was measured to determine the overall distance from the carotid to the femoral artery and determine the site for reproducibility. The ECG gated images were analyzed offline using the GE EchoPAC software to determine transit time. All imaging was conducted by a single echo technician and interpreted by one pediatric cardiologist. Both were blinded to the patient’s genotype at the time of data acquisition.
Peripheral artery tonometry
Peripheral artery tonometry was measured following an overnight fast and prior to physical activity in the left upper extremity using methods previously described (EndoPAT, Intamar Medical Inc, Caesarea, Israel) [10]. Baseline, occlusion (40–60 mmHg above baseline systolic pressure), and post-occlusion intervals were each 5 min.
Statistical analysis
ApoBR3500Q heterozygotes were compared to age-matched wild type sibling controls. ApoBR3500Q homozygotes were not included in statistical analysis due to small sample size but are included in tables and figures for completeness. Descriptive summaries with medians (min–max) or percentages are reported due to small sample sizes and the distributions of the data. For comparisons of median values between groups, we utilized a non-parametric test and report p-values using the Wilcoxon rank-sum test. The Fisher’s exact test was used for proportions. We used the non-parametric Spearman method in cases where correlations are reported and added a Bonferroni correction in cases where multiple correlations were calculated. In cases where the Bonferroni correction was used (Fig. 2 = 10 tests and Fig. 3 = 6 tests), we report the corrected p-value of the test calculated p-value multiplied by the number of tests so that all reported p-values (familywise or individual) can be assessed relative to our desired alpha. We assessed CIMT, PWV, and reactive hyperemia index (RHI) between the control and heterozygous groups using the Wilxoxon rank-sum test as well as the correlation with age using the Spearman method. In all cases, p-values < 0.05 were considered significant and analyses were conducted using Stata version 16.1 (College Station, TX).
Results
Cohort demographics
Twenty-five subjects [13 ApoBR3500Q heterozygotes, 3 ApoBR3500Q homozygotes, and 9 age-matched sibling controls, ages 3–28, 60% male] were included in the study. Families were identified by genetic screening for ApoBR3500Q among patients from the Clinic for Special Children, so index cases had medical comorbidities, including limb-girdle muscular dystrophy (n = 2), congenital CMV (n = 1), familial hypercholanemia (n = 1), Trisomy 21 (n = 1), Rett syndrome (n = 1), propionic acidemia (n = 1), and traumatic brain injury (n = 1). No ApoBR3500Q heterozygotes or homozygotes had xanthomas. Whole exome sequence analysis revealed no additional pathogenic variants in LDLR, APOB, or PCSK9 that could alter lipid homeostasis or atherosclerotic disease progression. Four control subjects were heterozygous for a pathogenic variant in ABCG8 (NM_022437.3; c.1720G > A; p.Gly574Arg), indicating carrier status for recessively inherited sitosterolemia.
There was no significant difference in average age, BMI, systolic blood pressure, free fatty acids, or markers of overall metabolic and organ function (hemoglobin A1c, homocysteine, alanine aminotransferase, estimated glomerular filtration rate, thyroid stimulating hormone) between ApoBR3500Q heterozygotes and sibling controls (Table 1). Inflammatory markers (high sensitivity CRP, fibrinogen, lipoprotein-associated phospholipase A2, and myeloperoxidase) were not significantly different in ApoBR3500Q heterozygotes.
Serum lipid analysis
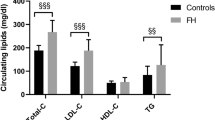
LDL cholesterol was significantly higher in ApoBR3500Q heterozygotes (median 165, range 101–219 mg/dL) compared to controls (median 95, range 59–154 mg/dL, P = 0.001), Table 1 and Fig. 1A. This was reflected in a quantitatively similar increase in total cholesterol in ApoBR3500Q heterozygotes (median 225, range 149–297 mg/dL) compared to controls (median 168, range 126–255 mg/dL, P = 0.021). HDL cholesterol and triglycerides were not different among genotypes.
Fasting serum lipid levels. A Cholesterol and triglyceride levels for controls (white circles), ApoBR3500Q heterozygotes (gray squares), and ApoBR3500Q homozygotes (black triangles). B Several ApoBR3500Q heterozygotes (gray bars) had LDL-C in the normal range (< 130 mg/dL) or below the threshold for suspecting heterozygous FH (160 mg/dL with a family history or 190 mg/dL). All ApoBR3500Q homozygotes (black bar) had LDL-C below 500 mg/dL, the level typically associated with homozygous FH. C LDL-C among controls, ApoBR3500Q heterozygotes, and ApoBR3500Q homozygotes (black triangles) compared to subject age. ApoBR3500Q homozygotes (black triangles) were not included in statistical analysis due to small sample size but are presented for completeness. LDL-C did not correlate significantly with age in controls or ApoBR3500Q heterozygotes. LDL-C association with age measured by Spearman’s rho (rs)
Heterozygous FH is typically suspected in children with LDL-C above 160 mg/dL and a positive family history or LDL-C above 190 mg/dL [6]. In this study of 13 ApoBR3500Q heterozygotes, 3 (23%) had LDL-C above 160 mg/dL and 5 (38%) had LDL above 190 mg/dL (Fig. 1B). All ApoBR3500Q homozygotes had LDL-C above 190 mg/dL, but none above 500 mg/dL, the level typically associated with homozygous FH [1]. LDL-C did not correlate significantly with age in controls or ApoBR3500Q heterozygotes (Fig. 1C).
ApoBR3500Q heterozygotes had significantly higher ApoB (P = 0.001), ApoB:ApoA-1 (P < 0.001), LDL-P (P = 0.001), and sdLDL-P (P = 0.003) compared to controls (Table 2). Small, dense LDL cholesterol (as a percent of LDL-C), Lipoprotein(a) [Lp(a)-P], ApoA-1, HDL-P, and HDL2-C were unaffected by genotype.
Several apolipoproteins, including ApoB, ApoB:ApoA-1, and LDL-P correlated strongly with LDL-C in both controls and ApoBR3500Q heterozygotes (Fig. 2). Small, dense LDL-C correlated with LDL-C in controls, but not ApoBR3500Q heterozygotes. Additional analysis of plant sterols and fatty acids found nominally significantly higher docosahexaenoic acid (DHA, P = 0.034) among ApoBR3500Q heterozygotes compared to controls. No significant differences were noted for campesterol, sitosterol, cholestanol, desmosterol, or omega-3, omega-6, cis-monounsaturated, saturated, or trans fatty acids. (Additional file 1: Supplemental Table 1).
Correlations between LDL-C and lipoproteins and lipid particles. LDL-C correlated significantly with Apolipoprotein B (ApoB), ApoB:ApoA and LDL particles (LDL-P) in controls (white circles) and ApoBR3500Q heterozygotes (gray squares). LDL-C correlated significantly with small dense LDL (sdLDL) in controls, but not ApoBR3500Q heterozygotes. ApoBR3500Q homozygotes (black triangles) were not included in statistical analysis due to small sample size but are presented for completeness. LDL-C association with individual small lipid particles measured by Spearman’s rho (rs)
Cardiovascular imaging
In age-adjusted analysis, CIMT, PWV, and RHI were not significantly different between ApoBR3500Q heterozygotes and controls. No subjects (controls, ApoBR3500Q heterozygotes or homozygotes) had supravalvular aortic stenosis or structural cardiac defects.
CIMT is a measure of atherosclerotic plaque and previous studies report CIMT between 0.38–0.5 mm in healthy children and young adults [11]. Our CIMT measurements were within this range for 10% of controls, 45% of ApoBR3500Q heterozygotes, and 66% of ApoBR3500Q homozygotes, likely reflecting variation in operator-dependent aspects of technique. CIMT measurements did not correlate with LDL-C. There was a correlation between CIMT and age when considering all study subjects in aggregate (overall rs = 0.77, P < 0.001) but no such correlations were found among genotype subgroups (controls or ApoBR3500Q heterozygotes, Fig. 3, top panels). Of note, the interaction effect of LDL-C and age was also significant (coefficient = 0.0001, P = 0.025) in predicting CIMT. We found no significant correlation between CIMT and sdLDL or LDL-P (data not shown).
Endothelial function, vascular stiffness, and atherosclerotic plaque. Carotid intima media thickness (CIMT, a surrogate for atherosclerotic plaque burden), pulse wave velocity (PWV, a measure of vascular stiffness), and reactive hyperemia index (RHI, a measure of endothelial function) were similar in ApoBR3500Q heterozygotes (gray squares) and controls (white circles) and did not correlate with LDL-C or age in either group. CIMT and RHI correlated with age in overall subjects. ApoBR3500Q homozygotes (black triangles) were not included in statistical analysis due to small sample size but are presented for completeness. Imaging values association with LDL-C or age measured by Spearman’s rho (rs)
Normal values for PWV are not firmly established, but previous studies report values between 4.1 and 10.9 m/s for control subjects [11]. Our PWV measurements were within or below this range in all subjects. PWV did not correlate with age or LDL-C in overall subjects, controls or ApoBR3500Q homozygotes (Fig. 3, middle panels). We found no significant correlation between PWV and sdLDL or LDL-P (data not shown).
RHI ≤ 1.67 reflects abnormal nitric oxide-dependent changes in vascular tone and presumably early evidence of CVD. In the current cohort, RHI values were below the previously described normal range (≤ 1.67) for the majority (n = 9, 75%) of subjects under age 20, regardless of genotype (Fig. 3, bottom panels). RHI did not correlate with LDL-C but correlated with age in overall subjects (overall rs = 0.74, P = 0.003), but not subgroups (controls or ApoBR3500Q heterozygotes). Of note, RHI values could not be obtained from 4 subjects (1 control, 1 ApoBR3500Q heterozygote, and 2 ApoBR3500Q homozygotes) due to disability or inability to be still for examination. Five additional values (1 control, 3 ApoBR3500Q heterozygotes, and 1 ApoBR3500Q homozygote) were excluded from the analysis due to poor data quality due to improper probe fitting on small fingers in young subjects. We found no significant correlation between RHI and sdLDL or LDL-P (data not shown).
Discussion
Here, we report advanced lipid testing, cardiovascular imaging, vascular stiffness, and endothelial function for a cohort of children and young adults with the Amish ApoBR3500Q founder variant. Previous studies in children with FH, predominantly LDL receptor defects, have demonstrated higher CIMT among affected children compared to unaffected siblings beginning at 8 to 12 years of age [12]. We found lower LDL-C levels than seen with other forms of FH, increased number of LDL particles, and absence of detectable atherosclerosis.
Advanced lipid testing
Apolipoprotein BR3500Q increases circulating LDL-C and coronary calcification in adults [5]. Comparing LDL-C between adults and children/adolescents with ApoBR3500Q is complicated by the fact that cholesterol levels normally peak around age 9–11 years, fall during adolescence, and increase progressively after age 17 years [13,14,15,16]. Collectively, we find children and adolescents with ApoBR3500Q have elevations of LDL-C compared to age-matched controls, similar to what is observed in adults with the same gene variant. However, our study size was not large enough to demonstrate the expected peak of LDL-C at age 9–11 years and after 17 years. Interestingly, several subjects with ApoBR3500Q had normal or only slightly elevated LDL-C and would thus be overlooked using current cholesterol screening guidelines [17]. This is consistent with previous reports of incomplete penetrance for ApoB variants [18] and underscores the importance of cascade genotyping (rather than lipid screening alone) to identify FH in at-risk family members. The variability in LDL-C elevations among ApoBR3500Q heterozygotes may also be influenced by diet or other environmental factors.
In addition to LDL-C, other serum lipids are associated with atherosclerosis risk in adults, but their predictive utility in pediatrics is largely unknown. All pro-atherogenic lipoproteins, including LDL-C, contain one ApoB surface protein, so serum measurements of ApoB or the ratio of ApoB to ApoA-1 (the primary lipoprotein on HDL, a protective lipoprotein) may more accurately reflect the number of atherogenic particles and better predict CVD risk. Some studies support this hypothesis [19,20,21,22,23,24], while others have found ApoB and ApoB:ApoA-1 to be equivocal to LDL-C in predicting CVD risk [25,26,27,28]. Consistent with this observation, ApoBR3500Q heterozygotes had elevations of ApoB and ApoB:ApoA-1 that were strongly correlated with LDL-C, suggesting that in patients with FH, these biomarkers might be interchangeable with predicted CVD risk.
Cholesterol content in LDL is variable; some particles are large and cholesterol-rich, whereas others are small and dense. LDL particle number (LDL-P) has been shown to be a stronger predictor of CVD risk compared to LDL-C in some studies, particularly when there is discordance between LDL-C and LDL-P [29,30,31], while others have shown its predictive value to be comparable to LDL-C [32]. ApoBR3500Q heterozygotes had significantly increased LDL-P and sdLDL-C and LDL-P correlated strongly with LDL-C.
Lipoprotein(a) consists of a single ApoB with a plasminogen-like protein [apoprotein(a)]. Lipoprotein(a) levels vary greatly among individuals and do not correlate with LDL-C, non-HDL-C, ApoB, or LDL particle number [33]. Lp(a) has been shown to be predictive of CVD risk in adults, independent of LDL-C, especially in those with elevated LDL-C and FH [34,35,36,37]. Lp(a) is higher in individuals with FH and it is postulated that severe elevations of Lp(a) have an FH-like clinical phenotype [38,39,40]. In our study, Lp(a) concentrations were similar among controls and ApoBR3500Q heterozygotes.
Our study also noted significantly increased docosahexaenoic acid (DHA) among ApoBR3500Q heterozygotes compared to controls. Previous studies among Amish adults demonstrated increased sitosterol, campesterol, and stigmasterol associated with heterozygosity for a variant in ABCG8 [41]. This is the first report, to our knowledge, of baseline differences in circulating levels of DHA in FH. Subjects were not asked to report dietary supplement use, so this may be an artifact of supplement use or a true increase in circulating DHA among those with a particular genotype.
Cardiovascular function and anatomy
Contrary to previous reports of FH in children, we did not find increases in CIMT among ApoBR3500Q heterozygotes [42]. In overall subjects, CIMT significantly correlated with age, but this relationship was not significant in controls or ApoBR3500Q heterozygote subgroups. The interaction effect of LDL-C and age was significant, suggesting a cumulative effect of age and LDL-C on CIMT. However, given the small cohort in our study it is difficult to draw a definitive conclusion from this interaction. The study cohort had lower LDL-C values than seen in other forms of FH, which may explain the lack of differences in CIMT.
Pulse wave velocity is a measure of vascular stiffness and can be measured by a variety of methods, including ultrasound and cardiac MRI. Studies using a variety of testing methods show PWV in healthy pediatric subjects to be quite variable [11] and increased with age [43]. Here, we measured PWV with ultrasound and detected values similar to previous reports for control subjects. In contrast to increased PWV previously reported in children with FH [44], PWV among ApoBR3500Q heterozygotes was indistinguishable from controls and we found no significant correlations between PWV and either age or LDL-C.
Abnormal flow-mediated dilation is found in children with a family history of cardiovascular events, FH, and familial combined hyperlipidemia in other studies [45]. We found similar “abnormal” flow mediated dilation in the majority of subjects under age 20 years, regardless of genotype, with improvement to normal ranges after age 20 years. This is contrary to what would be expected based on pathophysiology and raises concern that this imaging technique was inaccurate in our study subjects under age 20 years.
Strengths and limitations
There are few detailed studies of children with FH, and those that exist consist of subjects with a heterogeneous genetic makeup, most commonly LDL receptor defects. We focused on a cohort with identical ApoBR3500Q variants, similar lifestyle, and relatively homogeneous environmental exposures, and compared them to age-matched, sibling controls to limit variation from additional genetic and environmental factors. To further limit spurious or unforeseen background influences, we excluded any other pathogenic APOB, LDLR, PCSK9, or ABCG8 variants by exome sequencing for all study subjects. Several subjects had medical comorbidities unrelated to lipid metabolism that have not been described in previous FH cohorts. Finally, we utilized contemporary imaging techniques, including advanced lipid testing and cardiovascular imaging, which have mainly been studied in adults. The size of our cohort was small and the variant has incomplete penetrance, which may have constrained our ability to detect subtle changes in laboratory values or cardiovascular imaging indices that may have reached statistical significance with a larger sample size. Our study did not include dietary record analysis or supplement use, so relationships between nutrient intake and lipid levels and/or cardiovascular imaging could not be assessed.
Conclusions
We provide a detailed description of the serum lipids, atherosclerotic plaque burden, vascular stiffness, and endothelial function among children and young adults with FH due to ApoBR3500Q. Contrary to previous reports of middle-age and older adults with ApoBR3500Q [2], we did not find any difference in atherosclerotic measures in children and young adults with ApoBR3500Q compared to controls. Fasting LDL-C was higher in children with ApoBR3500Q compared to controls, but normal or below the threshold for suspecting FH for several ApoBR3500Q heterozygotes, emphasizing the importance of cascade genotyping (rather than fasting cholesterol levels alone) among related individuals for diagnosis and early treatment.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- FH:
-
Familial hypercholesterolemia
- CIMT:
-
Carotid intima media thickness
- PWV:
-
Pulse wave velocity
- LDL-C:
-
LDL cholesterol
- CVD:
-
Cardiovascular disease
- ApoB:
-
Apolipoprotein-B
- ApoA-1:
-
Apolipoprotein A-1
- LDL-P:
-
LDL particles
- HDL-P:
-
HDL particles
- sdLDL-C:
-
Small, dense LDL cholesterol
- Lp(a):
-
Lipoprotein(a)
- RHI:
-
Reactive hyperemia index
References
Wiegman A, Gidding SS, Watts GF, Chapman MJ, Ginsberg HN, Cuchel M, et al. Familial hypercholesterolaemia in children and adolescents: gaining decades of life by optimizing detection and treatment. Eur Heart J. 2015;36(36):2425–37.
Berenson GS, Srinivasan SR, Bao W, Newman WP, Tracy RE, Wattigney WA. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. The Bogalusa Heart Study. New Engl J Med. 1998;338(23):1650–6.
Freedman DS, Wattigney WA, Srinivasan S, Newman WP 3rd, Tracy RE, Byers T, et al. The relation of atherosclerotic lesions to antemortem and postmortem lipid levels: the Bogalusa Heart Study. Atherosclerosis. 1993;104(1–2):37–46.
Homma S, Troxclair DA, Zieske AW, Malcom GT, Strong JP. Pathobiological determinants of atherosclerosis in youth research G. Histological changes and risk factor associations in type 2 atherosclerotic lesions (fatty streaks) in young adults. Atherosclerosis. 2011;219(1):184–90.
Shen H, Damcott CM, Rampersaud E, Pollin TI, Horenstein RB, McArdle PF, et al. Familial defective Apolipoprotein B-100 and increased low-density lipoprotein cholesterol and coronary artery calcification in the old order amish. Arch Intern Med. 2010;170(20):1850–5.
Goldberg AC, Hopkins PN, Toth PP, Ballantyne CM, Rader DJ, Robinson JG, et al. Familial hypercholesterolemia: screening, diagnosis and management of pediatric and adult patients: clinical guidance from the National Lipid Association Expert Panel on Familial Hypercholesterolemia. J Clin Lipidol. 2011;5(3 Suppl):S1-8.
Luirink IK, Wiegman A, Kusters DM, Hof MH, Groothoff JW, de Groot E, et al. 20-Year follow-up of statins in children with familial hypercholesterolemia. N Engl J Med. 2019;381(16):1547–56.
Gidding SS, Bookstein LC, Chomka EV. Usefulness of electron beam tomography in adolescents and young adults with heterozygous familial hypercholesterolemia. Circulation. 1998;98(23):2580–3.
Strauss KA, Gonzaga-Jauregui C, Brigatti KW, Williams KB, King AK, Van Hout C, et al. Genomic diagnostics within a medically underserved population: efficacy and implications. Genet Med Off J Am College Med Genet. 2017.
Bonetti PO, Barsness GW, Keelan PC, Schnell TI, Pumper GM, Kuvin JT, et al. Enhanced external counterpulsation improves endothelial function in patients with symptomatic coronary artery disease. J Am Coll Cardiol. 2003;41(10):1761–8.
Urbina EM, Williams RV, Alpert BS, Collins RT, Daniels SR, Hayman L, et al. Noninvasive assessment of subclinical atherosclerosis in children and adolescents: recommendations for standard assessment for clinical research: a scientific statement from the American Heart Association. Hypertension. 2009;54(5):919–50.
Braamskamp MJ, Hutten BA, Wiegman A. Early initiation of statin treatment in children with familial hypercholesterolaemia. Curr Opin Lipidol. 2015;26(3):236–9.
Dai S, Fulton JE, Harrist RB, Grunbaum JA, Steffen LM, Labarthe DR. Blood lipids in children: age-related patterns and association with body-fat indices: project HeartBeat! Am J Prev Med. 2009;37(1 Suppl):S56-64.
Gooding HC, Rodday AM, Wong JB, Gillman MW, Lloyd-Jones DM, Leslie LK, et al. Application of pediatric and adult guidelines for treatment of lipid levels among US adolescents transitioning to young adulthood. JAMA Pediatr. 2015;169(6):569–74.
Hickman TB, Briefel RR, Carroll MD, Rifkind BM, Cleeman JI, Maurer KR, et al. Distributions and trends of serum lipid levels among United States children and adolescents ages 4–19 years: data from the Third National Health and Nutrition Examination Survey. Prev Med. 1998;27(6):879–90.
Kwiterovich PO Jr, Barton BA, McMahon RP, Obarzanek E, Hunsberger S, Simons-Morton D, et al. Effects of diet and sexual maturation on low-density lipoprotein cholesterol during puberty: the Dietary Intervention Study in Children (DISC). Circulation. 1997;96(8):2526–33.
Adolescents EPoIGfCHRRiCa, Institute NHLaB. Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: summary report. Pediatrics. 2011;128 Suppl 5:S213–56.
Fahed AC, Nemer GM. Familial hypercholesterolemia: the lipids or the genes? Nutr Metab (Lond). 2011;8(1):23.
Benn M, Nordestgaard BG, Jensen GB, Tybjaerg-Hansen A. Improving prediction of ischemic cardiovascular disease in the general population using Apolipoprotein B: the Copenhagen City Heart Study. Arterioscler Thromb Vasc Biol. 2007;27(3):661–70.
Chien KL, Hsu HC, Su TC, Chen MF, Lee YT, Hu FB. Apolipoprotein B and non-high density lipoprotein cholesterol and the risk of coronary heart disease in Chinese. J Lipid Res. 2007;48(11):2499–505.
Holme I, Aastveit AH, Jungner I, Walldius G. Relationships between lipoprotein components and risk of myocardial infarction: age, gender and short versus longer follow-up periods in the Apolipoprotein MOrtality RISk study (AMORIS). J Intern Med. 2008;264(1):30–8.
McQueen MJ, Hawken S, Wang X, Ounpuu S, Sniderman A, Probstfield J, et al. Lipids, lipoproteins, and apolipoproteins as risk markers of myocardial infarction in 52 countries (the INTERHEART study): a case-control study. Lancet. 2008;372(9634):224–33.
Parish S, Peto R, Palmer A, Clarke R, Lewington S, Offer A, et al. The joint effects of Apolipoprotein B, Apolipoprotein A1, LDL cholesterol, and HDL cholesterol on risk: 3510 cases of acute myocardial infarction and 9805 controls. Eur Heart J. 2009;30(17):2137–46.
Pischon T, Girman CJ, Sacks FM, Rifai N, Stampfer MJ, Rimm EB. Non-high-density lipoprotein cholesterol and Apolipoprotein B in the prediction of coronary heart disease in men. Circulation. 2005;112(22):3375–83.
Ingelsson E, Schaefer EJ, Contois JH, McNamara JR, Sullivan L, Keyes MJ, et al. Clinical utility of different lipid measures for prediction of coronary heart disease in men and women. JAMA. 2007;298(7):776–85.
Mora S, Otvos JD, Rifai N, Rosenson RS, Buring JE, Ridker PM. Lipoprotein particle profiles by nuclear magnetic resonance compared with standard lipids and apolipoproteins in predicting incident cardiovascular disease in women. Circulation. 2009;119(7):931–9.
Ridker PM, Rifai N, Cook NR, Bradwin G, Buring JE. Non-HDL cholesterol, Apolipoproteins A-I and B100, standard lipid measures, lipid ratios, and CRP as risk factors for cardiovascular disease in women. JAMA. 2005;294(3):326–33.
Shai I, Rimm EB, Hankinson SE, Curhan G, Manson JE, Rifai N, et al. Multivariate assessment of lipid parameters as predictors of coronary heart disease among postmenopausal women: potential implications for clinical guidelines. Circulation. 2004;110(18):2824–30.
Cromwell WC, Otvos JD, Keyes MJ, Pencina MJ, Sullivan L, Vasan RS, et al. LDL particle number and risk of future cardiovascular disease in the framingham offspring study—implications for LDL management. J Clin Lipidol. 2007;1(6):583–92.
Mora S, Buring JE, Ridker PM. Discordance of low-density lipoprotein (LDL) cholesterol with alternative LDL-related measures and future coronary events. Circulation. 2014;129(5):553–61.
Otvos JD, Mora S, Shalaurova I, Greenland P, Mackey RH, Goff DC Jr. Clinical implications of discordance between low-density lipoprotein cholesterol and particle number. J Clin Lipidol. 2011;5(2):105–13.
Blake GJ, Otvos JD, Rifai N, Ridker PM. Low-density lipoprotein particle concentration and size as determined by nuclear magnetic resonance spectroscopy as predictors of cardiovascular disease in women. Circulation. 2002;106(15):1930–7.
Feingold KR, Grunfeld C. Introduction to lipids and lipoproteins. In: De Groot LJ, Chrousos G, Dungan K, Feingold KR, Grossman A, Hershman JM, et al., editors. Endotext. South Dartmouth; 2000.
Alonso R, Andres E, Mata N, Fuentes-Jimenez F, Badimon L, Lopez-Miranda J, et al. Lipoprotein(a) levels in familial hypercholesterolemia: an important predictor of cardiovascular disease independent of the type of LDL receptor mutation. J Am Coll Cardiol. 2014;63(19):1982–9.
Bennet A, Di Angelantonio E, Erqou S, Eiriksdottir G, Sigurdsson G, Woodward M, et al. Lipoprotein(a) levels and risk of future coronary heart disease: large-scale prospective data. Arch Intern Med. 2008;168(6):598–608.
Collaboration ERF, Erqou S, Kaptoge S, Perry PL, Di Angelantonio E, Thompson A, et al. Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. JAMA. 2009;302(4):412–23.
O’Donoghue ML, Morrow DA, Tsimikas S, Sloan S, Ren AF, Hoffman EB, et al. Lipoprotein(a) for risk assessment in patients with established coronary artery disease. J Am Coll Cardiol. 2014;63(6):520–7.
Nordestgaard BG, Langsted A. Lipoprotein(a) as a cause of cardiovascular disease: insights from epidemiology, genetics, and biology. J Lipid Res. 2016;57(11):1953–75.
Orso E, Ahrens N, Kilalic D, Schmitz G. Familial hypercholesterolemia and lipoprotein(a) hyperlipidemia as independent and combined cardiovascular risk factors. Atheroscler Suppl. 2009;10(5):74–8.
Schmitz G, Orso E. Lipoprotein(a) hyperlipidemia as cardiovascular risk factor: pathophysiological aspects. Clin Res Cardiol Suppl. 2015;10:21–5.
Horenstein RB, Mitchell BD, Post WS, Lutjohann D, von Bergmann K, Ryan KA, et al. The ABCG8 G574R variant, serum plant sterol levels, and cardiovascular disease risk in the Old Order Amish. Arterioscler Thromb Vasc Biol. 2013;33(2):413–9.
Kusters DM, Wiegman A, Kastelein JJ, Hutten BA. Carotid intima-media thickness in children with familial hypercholesterolemia. Circ Res. 2014;114(2):307–10.
Avolio AP, Deng FQ, Li WQ, Luo YF, Huang ZD, Xing LF, et al. Effects of aging on arterial distensibility in populations with high and low prevalence of hypertension: comparison between urban and rural communities in China. Circulation. 1985;71(2):202–10.
Riggio S, Mandraffino G, Sardo MA, Iudicello R, Camarda N, Imbalzano E, et al. Pulse wave velocity and augmentation index, but not intima-media thickness, are early indicators of vascular damage in hypercholesterolemic children. Eur J Clin Invest. 2010;40(3):250–7.
Mietus-Snyder M, Malloy MJ. Endothelial dysfunction occurs in children with two genetic hyperlipidemias: improvement with antioxidant vitamin therapy. J Pediatr. 1998;133(1):35–40.
Acknowledgements
We sincerely thank the families who participated in the study and contributed to our growing knowledge. Their commitment to better understanding of genetic disorders and improved care for children with rare disease continues to inspire us.
Funding
Research was funded by private donations and community fundraising for the Clinic for Special Children. Funds were used to purchase medical equipment needed for the study. Donors and fundraisers did not participate in the study design, execution, data analysis, or manuscript preparation.
Author information
Authors and Affiliations
Contributions
KBW contributed to study design, data acquisition, data analysis, and drafted the initial manuscript. MH conducted data analysis and critically reviewed the manuscript. MY and CP contributed to data acquisition, data analysis, and critically reviewed the manuscript. EGP, KWB, and CGJ contributed to study design, data acquisition, and critically reviewed the manuscript. ARS and SG contributed to study design and critically reviewed the manuscript. KAS contributed to study design, data analysis, and critically reviewed the manuscript. DC contributed to study design, data acquisition, data analysis, and critically reviewed the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Institutional Review Board of Penn Medicine-Lancaster General Hospital (Lancaster, PA) approved the research study. Written informed consent was obtained from study participants age 18 years and older to participate in the research study. Written informed consent was obtained from parents on behalf of their children age less than 18 years to participate in the research study. Study was conducted in accordance with the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
SG serves as a consultant for Esperion Therapeutics, Inc. and as part of the advisory board for Silence Therapeutics. ARS and CGJ are employees of Regeneron Pharmaceuticals, Inc. and receive compensation in the form of salary and stock incentives for their employment. Remaining authors have no declarations of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
. Supplemental Table 1: Plant sterols and fatty acids. Fasting plant sterol and fatty acid levels for ApoBR3500Q heterozygotes, homozygotes and age-matched sibling controls. Median (min-max) compared by the exact Wilcoxon rank-sum test.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Williams, K.B., Horst, M., Young, M. et al. Clinical characterization of familial hypercholesterolemia due to an amish founder mutation in Apolipoprotein B. BMC Cardiovasc Disord 22, 109 (2022). https://doi.org/10.1186/s12872-022-02539-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12872-022-02539-3