Abstract
Background
Transcatheter aortic valve replacement (TAVR) has gained increasing acceptance for patients with aortic disease. Both transfemoral (TF-TAVR) and transapical (TA-TAVR) approach were widely adopted while their performances are limited to a few studies with controversial results. This meta-analysis aimed to compare the mortality and morbidity of complications between TF- versus TA-TAVR based on the latest data.
Methods
Electronic databases were searched until April 2021. RCTs and observational studies comparing the outcomes between TF-TAVR versus TA-TAVR patients were included. Heterogeneity assumption was assessed by an I2 test. The pooled odds ratios(OR) or mean differences with corresponding 95% confidence intervals (CI) were used to evaluate the difference for each end point using a fixed-effect model or random-effect model based on I2 test.
Results
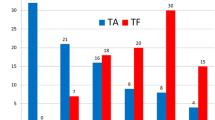
The meta-analysis included 1 RCT and 20 observational studies, enrolling 19,520 patients (TF-TAVR, n = 11,986 and TA-TAVR, n = 7,534). Compared with TA-TAVR, TF-TAVR patients showed significantly lower rate of postoperative in-hospital death (OR = 0.67, 95% CI 0.59–0.77, P < 0.001) and 1-year death (OR = 0.53, 95% CI 0.41–0.69, P < 0.001). Incidence of major bleeding and acute kidney injury were lower and length of hospital stay was shorter, whereas those of permanent pacemaker and major vascular complication were higher in TF-TAVR patients. There were no significant differences between TF-TAVR versus TA-TAVR for stroke and mid-term mortality.
Conclusions
There were fewer early deaths in patients with transfemoral approach, whereas the number of mid-term deaths and stroke was not significantly different between two approaches. TF-TAVR was associated with lower risk of bleeding, acute kidney injury as well as shorter in-hospital stay, but higher incidence of vascular complication and permanent pacemaker implantation.
Similar content being viewed by others
Background
Transcatheter aortic valve replacement (TAVR) is a recognized alternative to surgical aortic valve replacement (SAVR) with superior in mini-invasiveness and noninferior outcomes of postoperative myocardial infarctions, cerebrovascular events, mid-term mortality and stroke [1]. Trials like PARTNER and CoreValve Pivotal Trial have resulted in a Class I, Level of Evidence: a recommendation for patients with symptomatic severe aortic stenosis (AS) and high surgical mortality risk to undergo TAVR [2, 3]. The Indications of TAVR would be further expanded since some recent RCT trials provide promising interim results in low risk patients [4]. As the exclusive percutaneous approach, transfemoral (TF) access is the most preferred and widely adopted route for TAVR for its safety and less-invasiveness [5]. However, approximately 10–15% of the patients with unsuitable iliofemoral anatomy (iliofemoral arteriopathy, tortuosity, severe calcifications, aortic aneurysm, mural thrombus, previous vascular surgery, or small size) requiring alternative approaches for valve deployment [6]. Differed from the retrograde TF approach, another main access- transapical (TA) TAVR—can be achieved by using anterograde access with left-anterior mini-thoracotomy. TA approach extends the feasibility and broadens indication of TAVR, therefore, it is performed in a reasonable proportion of patients [3]. Nevertheless, TA-TAVR is a more invasive procedure associated with high risk of mortality and morbidity, especially for elder patient with severe comorbidities. Some researchers have suggested that TA-TAVR showed poor outcomes compared with SAVR [7, 8]. While most of the previous studies have assessed the performance of TF and TA approaches separately, comparative studies regarding the safety, efficacy, and efficiency between the two approaches were rarely performed. Thus, we systematically reviewed the latest literature regarding this topic and employed a meta-analytic strategy to determine the short and mid-term mortality as well as incidence of major adverse events between TF- versus TA-TAVR.
Methods
This meta-analysis was performed in accordance with the PRISMA guidelines statement [9], the MOOSE statement [10] and the Cochrane Handbook Cochrane Handbook recommendations [11]. A systematic literature search was conducted through online databases including PubMed, ClinicalKey, the Web of Science and Google Scholar up till April 2021. For peer- reviewed publications, the language is not limited. The following key words and Medical Subject Headings (MeSH) terms were used: “transcatheter aortic valve replacement (MeSH)”, “transcatheter aortic valve implantation”, “TAVR”, “TAVI”, “transfemoral”, “transapical”, ‘transapical aortic valve implantation’, ‘transfemoral aortic valve implantation’, ‘transapical aortic valve replacement’ and ‘transfemoral aortic valve replacement’. The search string used for PubMed was ‘(((((((((transcatheter aortic valve replacement) OR (transcatheter aortic valve implantation)) OR (TAVR)) OR (TAVI)) AND (transfemoral)) OR (transapical)) OR (transapical aortic valve implantation)) OR (transfemoral aortic valve implantation)) OR (transapical aortic valve replacement)) OR (transfemoral aortic valve replacement)’. References of original articles were reviewed manually and cross-checked. Two investigators (R.G. and M.X.) conducted the search. Two or more studies published from the same database were included if the studies reported outcomes from different follow-up periods or compared different groups.
Studies were included if they fulfilled the following criteria: (1) randomized controlled trials (RCTs) or observational studies published as original articles; (2) compared TF-TAVR versus TA-TAVR; (3) reported at least one of the following events: death (in-hospital, 1-year, and mid-term), stroke, major vascular events, major bleeding, pacemaker implantation, acute kidney injury, reintervention, endocarditis and length of hospital stay; (4) sample size per group of at least 10 patients. Two investigators (R.G. and M.X.) selected the studies for the inclusion, and studies did not meet any of these criteria were excluded. Conflicts between the two investigators were resolved by consensus.
The eligibility and quality of included studies was evaluated independently by two reviewers (Y.W. and X.H.), and a standardized data collection sheet was used for data extraction. Data on investigators, year, journal, design, study period, follow-up duration, procedural approach, sample size, patient characteristics and outcomes were extracted. Disagreements were resolved by consensus. The quality of RCTs and observational studies was appraised by utilizing the components recommended by the Cochrane Collaboration [12], and ROBINS-I (Risk of Bias in Nonrandomized Studies-of Interventions) respectively [13].
The primary outcome of interest was postoperative in hospital death occuring at 1-year as well as 1 to 5 years which is referred to as mid-term mortality. Secondary outcomes included stroke, major vascular events, major bleeding, pacemaker implantation, acute kidney injury.
The pooled odds ratio (OR) or mean difference and corresponding 95% confidence interval (CI) was calculated for dichotomous and continuous outcomes, respectively. Heterogeneity of the studies was assessed using the Higgins I2 statistic for each outcome. An I2 of 0–25% renders insignificant heterogeneity, 26–50% low heterogeneity, 51–75% moderate heterogeneity and > 75% high heterogeneity [14]. Fixed-effect models of Mantel–Haenszel were used for studies that were homogenous, while Random-effect models of Inverse Variance were used for studies that were heterogenous. Publication bias was assessed visually using a funnel-plot method. Sensitivity analysis was performed by removing studies with the study period finished before 2010. All tests were 2-tailed with a p value of < 0.05 considered significant. Analyses were performed using Review Manager Software from the Cochrane Collaboration (Version 5.3, Copenhagen, Denmark).
Results
Twenty-one studies enrolling 19,520 patients (11,986 undergoing TF-TAVR and 7,534 undergoing TA-TAVR) met the inclusion criteria and were included for the final meta-analysis [6, 15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34]. The search and selection process are shown in Fig. 1. The main characteristics of the included studies are shown in Table 1. Of the 21 studies, 1 was RCT, 9 were prospective observational studies and 11 were retrospective observational studies. The Study quality assessment is summarized in Table 2. The quality of RCT study was high, and among the 20 observational studies, the assessment result of 14 studies was moderate bias, while the remaining 6 studies have serious bias.
Publication bias and heterogeneity for each outcome are listed in Table 3.
Mortality
Postoperative in-hospital mortality was reported in 20 studies. One RCT and 19 observational studies with 18,492 patients were included. In the pooled analysis, in-hospital mortality was significantly lower with TF-TAVR compared with TA-TAVR (OR = 0.67, 95% CI 0.59–0.77, P < 0.001, Fig. 2a).Postoperative 1-year mortality was reported in 5 studies. One RCT and 4 observational studies with 2,313 patients were included. In the pooled analysis, 1-year mortality remained significantly lower in TF-TAVR compared with TA-TAVR (OR = 0.53, 95% CI 0.41–0.69, P < 0.001, Fig. 2b). Postoperative mid-term mortality was reported in 4 observational studies with 5,907 patients. The pooled analysis did not demonstrate a statistically significant difference in the risk of mid-term mortality when comparing TF-TAVR versus TA-TAVR (OR = 0.68, 95% CI 0.46–1.01, P = 0.06, Fig. 2c).
Forest plot of direct comparison meta-analysis of postoperative mortality rate between TF-TAVR versus TA-TAVR: a in-hospital mortality evaluated by M-H fixed-effect model; b 1-year mortality evaluated by M–H fixed-effect model; c mid-term mortality evaluated IV random-effects model. TF: transfemoral; TA: transapical; TAVR: transcatheter aortic valve replacement; OR, odds ratio; CI, confidence interval; H–M, Mantel–Haenszel; IV, inverse variance
Morbidity and other complications
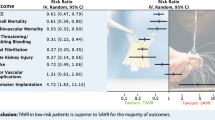
Results for the other outcomes are summarized in Fig. 3. The pooled analysis of 13 studies (12,023 patients) demonstrated a higher risk of major vascular complication with TF-TAVR compared with TA-TAVR (OR = 2.85, 95% CI 1.72–4.71, P < 0.001, Fig. 3a). Meanwhile, in the pooled analysis of 17 studies (n = 8,967), there was a significantly higher incidence of pacemaker implantation in the TF-TAVR group (OR = 1.31, 95% CI 1.12–1.53, P < 0.001, Fig. 3b).
Forest plot of direct comparison meta-analysis of postoperative event rate between TF-TAVR versus TA-TAVR: a major vascular complication evaluated by IV random-effect model; b pacemaker implantation evaluated by M–H fixed-effect model; c major bleeding by IV random-effect model. TF: transfemoral; TA: transapical; TAVR: transcatheter aortic valve. d acute kidney injury evaluated by IV random-effect model; e length of hospital stay evaluated by IV random-effect model; f stroke evaluated by M–H fixed-effect model. TF: transfemoral; TA: transapical; TAVR: transcatheter aortic valve replacement; OR, odds ratio; CI, confidence interval; H–M, Mantel–Haenszel; IV, inverse variance
On the other hand, pooled analyses revealed that TF-TAVR was associated with lower risk for major bleeding (11 studies, 11,741patients, OR = 0.60, 95% CI 0.41–0.86, P = 0.006, Fig. 3c) and acute kidney injury (14 studies, 12,189 patients, OR = 0.41, 95% CI 0.27–0.63, P < 0.001, Fig. 3d), as well as shorter length of hospital stay (8 studies, 13,457 patients, mean difference = -2.88 days, 95% CI -3.56 to -2.19, P < 0.001, Fig. 3e). Pooled analysis of 12 studies (12,293 patients) demonstrated no statistically significant difference in the risk of stroke among patients assigned to TF-TAVR versus TA-TAVR (OR = 0.84 95% CI 0.69–1.02, P = 0.07, Fig. 3f).
Funnel plots for each outcome are shown in Additional file 1: Fig. S1. No significant publication biases were detected. The results of the sensitivity analyses were consistent with the primary analysis for all the endpoints (Additional file 1: Table S1).
Discussion
Since its first clinical application in 2002, TAVR has gone through several generations of evolution and expanded rapidly to be a nonnegligible alternative to SAVR in patients with high and intermediate procedural risk. It is foreseen that the number of TAVR procedures will continue to increase with the appearance of novel generations of prosthetic valves and delivery devices, as well as expanded indications from high-risk and inoperable elder patients to younger and low-risk patients [35]. In addition, patients with native aortic valve regurgitation can also be treated successfully with TAVR with randomized trials under designing aimed to prove its mid and long-term performance [36, 37]. Minimally invasive surgery is the most attractive merit of TAVR, which makes TF approach the preferred one, given its less inherent risk for postoperation complications by avoiding more invasive steps such as mini-thoracotomy and left ventricular puncture in TA-TAVR. However, despite the improvement in device profiles and procedure techniques, TF access is faced with technical limitations such as the sheath size and the prosthetic orifice area, which cannot be performed in a considerable proportion of patients. Thus, TA access remained applicable during these scenario in clinic practice. The attendant problem is whether these two different approaches have similar performances. Several previous studies have compared the outcomes of TF-TAVR versus those of TA-TAVR based on observational studies with relatively early data (before 2014) and small sample size and drew contradictory results. Panchal et al. reported that 1-year mortality was similar in both approaches while TF approach resulted in lower 30-day mortality [38]. Liu et al. concluded a comparable result for both 30-day and 1-year mortality [39]. Conversely, Ghatak et al. reported superior 30-day and mid-term mortality with TF-TAVR [40]. The discrepancy will cause dilemma and confusion for treatment decisions.
By pooling data from 1 RCT and 18 observational trials, this large sample volume meta-analysis has included the latest and most comprehensive studies in this area. The results demonstrated that the mid-term deaths and stroke incidences were comparable between TF-versus TA-TAVR, while the number of early deaths (30-day and 1-year) was smaller with TF approach than with TA approach. Since there was no obvious difference in patient risk factors (using STS or EuroSCORE in different studies) between two approaches, it may be speculated that the higher early mortality with TA approach could be related to (i) the physical damage to the myocardium through direct puncture of the apex, (ii) surgical chest trauma, and (iii) effects of general anesthesia. TA-TAVR has been also associated with cardiac biomarkers level elevation and poorer cardiac function improvement [41]. These perioperative complications appeared to have early rather than mid to long-term consequences. Therefore, performance of patients in TA group surviving beyond the early postoperative convalescence would gradually catch up with those in TF group.
The postoperative complications requiring special attention during the early convalescence in patients with TA-TAVR are acute kidney injury and major bleeding events since they present significantly higher occurrences than those in TF group. Worth noting, these two complications had previously been identified as predictors of adverse outcome including mortality and longer hospital stay following TAVR [42, 43]. Postoperative renal dysfunction is not uncommon in TAVR patients due to the side-effect of contrast media and inadequate renal perfusion during the hemodynamic alterations during the procedure. Moreover, the high incidence of AKI in TA-TAVR patients can also be ascribed to the transfusion for there were more major bleeding events in TA group. Transfusion has been proved to be an independent predictor of AKI as it is associated with the coadministration of some other causative molecular and cellular substances causing kidney injury, such as interleukin-8 which typically accumulates in stored packed red cells [44]. So it is reasonable to emphasize the importance of close monitoring of perioperative renal function, as well as a strict surgical discipline in the execution of TA-TAVR by—among others—strict control of hemostasis, especially the puncture site on heart.
Despite accumulated experiences and meticulous efforts to redesign the transcatheter prosthesis and sheath (smallest sheath size has been reduced to 14 Fr or equivalent nowadays), vascular complications and conduction abnormalities were increasingly observed with TF-TAVR. Consistently, higher incidence of vascular complications may reflect the inherent defect of TF approach. Recent echo-guided puncture and closure devices had emerged to ensure proper entry and hemostasis of the femoral artery. However, vascular complications may be inevitable in patients with poor arterial condition and the key for prevention is a comprehensive preoperative assessment and proper patient selection. On the other hand, the higher incidence of pacemaker implantation in TF-TAVR patients may lead to adverse clinical sequelae on their long-term outcomes through the loss of atrioventricular synchrony, lack of physiological rate control, and unphysiological right ventricular stimulation. The mechanism of conduction tissue injury is speculated to be due to the mechanical pressure from metal struts. Some researchers suggested that the likelihood of pacemaker implantation differs according to valve design (significantly higher with self-expandable valves, marginally elevated with balloon-expandable valves) [45, 46]. The higher rate of pacemaker implantation in TF-TAVR patients may be associated with the position difficulty and repeated attempts during the angiographic deployment. Hence, further technical refinements in valve and sheath design as well as precise image-guided puncture and positioning are warranted to improve the performance of TF-TAVR given the significant impact of conduction abnormalities and major vascular complications.
Several limitations to the current meta-analysis need to be acknowledged. The baseline characteristics between the two approaches could not be compared entirely, attributed to the inherent nature of the meta-analysis. The use of various type and generations of prostheses in these studies may limit the validity of the findings in the current meta-analysis, since there are certain, albeit minor, differences in different TAVR prostheses. Part of these trials were small volumes with limited data to assess outcomes, thus some of these studies may have been underpowered. The overall follow-up period was short to intermediate, that is why some other crucial outcomes such as durability of the prostheses is not investigated. Because of the unavailability of combined MACCE outcomes data in the original studies, we were unable to include them in our analysis. Finally, the data analyzed in this study are mainly observational and with only one randomized concerning transfemoral and transapical access, leading to an indication bias. However, in the shortage of randomized data, the findings of our analysis can further advise the practice of TAVR clinicians and influence future studies. In the future, more randomized controlled trials and comprehensive registries with longer follow-up (> 5-year) will help us to better define the safety and durability, and subsequently, indications of the technique, and the respective places of transfemoral and transapical approaches.
Conclusions
Nowadays, not only elder patients at very high surgical risk or with contraindications to SAVR, but also younger and low-risk patients with aortic valve disease will benefit from TAVR, The availability of both transfemoral and transapical approaches can increase the number of patients who can be treated. In our analysis, the mid-term mortality and risk of stroke are similar with TA- and TF-TAVR. TF-TAVR has significantly less early mortality, but with a higher incidence of major vascular complications and pacemaker implantation. On the other hand, TA-TAVR is associated with a significant increase in the risk of major bleeding, AKI, and has a longer length of hospitalization. Hereby, both TA and TF are effective approaches with satisfactory short to mid-term outcomes for patients need TAVR treatment. However, it is reasonable to make the approach choice based on detailed individualized evaluation and the experience of local heart teams.
Availability of data and materials
The datasets generated and/or analysed during the current study are available in the PubMed (www.pubmed.ncbi.nlm.nih.gov), ClinicalKey (www.clinicalkey.com), the Web of Science (www.webofknowledge.com) and Google Scholar (www.scholar.google.com).
Abbreviations
- AKI:
-
Acute kidney injury
- CI:
-
Confidence interval
- MACCE:
-
Main adverse cardiovascular and cerebrovascular events
- OR:
-
Odds ratio
- RCT:
-
Randomized controlled trial
- SAVR:
-
Surgical aortic valve replacement
- STS:
-
The society of thoracic surgeons
- TA:
-
Transapical
- TAVR:
-
Transcatheter aortic valve replacement
- TAVI:
-
Transcatheter aortic valve implantation
- TF:
-
Transfemoral
References
Panchal HB, Ladia V, Desai S, Shah T, Ramu V. A meta-analysis of mortality and major adverse cardiovascular and cerebrovascular events following transcatheter aortic valve implantation versus surgical aortic valve replacement for severe aortic stenosis. Am J Cardiol. 2013;112(6):850–60.
Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP 3rd, Fleisher LA, Jneid H, Mack MJ, McLeod CJ, O’Gara PT, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. J Am Coll Cardiol. 2017;70(2):252–89.
Holmes DR Jr, Nishimura RA, Grover FL, Brindis RG, Carroll JD, Edwards FH, Peterson ED, Rumsfeld JS, Shahian DM, Thourani VH, et al. Annual outcomes with transcatheter valve therapy: from the STS/ACC TVT registry. Ann Thorac Surg. 2016;101(2):789–800.
Mack MJ, Leon MB, Thourani VH, Makkar R, Kodali SK, Russo M, Kapadia SR, Malaisrie SC, Cohen DJ, Pibarot P, et al. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med. 2019;380(18):1695–705.
Auffret V, Lefevre T, Van Belle E, Eltchaninoff H, Iung B, Koning R, Motreff P, Leprince P, Verhoye JP, Manigold T, et al. Temporal trends in transcatheter aortic valve replacement in France: FRANCE 2 to FRANCE TAVI. J Am Coll Cardiol. 2017;70(1):42–55.
Thomas M, Schymik G, Walther T, Himbert D, Lefevre T, Treede H, Eggebrecht H, Rubino P, Colombo A, Lange R, et al. One-year outcomes of cohort 1 in the Edwards SAPIEN Aortic Bioprosthesis European Outcome (SOURCE) registry: the European registry of transcatheter aortic valve implantation using the Edwards SAPIEN valve. Circulation. 2011;124(4):425–33.
Nielsen HH, Klaaborg KE, Nissen H, Terp K, Mortensen PE, Kjeldsen BJ, Jakobsen CJ, Andersen HR, Egeblad H, Krusell LR et al. A prospective, randomised trial of transapical transcatheter aortic valve implantation vs. surgical aortic valve replacement in operable elderly patients with aortic stenosis: the STACCATO trial. EuroIntervention 2012, 8(3):383–389.
Mack MJ, Leon MB, Smith CR, Miller DC, Moses JW, Tuzcu EM, Webb JG, Douglas PS, Anderson WN, Blackstone EH, et al. 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet. 2015;385(9986):2477–84.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009; 339:b2700.
Bushe CJ, Bradley AJ, Wildgust HJ, Hodgson RE. Schizophrenia and breast cancer incidence: a systematic review of clinical studies. Schizophr Res. 2009;114(1–3):6–16.
Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, Thomas J. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev 2019;10:ED000142.
Corbett MS, Higgins JP, Woolacott NF. Assessing baseline imbalance in randomised trials: implications for the Cochrane risk of bias tool. Res Synth Methods. 2014;5(1):79–85.
Sterne JA, Hernan MA, Reeves BC, Savovic J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron I et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355:i4919.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60.
Thomas M, Schymik G, Walther T, Himbert D, Lefevre T, Treede H, Eggebrecht H, Rubino P, Michev I, Lange R, et al. Thirty-day results of the SAPIEN aortic Bioprosthesis European Outcome (SOURCE) registry: a European registry of transcatheter aortic valve implantation using the Edwards SAPIEN valve. Circulation. 2010;122(1):62–9.
Bosmans JM, Kefer J, De Bruyne B, Herijgers P, Dubois C, Legrand V, Verheye S, Rodrigus I, Belgian TRP. Procedural, 30-day and one year outcome following CoreValve or Edwards transcatheter aortic valve implantation: results of the Belgian national registry. Interact Cardiovasc Thorac Surg. 2011;12(5):762–7.
Ewe SH, Delgado V, Ng AC, Antoni ML, van der Kley F, Marsan NA, de Weger A, Tavilla G, Holman ER, Schalij MJ, et al. Outcomes after transcatheter aortic valve implantation: transfemoral versus transapical approach. Ann Thorac Surg. 2011;92(4):1244–51.
Eltchaninoff H, Prat A, Gilard M, Leguerrier A, Blanchard D, Fournial G, Iung B, Donzeau-Gouge P, Tribouilloy C, Debrux JL, et al. Transcatheter aortic valve implantation: early results of the FRANCE (FRench Aortic National CoreValve and Edwards) registry. Eur Heart J. 2011;32(2):191–7.
Wenaweser P, Pilgrim T, Roth N, Kadner A, Stortecky S, Kalesan B, Meuli F, Bullesfeld L, Khattab AA, Huber C, et al. Clinical outcome and predictors for adverse events after transcatheter aortic valve implantation with the use of different devices and access routes. Am Heart J. 2011;161(6):1114–24.
Dworakowski R, MacCarthy PA, Monaghan M, Redwood S, El-Gamel A, Young C, Bapat V, Hancock J, Wilson K, Brickham B, et al. Transcatheter aortic valve implantation for severe aortic stenosis-a new paradigm for multidisciplinary intervention: a prospective cohort study. Am Heart J. 2010;160(2):237–43.
Murashita T, Greason KL, Pochettino A, Sandhu GS, Nkomo VT, Bresnahan JF, Reeder GS, Holmes DR, Rihal CS, Mathew V. Clinical outcomes after transapical and transfemoral transcatheter aortic valve insertion: an evolving experience. Ann Thorac Surg. 2016;102(1):56–61.
Biancari F, Rosato S, D’Errigo P, Ranucci M, Onorati F, Barbanti M, Santini F, Tamburino C, Santoro G, Grossi C, et al. Immediate and intermediate outcome after transapical versus transfemoral transcatheter aortic valve replacement. Am J Cardiol. 2016;117(2):245–51.
Smith CR, Leon MB, Mack MJ, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364(23):2187–98.
Thourani VH, Kodali S, Makkar RR, Herrmann HC, Williams M, Babaliaros V, Smalling R, Lim S, Malaisrie SC, Kapadia S et al. Transcatheter aortic valve replacement versus surgical valve replacement in intermediate-risk patients: a propensity score analysis. Lancet 2016;387(10034):2218–2225.
Blackstone EH, Suri RM, Rajeswaran J, Babaliaros V, Douglas PS, Fearon WF, Miller DC, Hahn RT, Kapadia S, Kirtane AJ, et al. Propensity-matched comparisons of clinical outcomes after transapical or transfemoral transcatheter aortic valve replacement: a placement of aortic transcatheter valves (PARTNER)-I trial substudy. Circulation. 2015;131(22):1989–2000.
Al-Hijji MA, Zack CJ, Nkomo VT, Pislaru SV, Pellikka PA, Reeder GS, Greason KL, Rihal CS, Eleid MF. Left ventricular remodeling and function after transapical versus transfemoral transcatheter aortic valve replacement. Catheter Cardiovasc Interv. 2019;94(5):738–44.
Arai T, Romano M, Lefevre T, Hovasse T, Farge A, Le Houerou D, Hayashida K, Watanabe Y, Garot P, Benamer H, et al. Direct comparison of feasibility and safety of transfemoral versus transaortic versus transapical transcatheter aortic valve replacement. JACC Cardiovasc Interv. 2016;9(22):2320–5.
Koifman E, Magalhaes M, Kiramijyan S, Escarcega RO, Didier R, Torguson R, Ben-Dor I, Corso P, Shults C, Satler L, et al. Impact of transfemoral versus transapical access on mortality among patients with severe aortic stenosis undergoing transcatheter aortic valve replacement. Cardiovasc Revasc Med. 2016;17(5):318–21.
Schymik G, Wurth A, Bramlage P, Herbinger T, Heimeshoff M, Pilz L, Schymik JS, Wondraschek R, Suselbeck T, Gerhardus J et al. Long-term results of transapical versus transfemoral TAVI in a real world population of 1000 patients with severe symptomatic aortic stenosis. Circ Cardiovasc Interv 2015;8(1).
Gilard M, Eltchaninoff H, Donzeau-Gouge P, Chevreul K, Fajadet J, Leprince P, Leguerrier A, Lievre M, Prat A, Teiger E, et al. Late outcomes of transcatheter aortic valve replacement in high-risk patients: the FRANCE-2 registry. J Am Coll Cardiol. 2016;68(15):1637–47.
Rodes-Cabau J, Webb JG, Cheung A, Ye J, Dumont E, Feindel CM, Osten M, Natarajan MK, Velianou JL, Martucci G, et al. Transcatheter aortic valve implantation for the treatment of severe symptomatic aortic stenosis in patients at very high or prohibitive surgical risk: acute and late outcomes of the multicenter Canadian experience. J Am Coll Cardiol. 2010;55(11):1080–90.
Al-Attar N, Himbert D, Descoutures F, Iung B, Raffoul R, Messika-Zeitoun D, Brochet E, Francis F, Ibrahim H, Vahanian A, et al. Transcatheter aortic valve implantation: selection strategy is crucial for outcome. Ann Thorac Surg. 2009;87(6):1757–62.
Reents W, Barth S, Griese DP, Winkler S, Babin-Ebell J, Kerber S, Diegeler A, Zacher M, Hamm K. Transfemoral versus transapical transcatheter aortic valve implantation: a single-centre experience. Eur J Cardiothorac Surg. 2019;55(4):744–50.
Elbadawi A, Mohamed AH, Elgendy IY, Ogunbayo GO, Megaly M, Shahin HI, Mahmoud K, Omer MA, Abuzaid A, Fujise K, et al. Comparative outcomes of transapical versus transfemoral access for transcatheter aortic valve replacement in diabetics. Cardiol Ther. 2020;9(1):107–18.
Barbash IM, Finkelstein A, Barsheshet A, Segev A, Steinvil A, Assali A, Ben Gal Y, Vaknin Assa H, Fefer P, Sagie A, et al. Outcomes of patients at estimated low, intermediate, and high risk undergoing transcatheter aortic valve implantation for aortic stenosis. Am J Cardiol. 2015;116(12):1916–22.
Pellikka PA, Dangas G. TAVR for severe aortic regurgitation: advancing the frontier. J Am Coll Cardiol. 2017;70(22):2764–5.
Belardi J, Lamelas P. TAVR for aortic valve regurgitation: ready for a randomized trial? Catheter Cardiovasc Interv. 2019;93(2):354–5.
Panchal HB, Ladia V, Amin P, Patel P, Veeranki SP, Albalbissi K, Paul T. A meta-analysis of mortality and major adverse cardiovascular and cerebrovascular events in patients undergoing transfemoral versus transapical transcatheter aortic valve implantation using edwards valve for severe aortic stenosis. Am J Cardiol. 2014;114(12):1882–90.
Liu Z, He R, Wu C, Xia Y. Transfemoral versus transapical aortic implantation for aortic stenosis based on no significant difference in logistic EuroSCORE: a meta-analysis. Thorac Cardiovasc Surg. 2016;64(5):374–81.
Ghatak A, Bavishi C, Cardoso RN, Macon C, Singh V, Badheka AO, Padala S, Cohen MG, Mitrani R, O’Neill W, et al. Complications and mortality in patients undergoing transcatheter aortic valve replacement with edwards SAPIEN & SAPIEN XT valves: a meta-analysis of world-wide studies and registries comparing the transapical and transfemoral accesses. J Interv Cardiol. 2015;28(3):266–78.
Rodes-Cabau J, Gutierrez M, Bagur R, De Larochelliere R, Doyle D, Cote M, Villeneuve J, Bertrand OF, Larose E, Manazzoni J, et al. Incidence, predictive factors, and prognostic value of myocardial injury following uncomplicated transcatheter aortic valve implantation. J Am Coll Cardiol. 2011;57(20):1988–99.
Nuis RJ, Van Mieghem NM, Tzikas A, Piazza N, Otten AM, Cheng J, van Domburg RT, Betjes M, Serruys PW, de Jaegere PP. Frequency, determinants, and prognostic effects of acute kidney injury and red blood cell transfusion in patients undergoing transcatheter aortic valve implantation. Catheter Cardiovasc Interv. 2011;77(6):881–9.
Bagur R, Webb JG, Nietlispach F, Dumont E, De Larochelliere R, Doyle D, Masson JB, Gutierrez MJ, Clavel MA, Bertrand OF, et al. Acute kidney injury following transcatheter aortic valve implantation: predictive factors, prognostic value, and comparison with surgical aortic valve replacement. Eur Heart J. 2010;31(7):865–74.
Merchant AM, Neyra JA, Minhajuddin A, Wehrmann LE, Mills RA, Gualano SK, Kumbhani DJ, Huffman LC, Jessen ME, Fox AA. Packed red blood cell transfusion associates with acute kidney injury after transcatheter aortic valve replacement. BMC Anesthesiol. 2019;19(1):99.
Siontis GC, Praz F, Pilgrim T, Mavridis D, Verma S, Salanti G, Sondergaard L, Juni P, Windecker S. Transcatheter aortic valve implantation vs surgical aortic valve replacement for treatment of severe aortic stenosis: a meta-analysis of randomized trials. Eur Heart J. 2016;37(47):3503–12.
Siontis GC, Juni P, Pilgrim T, Stortecky S, Bullesfeld L, Meier B, Wenaweser P, Windecker S. Predictors of permanent pacemaker implantation in patients with severe aortic stenosis undergoing TAVR: a meta-analysis. J Am Coll Cardiol. 2014;64(2):129–40.
Acknowledgements
Not applicable.
Funding
This study was financially supported by the National Natural Science Foundation of China (No. 81700317) to Y.W. and (No. 81400290) to X.H.
Author information
Authors and Affiliations
Contributions
Conceptualization, Y.W. and X.H.; Methodology, Y.W. and X.H.; Software, W.W.; Formal Analysis, W.W. and W.J.; Data Curation, M.X. and R.G. and W.Y.; Writing—Original Draft Preparation, M.X. and R.G. and W.Y.; Writing—Review & Editing, Y.W. and X.H.; Visualization, W.J.; Supervision, X.H. and Y.W.; Project Administration, M.X. and R.G. and W.Y.; Funding Acquisition, Y.W. and X.H. All authors had read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable as this is a meta-analysis.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Table S1: Results of sensitivity analysis
Additional file 2: Figure S1:
Funnel plot of comparison meta-analysis outcomes between TF-TAVR versus TA-TAVR: (A) Major bleeding events; (B) 30-day mortality; (C) Major vascular complications; (D) Pacemaker implantation; (E) Acute kidney injury; (F) Length of hospital stay; (G)Mid-term mortality; (H) 1-year mortality; (I)Stroke. Guideline for methodology: PRISMA 2020_Checklist
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Guo, R., Xie, M., Yim, W.Y. et al. Dose approach matter? A meta-analysis of outcomes following transfemoral versus transapical transcatheter aortic valve replacement. BMC Cardiovasc Disord 21, 358 (2021). https://doi.org/10.1186/s12872-021-02158-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12872-021-02158-4